Characterization of Coronary Artery Disease in Sepsis Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Ethical Approval
2.3. Clinical Data Collection
2.4. CTCA
2.5. ICA
2.6. Transthoracic Echocardiography (TTE)
2.7. Statistical Analysis
3. Results
3.1. Clinical Data of Sepsis Survivors
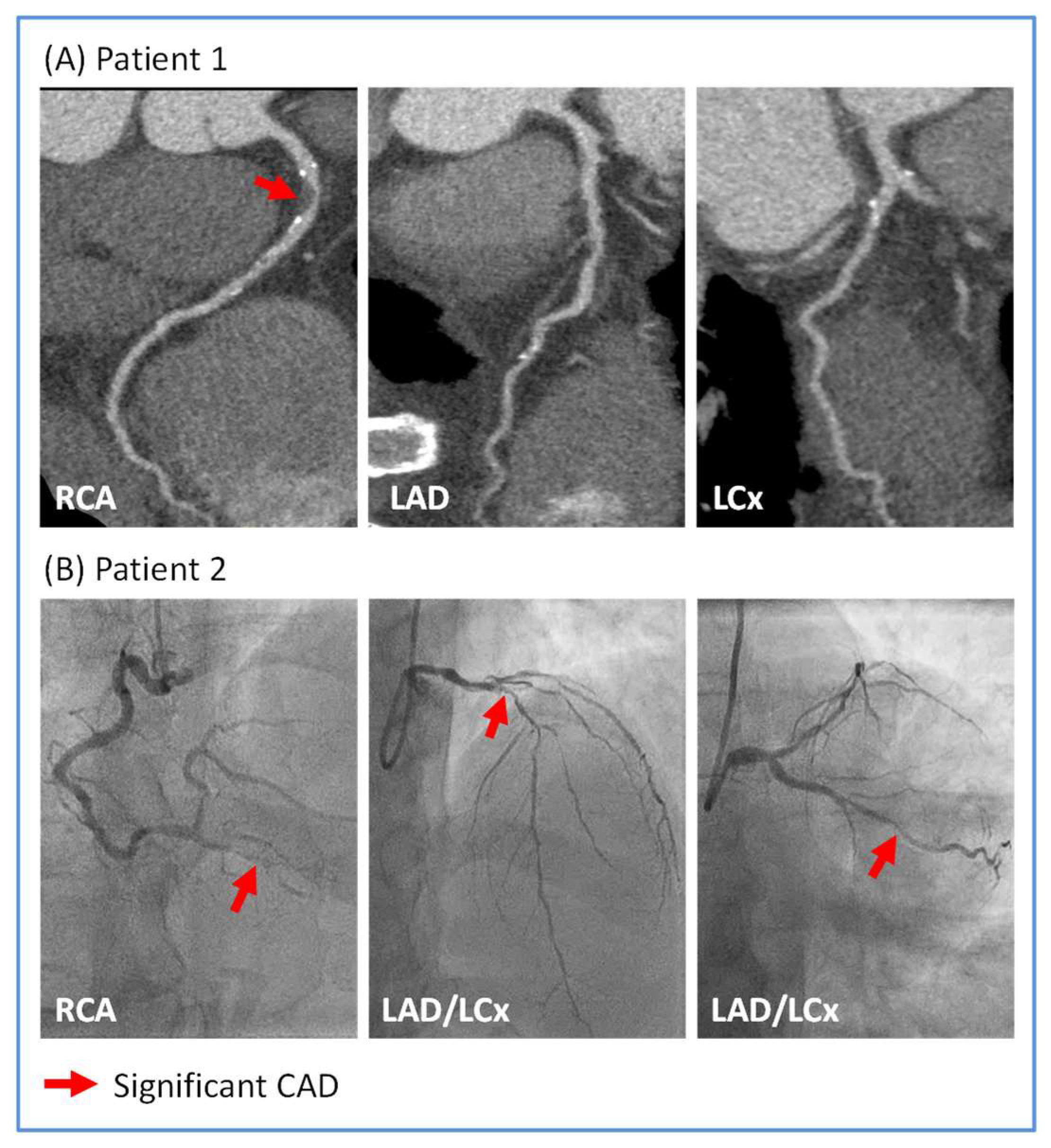
3.2. Distribution of CAD in Sepsis Survivors
3.3. Relationship Between CAD and TTE/Outcomes Data in Sepsis Survivors
4. Discussion
4.1. The Post-Sepsis Cardiovascular Risk
4.2. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Lawler, P.R.; Van Houten, H.K.; Yao, X.; Kashani, K.B.; Dunlay, S.M. Cardiovascular Events Among Survivors of Sepsis Hospitalization: A Retrospective Cohort Analysis. J. Am. Heart Assoc. 2023, 12, e027813. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Štěchovský, C.; Šakalyte, G.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Muehlberg, F.; Blaszczyk, E.; Will, K.; Wilczek, S.; Brederlau, J.; Schulz-Menger, J. Characterization of critically ill patients with septic shock and sepsis-associated cardiomyopathy using cardiovascular MRI. ESC Heart Fail. 2022, 9, 2147–2156. [Google Scholar] [CrossRef]
- Malomo, S.; Oswald, T.; Stephenson, E.; Yip, A.; Alway, T.; Hadjivassilev, S.; Coombs, S.; Ellery, S.; Lee, J.; James, R.; et al. Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance. Diagnostics 2025, 15, 997. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADS™ 2.0—2022 Coronary Artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2022, 16, 536–557. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Yende, S.; Angus, D.C. Long-term impact of sepsis on cardiovascular health. Intensive Care Med. 2019, 45, 78–81. [Google Scholar] [CrossRef]
- Merdji, H.; Schini-Kerth, V.; Meziani, F.; Toti, F. Long-term cardiovascular complications following sepsis: Is senescence the missing link? Ann. Intensive Care 2021, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Merdji, H.; Siegemund, M.; Meziani, F. Acute and Long-Term Cardiovascular Complications among Patients with Sepsis and Septic Shock. J. Clin. Med. 2022, 11, 7362. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.M.; Shelhamer, J.H.; Bacharach, S.L.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984, 100, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.M.; Suffredini, A.F.; Natanson, C.; Ognibene, F.P.; Shelhamer, J.H.; Parrillo, J.E. Responses of left ventricular function in survivors and nonsurvivors of septic shock. J. Crit. Care 1989, 4, 19–25. [Google Scholar] [CrossRef]
- Gupta, V.A.; Sousa, M.; Kraitman, N.; Annabathula, R.; Vsevolozhskaya, O.; Leung, S.W.; Sorrell, V.L. Coronary artery calcification predicts cardiovascular complications after sepsis. J. Crit. Care 2018, 44, 261–266. [Google Scholar] [CrossRef]
