Predictors of Poor Long-Term Outcomes in Patients with Newly Diagnosed Asymptomatic Cardiac Sarcoidosis: A Cardiovascular Magnetic Resonance Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Protocol
2.3. Reproducibility Analysis
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. Results of Multimodality Imaging
3.3. Clinical Outcomes
3.4. Predictors of Adverse Events
4. Discussion
4.1. Predictors of Adverse Outcomes
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CMR | Cardiovascular magnetic resonance |
| CS | Cardiac sarcoidosis |
| GLS | Global longitudinal strain |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| LGE | Late gadolinium enhancement |
| PET | Positron emission tomography |
| ROC | Receiver-operating characteristic |
| RV | Right ventricle |
| RVFW | Right ventricular free wall |
| VT | Ventricular tachycardia |
References
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S.B. Cardiac Sarcoidosis. JACC 2016, 68, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kandolin, R.; Lehtonen, J.; Airaksinen, J.; Vihinen, T.; Miettinen, H.; Ylitalo, K.; Kaikkonen, K.; Tuohinen, S.; Haataja, P.; Kerola, T.; et al. Cardiac Sarcoidosis: Epidemiology, Characteristics, and Outcome over 25 Years in a Nationwide Study. Circulation 2015, 131, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.; Uusitalo, V.; Pöyhönen, P.; Mäyränpää, M.I.; Kupari, M. Cardiac Sarcoidosis: Phenotypes, Diagnosis, Treatment, and Prognosis. Eur. Heart J. 2023, 44, 1495–1510. [Google Scholar] [CrossRef]
- Aitken, M.; Chan, M.V.; Urzua Fresno, C.; Farrell, A.; Islam, N.; McInnes, M.D.F.; Iwanochko, M.; Balter, M.; Moayedi, Y.; Thavendiranathan, P.; et al. Diagnostic Accuracy of Cardiac MRI versus FDG PET for Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis. Radiology 2022, 304, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Lancellotti, P.; Hyafil, F.; Blankstein, R.; Schwartz, R.G.; Jaber, W.A.; Russell, R.; Gimelli, A.; Rouzet, F.; et al. A Joint Procedural Position Statement on Imaging in Cardiac Sarcoidosis: From the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J. Nucl. Cardiol. 2018, 25, 298–319. [Google Scholar] [CrossRef]
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of Myocardial Damage in Patients with Sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef]
- Kouranos, V.; Tzelepis, G.E.; Rapti, A.; Mavrogeni, S.; Aggeli, K.; Douskou, M.; Prasad, S.; Koulouris, N.; Sfikakis, P.; Wells, A.; et al. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 1437–1447. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis. Heart Rhythm. 2014, 11, 1304–1323. [Google Scholar] [CrossRef]
- Cheng, R.K.; Kittleson, M.M.; Beavers, C.J.; Birnie, D.H.; Blankstein, R.; Bravo, P.E.; Gilotra, N.A.; Judson, M.A.; Patton, K.K.; Rose-Bovino, L.; et al. Diagnosis and Management of Cardiac Sarcoidosis: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1197–e1216. [Google Scholar] [CrossRef]
- Society for Cardiovascular Magnetic Resonance; Board of Trustees Task Force on Standardized Protocols; Kramer, C.M.; Barkhausen, J.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized Cardiovascular Magnetic Resonance (CMR) Protocols 2013 Update. J. Cardiovasc. Magn. Reson. 2013, 15, 91. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Chapelon-Abric, C.; Resche-Rigon, M.; Saadoun, D.; Desbois, A.C.; Biard, L. Cardiac Sarcoidosis: A Long Term Follow up Study. PLoS ONE 2020, 15, e0238391. [Google Scholar] [CrossRef] [PubMed]