- Alnabelsi, T.S.; Gupta, V.A.; Su, L.C.; Thompson, K.L.; Leung, S.W.; Sorrell, V.L. Usefulness of Findings by Multimodality Imaging to Stratify Risk of Major Adverse Cardiac Events After Sepsis at 1 and 12 months. Am. J. Cardiol. 2020, 125, 1732–1737. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Crouser, E.D.; Raman, S.V. Nonischemic myocardial changes detected by cardiac magnetic resonance in critical care patients with sepsis. Am. J. Respir. Crit. Care Med. 2013, 188, 1037–1039. [Google Scholar] [CrossRef]
- Fouda, S.; Godfrey, R.; Pavitt, C.; Alway, T.; Coombs, S.; Ellery, S.M.; Parish, V.; Silberbauer, J.; Liu, A. Cardiac Sarcoidosis and Inherited Cardiomyopathies: Clinical Masquerade or Overlap? J. Clin. Med. 2025, 14, 1609. [Google Scholar] [CrossRef]
- Liu, A.; Munemo, L.T.; Martins, N.; Kouranos, V.; Wells, A.U.; Sharma, R.K.; Wechalekar, K. Assessment of Cardiac Sarcoidosis with PET/CT. J. Nucl. Med. Technol. 2025, 53. [Google Scholar] [CrossRef]
- Liu, A.; Ahmed, R.; Dulay, M.S.; Okafor, J.; Azzu, A.; Ramphul, K.; Shi, R.; Ballo, G.; Baksi, J.A.; Wechalekar, K.; et al. Outcomes of cardiac resynchronization therapy (CRT) in cardiac sarcoidosis patients with a range of ejection fractions. ESC Heart Fail. 2025, 12, 592–602. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Ke, Z.; Ying, B.; Qiao, B.; Yuan, L. The diagnostic and prognostic role of myocardial injury biomarkers in hospitalized patients with COVID-19. Clin. Chim. Acta 2020, 510, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hammond, R.; Chan, K.; Chukwuenweniwe, C.; Johnson, R.; Khair, D.; Duck, E.; Olubodun, O.; Barwick, K.; Banya, W.; et al. Normal high-sensitivity cardiac troponin for ruling-out inpatient mortality in acute COVID-19. PLoS ONE 2023, 18, e0284523. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hammond, R.; Donnelly, P.D.; Kaski, J.C.; Coates, A.R.M. Effective prognostic and clinical risk stratification in COVID-19 using multimodality biomarkers. J. Intern. Med. 2023, 294, 21–46. [Google Scholar] [CrossRef]
- Fouda, S.; Hammond, R.; Donnelly, P.D.; Coates, A.R.M.; Liu, A. COVID-19 Pathophysiology: Inflammation to Cardiac Injury. Hearts 2024, 5, 628–644. [Google Scholar] [CrossRef]
- Han, Y.; Chen, T.; Bryant, J.; Bucciarelli-Ducci, C.; Dyke, C.; Elliott, M.D.; Ferrari, V.A.; Friedrich, M.G.; Lawton, C.; Manning, W.J.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J. Cardiovasc. Magn. Reson. 2020, 22, 26. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2016, 19, 75. [Google Scholar] [CrossRef]
- Jia, S.; Li, J.; Zhang, C.; Liu, Y.; Yuan, D.; Xu, N.; Zhao, X.; Gao, R.; Yang, Y.; Xu, B.; et al. Long-Term Prognosis of Moderate to Severe Coronary Artery Calcification in Patients Undergoing Percutaneous Coronary Intervention. Circ. J. Off. J. Jpn. Circ. Soc. 2020, 85, 50–58. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Morgan, H.; Nazir, M.S.; Li Kam Wa, M.E.; McCann, G.P.; Greenwood, J.P.; McDiarmid, A.K.; Dodd, M.; Ryan, M.; Perera, D.; Chiribiri, A.; et al. Prognostic impact of inducible ischaemia in ischaemic left ventricular dysfunction: The REVIVED-BCIS2 trial. Eur. Heart J. 2024, 46, 487–490. [Google Scholar] [CrossRef]