- Nordenswan, H.-K.; Pöyhönen, P.; Lehtonen, J.; Ekström, K.; Uusitalo, V.; Niemelä, M.; Vihinen, T.; Kaikkonen, K.; Haataja, P.; Kerola, T.; et al. Incidence of Sudden Cardiac Death and Life-Threatening Arrhythmias in Clinically Manifest Cardiac Sarcoidosis with and Without Current Indications for an Implantable Cardioverter Defibrillator. Circulation 2022, 146, 964–975. [Google Scholar] [CrossRef]
- Ekström, K.; Lehtonen, J.; Nordenswan, H.-K.; Mäyränpää, M.I.; Räisänen-Sokolowski, A.; Kandolin, R.; Simonen, P.; Pietilä-Effati, P.; Alatalo, A.; Utriainen, S.; et al. Sudden Death in Cardiac Sarcoidosis: An Analysis of Nationwide Clinical and Cause-of-Death Registries. Eur. Heart J. 2019, 40, 3121–3128. [Google Scholar] [CrossRef]
- Kitai, T.; Nabeta, T.; Naruse, Y.; Taniguchi, T.; Yoshioka, K.; Miyakoshi, C.; Kurashima, S.; Miyoshi, Y.; Tanaka, H.; Okumura, T.; et al. Comparisons between Biopsy-Proven versus Clinically Diagnosed Cardiac Sarcoidosis. Heart 2022, 108, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Nabeta, T.; Kitai, T.; Naruse, Y.; Taniguchi, T.; Yoshioka, K.; Tanaka, H.; Okumura, T.; Sato, S.; Baba, Y.; Kida, K.; et al. Risk Stratification of Patients with Cardiac Sarcoidosis: The ILLUMINATE-CS Registry. Eur. Heart J. 2022, 43, 3450–3459. [Google Scholar] [CrossRef]
- Hulten, E.; Agarwal, V.; Cahill, M.; Cole, G.; Vita, T.; Parrish, S.; Bittencourt, M.S.; Murthy, V.L.; Kwong, R.; Di Carli, M.F.; et al. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients with Suspected Cardiac Sarcoidosis Is Associated with Adverse Cardiovascular Prognosis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2016, 9, e005001. [Google Scholar] [CrossRef]