- Zhou, W.; Sin, J.; Yan, A.T.; Wang, H.; Lu, J.; Li, Y.; Kim, P.; Patel, A.R.; Ng, M.Y. Qualitative and Quantitative Stress Perfusion Cardiac Magnetic Resonance in Clinical Practice: A Comprehensive Review. Diagnostics 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Catania, R.; Quinn, S.; Rahsepar, A.A.; Agirlar Trabzonlu, T.; Bisen, J.B.; Chow, K.; Lee, D.C.; Avery, R.; Kellman, P.; Allen, B.D. Quantitative Stress First-Pass Perfusion Cardiac MRI: State of the Art. Radiographics: A review publication of the Radiological Society of North America, Inc. Radiographics 2025, 45, e240115. [Google Scholar] [CrossRef]
- Hoek, R.; van Diemen, P.A.; Somsen, Y.B.O.; de Winter, R.W.; Jukema, R.A.; Dahdal, J.E.; Raijmakers, P.G.; Driessen, R.S.; Danad, I.; Knaapen, P. Myocardial perfusion imaging in advanced coronary artery disease. Eur. J. Clin. Invest. 2025, 18, e70024. [Google Scholar] [CrossRef]
- Weberling, L.D.; Lossnitzer, D.; Frey, N.; André, F. Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease. Diagnostics 2023, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Guglielmo, M.; Serra, A.; Gatti, M.; Volpato, V.; Schoepf, U.J.; Saba, L.; Cau, R.; Faletti, R.; McGill, L.J.; et al. Multimodality Imaging in Ischemic Chronic Cardiomyopathy. J. Imaging 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, P.; Georgiopoulos, G.; Figliozzi, S.; Klettas, D.; Nicoli, F.; Masci, P.G. Multi-Modality Imaging in Dilated Cardiomyopathy: With a Focus on the Role of Cardiac Magnetic Resonance. Front. Cardiovasc. Med. 2020, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Mansencal, N.; Réant, P.; Piriou, N.; Barone-Rochette, G. Role of multimodality imaging in the diagnosis and management of cardiomyopathies. Arch. Cardiovasc. Dis. 2019, 112, 615–629. [Google Scholar] [CrossRef]
- Casas, G.; Rodríguez-Palomares, J.F. Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis. J. Clin. Med. 2022, 11, 578. [Google Scholar] [CrossRef]
| Patients (n = 30) | |
|---|---|
| Age, years | 57 ± 12 |
| Male | 15 (50) |
| BMI, kg/m2 | 29 ± 5 |
| Sepsis cause | |
| Pneumonia | 12 (40) |
| Unknown origin | 4 (13) |
| Endocarditis | 5 (17) |
| Abscess/Soft tissue | 4 (13) |
| Gastrointestinal | 2 (7) |
| Cellulitis | 2 (7) |
| Urinary tract infection | 1 (3) |
| Care escalation | |
| ICU | 14 (47) |
| HDU/CCU | 4 (13) |
| Ward-based care | 12 (40) |
| Support requirements | |
| Intubation | 5 (17) |
| Vasopressor | 5 (17) |
| Inotrope | 4 (13) |
| Serum biomarkers | |
| Peak CRP, mg/L | 244 [71–316] |
| Peak WCC, ×109/L | 16.5 [12.0–22.9] |
| Peak Hs-cTnT, ng/L | 108 [16–785] |
| Patients (n = 30) | |
|---|---|
| Cardiac symptoms | |
| Chest pain | 14 (47) |
| Dyspnoea | 10 (33) |
| Palpitations | 4 (13) |
| Pre-syncope/Syncope | 1 (3) |
| Co-morbidities | |
| Atrial fibrillation | 10 (33) |