- Kusano, K.; Ishibashi, K.; Noda, T.; Nakajima, K.; Nakasuka, K.; Terasaki, S.; Hattori, Y.; Nagayama, T.; Mori, K.; Takaya, Y.; et al. Prognosis and Outcomes of Clinically Diagnosed Cardiac Sarcoidosis Without Positive Endomyocardial Biopsy Findings. JACC Asia 2021, 1, 385–395. [Google Scholar] [CrossRef]
- Ise, T.; Hasegawa, T.; Morita, Y.; Yamada, N.; Funada, A.; Takahama, H.; Amaki, M.; Kanzaki, H.; Okamura, H.; Kamakura, S.; et al. Extensive Late Gadolinium Enhancement on Cardiovascular Magnetic Resonance Predicts Adverse Outcomes and Lack of Improvement in LV Function after Steroid Therapy in Cardiac Sarcoidosis. Heart 2014, 100, 1165–1172. [Google Scholar] [CrossRef]
- Niemelä, M.; Uusitalo, V.; Pöyhönen, P.; Schildt, J.; Lehtonen, J.; Kupari, M. Incidence and Predictors of Atrial Fibrillation in Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2022, 15, 1622–1631. [Google Scholar] [CrossRef]
- Schuller, J.L.; Zipse, M.; Crawford, T.; Bogun, F.; Beshai, J.; Patel, A.R.; Sweiss, N.J.; Nguyen, D.T.; Aleong, R.G.; Varosy, P.D.; et al. Implantable Cardioverter Defibrillator Therapy in Patients with Cardiac Sarcoidosis. J. Cardiovasc. Electrophysiol. 2012, 23, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.; Mueller, G.; Sarsam, S.; Prasitdumrong, H.; Chaiyen, N.; Gu, X.; Schuller, J.; Kron, J.; Nour, K.A.; Cheng, A.; et al. Magnetic Resonance Imaging for Identifying Patients with Cardiac Sarcoidosis and Preserved or Mildly Reduced Left Ventricular Function at Risk of Ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 2014, 7, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Osborne, M.; Naya, M.; Waller, A.; Kim, C.K.; Murthy, V.L.; Kazemian, P.; Kwong, R.Y.; Tokuda, M.; Skali, H.; et al. Cardiac Positron Emission Tomography Enhances Prognostic Assessments of Patients with Suspected Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2014, 63, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Albakaa, N.K.; Sato, K.; Iida, N.; Yamamoto, M.; Machino-Ohtsuka, T.; Ishizu, T.; Ieda, M. Association between Right Ventricular Longitudinal Strain and Cardiovascular Events in Patients with Cardiac Sarcoidosis. J. Cardiol. 2022, 80, 549–556. [Google Scholar] [CrossRef]
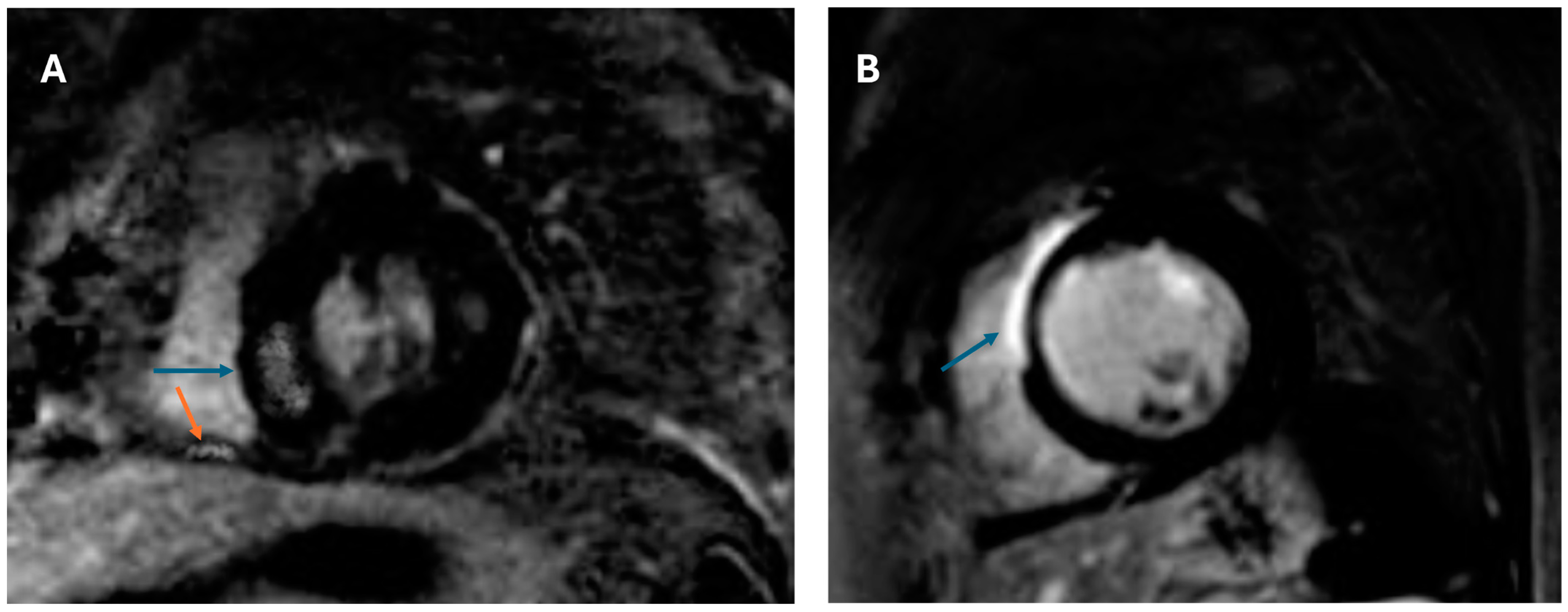
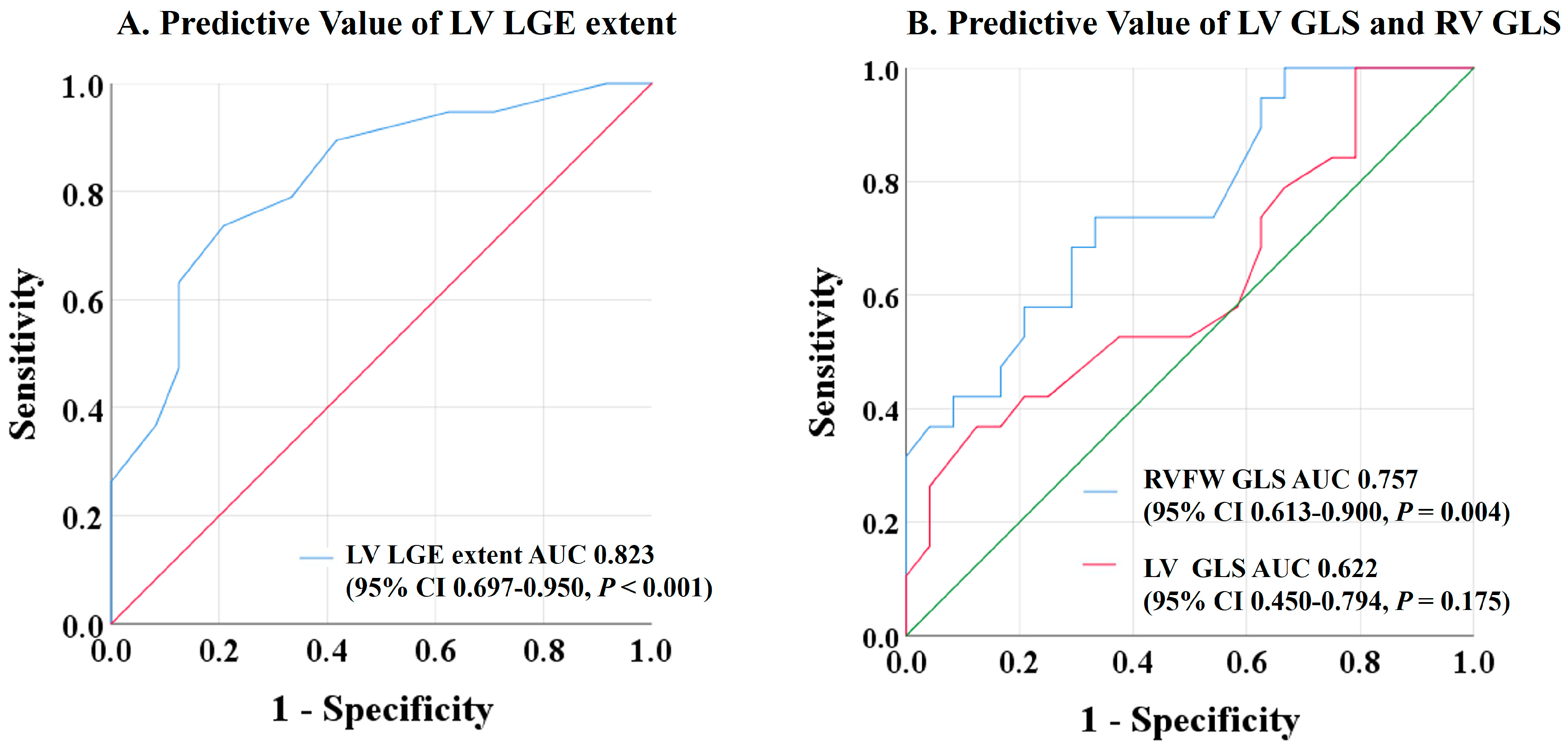
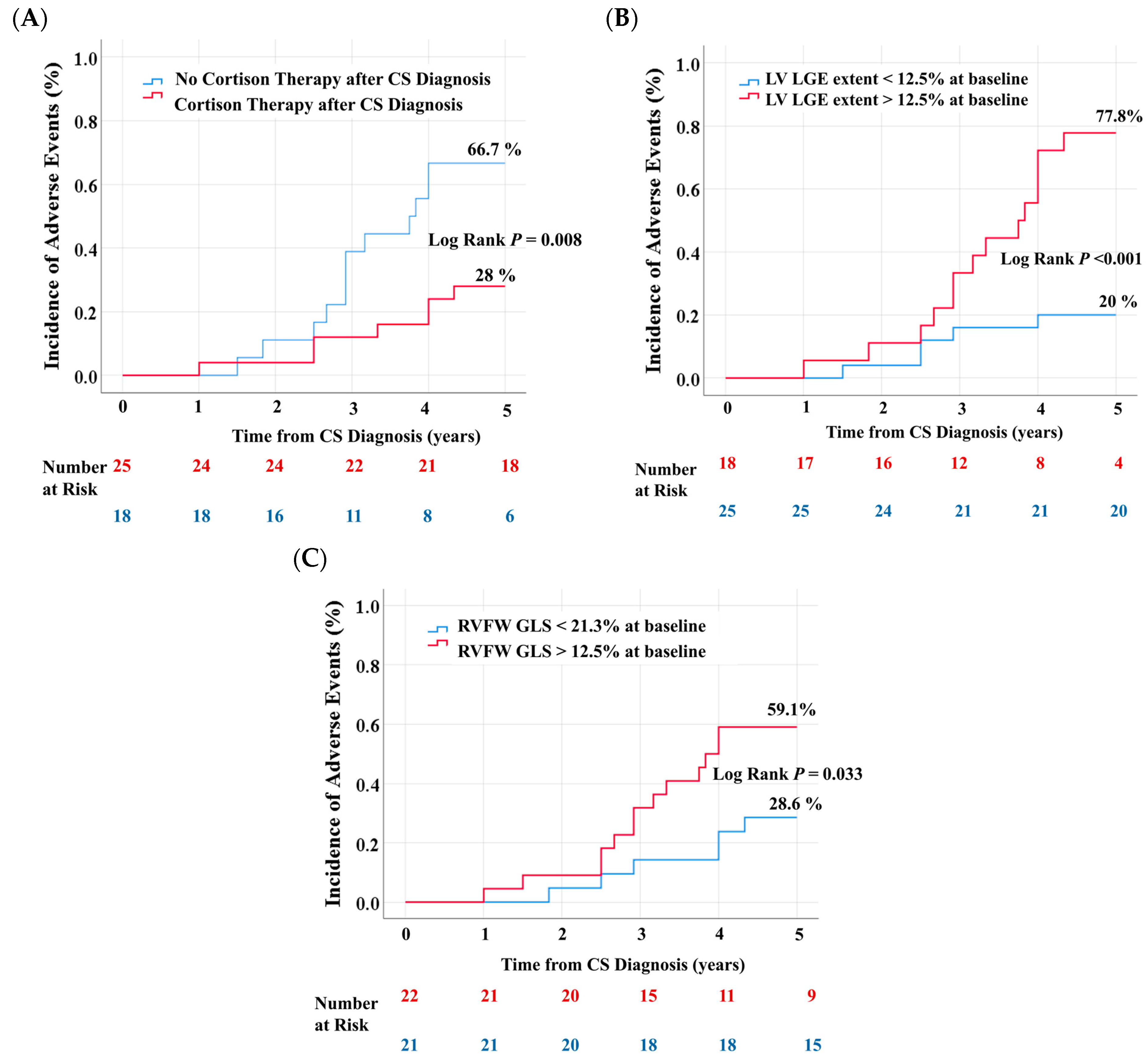
| No Event (n = 24) | Event (n = 19) | p Value | |
|---|---|---|---|
| Age | 54 ± 9 | 59 ± 12 | 0.085 |
| Female | 11 (45.8) | 14 (73.7) | 0.066 |
| Body Mass Index | 1.9 ± 0.2 | 1.9 ± 0.3 | 0.765 |
| Comorbidities | |||
| Hypertension | 7 (29.2) | 5 (26.3) | 0.836 |
| Diabetes Mellitus | 1 (4.2) | 2 (10.5) | 0.416 |
| Coronary artery disease | 2 (8.3) | 0 (0) | 0.198 |
| Sleep apnea | 1 (4.2) | 3 (15.8) | 0.193 |
| Definite diagnosis | 4 (16.7) | 3 (15.8) | 0.938 |
| Probable diagnosis | 20 (83.3) | 16 (84.2) | 0.938 |
| Extracardiac sarcoidosis | 23 (95.8) | 19 (100) | 0.368 |
| Extracardiac organ involvement | |||
| Lung | 22 (91.7) | 18 (94.7) | 0.695 |
| Lymph Node | 10 (41.7) | 7 (36.8) | 0.748 |
| Skin | 3 (12.5) | 3 (15.8) | 0.757 |
| Laboratory values | |||
| Troponin T (pg/mL) | 8 ± 3 | 10 ± 3 | 0.182 |
| NT-proBNP (ng/L) | 291 ± 156 | 355 ± 129 | 0.160 |
| GFR (mL/min) | 81 ± 14 | 69 ± 121 | 0.030 |
| No Event (n = 24) | Event (n = 19) | p Value | |
|---|---|---|---|
| CMR | |||
| LVEDVI, mL/m2 | 90 ± 13 | 89 ± 13 | 0.876 |
| LVEF % | 58 ± 4 | 57 ± 2 | 0.424 |
| LV Mass g/m2 | 62 ± 15 | 62 ± 14 | 0.923 |
| LGE extent, % of LV mass | 11 ± 3 | 14 ± 3 | <0.001 |
| RVEDVI, mL/m2 | 86 ± 12 | 85 ± 14 | 0.857 |