| Hypertension | 7 (23) |
| Diabetes mellitus | 6 (20) |
| Smoking (ex- or current) | 9 (30) |
| Hypercholesterolaemia | 9 (30) |
| CKD | 2 (7) |
| Medications | |
| Anti-platelet drugs | 12 (40) |
| ACE-inhibitor/ARB | 13 (43) |
| Beta-blocker | 15 (50) |
| Sacubitril/Valsartan | 3 (10) |
| MRA | 8 (27) |
| SGLT-2 inhibitor | 7 (23) |
| Calcium channel blockers | 2 (7) |
| Loop diuretics | 6 (20) |
| Statin | 13 (43) |
| Anticoagulation | 8 (27) |
| Patients (n = 30) | |
|---|---|
| Coronary artery assessment | |
| CTCA | 21 (70) |
| ICA | 9 (30) |
| Days from sepsis episode | 39 [12–152] |
| Per-patient CAD (one or more of the following) | |
| Severe stenosis (>70%) | 6 (23) |
| Moderate stenosis (50–70%) | 2 (7) |
| Per-vessel CAD | |
| Severe stenosis (>70%) | |
| Total | 8 (27) |
| LMS | 0 (0) |
| LAD | 4 (13) |
| LCx | 1 (3) |
| RCA | 3 (10) |
| Moderate stenosis (50–70%) | |
| Total | 6 (20) |
| LMS | 0 (0) |
| LAD | 2 (7) |
| LCx | 2 (7) |
| RCA | 2 (7) |
| Multi-vessel disease † | 4 (13) |
| CT coronary artery calcium score | |
| CAD positive (≥50% stenosis) | 638 [368–1015] |
| CAD negative (<50% stenosis) | 4 [1–72] |
| Intervention | |
| PCI and medical therapy | 2 (7) |
| Medical therapy alone | 6 (23) |
| Echocardiography data | |
| LVEF ≥50% | 19 (63) |
| LVEF <50% | 11 (37) |
| LVIDd, cm | 4.9 ± 0.8 |
| LVIDs, cm | 3.1 [2.6–3.5] |
| Septal wall thickness, cm | 1.0 [0.9–1.2] |
| Posterior wall thickness, cm | 1.1 [0.9–1.2] |
| TAPSE, cm | 2.1 ± 0.4 |
| RV S’ | 13 ± 4 |
| LA volume, cm2 | 48 [40–72] |
| Clinical outcomes data | |
| Follow up period, months | 16 [2–40] |
| Total composite end points | 7 (23) |
| Death | 5 (17) |
| Heart failure hospitalization | 2 (7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malomo, S.; Oswald, T.; Alway, T.; Hadjivassilev, S.; Coombs, S.; Ellery, S.; Lee, J.; Phillips, C.; Philips, B.; James, R.; et al. Characterization of Coronary Artery Disease in Sepsis Survivors. Biomedicines 2025, 13, 1181. https://doi.org/10.3390/biomedicines13051181
Malomo S, Oswald T, Alway T, Hadjivassilev S, Coombs S, Ellery S, Lee J, Phillips C, Philips B, James R, et al. Characterization of Coronary Artery Disease in Sepsis Survivors. Biomedicines. 2025; 13(5):1181. https://doi.org/10.3390/biomedicines13051181
Chicago/Turabian StyleMalomo, Samuel, Thomas Oswald, Thomas Alway, Stanislav Hadjivassilev, Steven Coombs, Susan Ellery, Joon Lee, Claire Phillips, Barbara Philips, Rachael James, and et al. 2025. "Characterization of Coronary Artery Disease in Sepsis Survivors" Biomedicines 13, no. 5: 1181. https://doi.org/10.3390/biomedicines13051181
APA StyleMalomo, S., Oswald, T., Alway, T., Hadjivassilev, S., Coombs, S., Ellery, S., Lee, J., Phillips, C., Philips, B., James, R., Hildick-Smith, D., Parish, V., & Liu, A. (2025). Characterization of Coronary Artery Disease in Sepsis Survivors. Biomedicines, 13(5), 1181. https://doi.org/10.3390/biomedicines13051181