| RVEF % | 58 ± 3 | 56 ± 4 | 0.230 |
| RV LGE | 1 (4.2) | 5 (26.3) | 0.037 |
| RVFW-GLS (%) | −21.7 ± 1.7 | −19.6 ± 2.2 | 0.001 |
| LV-GLS (%) | −13.2 ± 1.5 | −12.6 ± 1.2 | 0.142 |
| Global Native T1, ms a | 1078 ± 36 | 1166 ± 85 | 0.021 |
| Global ECV | 27.4 ± 2.5 | 31 ± 3.4 | 0.019 |
| Global T2, ms | 55 ± 8 | 57 ± 5 | 0.390 |
| PET b | |||
| Ventricular 18F-FDG uptake | 6/7 (85.7) | 8/11 (72.7) | 0.518 |
| HR (95% CI) | p Value | |
|---|---|---|
| Demographics/Comorbidities | ||
| Age | 1.05 (0.99–1.12) | 0.091 |
| Gender | 3.30 (0.90–12.13) | 0.071 |
| Hypertension | 1.15 (0.30–4.43) | 0.836 |
| Lack of cortison therapy after CS diagnosis | 5.14 (1.38–19.10) | 0.014 |
| Diagnosis through myocardial biopsy | 0.938 (0.183–4.80) | 0.938 |
| Laboratory Variables | ||
| NT-proBNP (ng/mL) | 1.003(0.999–1.007) | 0.159 |
| Troponin (pg/mL) | 1.61 (0.93–1.44) | 0.182 |
| GFR (mL/min) | 0.96 (0.92–0.99) | 0.042 |
| Imaging Variables | ||
| LVEDVI, mL/m2 | 0.99 (0.95–1.05) | 0.873 |
| RVEDVI, mL/m2 | 0.99 (0.95–1.04) | 0.853 |
| RVFW-GLS (%) | 0.57 (0.38–0.84) | 0.005 |
| LV-GLS (%) | 0.69 (0.43–1.13) | 0.147 |
| Ventricular 18F-FDG uptake | 2.25 (0.18–27.36) | 0.525 |
| Global Native T1 Mapping, ms | 1.02 (0.99–1.04) | 0.058 |
| Global ECV (%) | 1.49 (1.05–2.20) | 0.042 |
| Global T2, ms | 1.08 (0.91–1.28) | 0.369 |
| LVEF (%) | 0.93 (0.78–1.10) | 0.418 |
| LV LGE extent (%) | 1.54 (1.18–2.02) | 0.002 |
| LV Mass, g/m2 | 0.99 (0.96–1.04) | 0.921 |
| RVEF (%) | 0.90 (0.76–1.07) | 0.229 |
| RV LGE | 8.21 (0.68–77.77) | 0.066 |
| Model 1 (n = 43, e = 19) | Model 2 (n = 43, e = 19) | Model 3 (n = 43, e = 19) | Model 4 (n = 43, e = 19) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.08 (0.99–1.18) | 0.077 | - | N/A | 1.07 (0.99–17) | 0.083 | - | N/A |
| Cortison | - | N/A | - | N/A | 4.76 (1.04–13.30) | 0.050 | 7.69 (1.11–11.11) | 0.044 |
| GFR | - | N/A | 0.95 (0.89–1.01) | 0.106 | - | N/A | - | N/A |
| RVFW GLS (%) | 0.46 (0.24–0.68) | 0.015 | 0.56 (0.32–0.89) | 0.044 | - | N/A | 0.39 (0.19–0.83) | 0.014 |
| LV LGE (%) | 1.61 (1.16–2.04) | 0.004 | 1.65 (1.17–2.13) | 0.004 | 1.55 (1.13–2.12) | 0.007 | 1.58 (1.14–2.08) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nita, N.; Felbel, D.; Melnic, R.; Paukovitsch, M.; Rottbauer, W.; Buckert, D.; Mörike, J. Predictors of Poor Long-Term Outcomes in Patients with Newly Diagnosed Asymptomatic Cardiac Sarcoidosis: A Cardiovascular Magnetic Resonance Study. Biomedicines 2025, 13, 1093. https://doi.org/10.3390/biomedicines13051093
Nita N, Felbel D, Melnic R, Paukovitsch M, Rottbauer W, Buckert D, Mörike J. Predictors of Poor Long-Term Outcomes in Patients with Newly Diagnosed Asymptomatic Cardiac Sarcoidosis: A Cardiovascular Magnetic Resonance Study. Biomedicines. 2025; 13(5):1093. https://doi.org/10.3390/biomedicines13051093
Chicago/Turabian StyleNita, Nicoleta, Dominik Felbel, Rima Melnic, Michael Paukovitsch, Wolfgang Rottbauer, Dominik Buckert, and Johannes Mörike. 2025. "Predictors of Poor Long-Term Outcomes in Patients with Newly Diagnosed Asymptomatic Cardiac Sarcoidosis: A Cardiovascular Magnetic Resonance Study" Biomedicines 13, no. 5: 1093. https://doi.org/10.3390/biomedicines13051093
APA StyleNita, N., Felbel, D., Melnic, R., Paukovitsch, M., Rottbauer, W., Buckert, D., & Mörike, J. (2025). Predictors of Poor Long-Term Outcomes in Patients with Newly Diagnosed Asymptomatic Cardiac Sarcoidosis: A Cardiovascular Magnetic Resonance Study. Biomedicines, 13(5), 1093. https://doi.org/10.3390/biomedicines13051093






