Minor Visual Phenomena in Lewy Body Disease: A Systematic Review
Abstract
1. Introduction
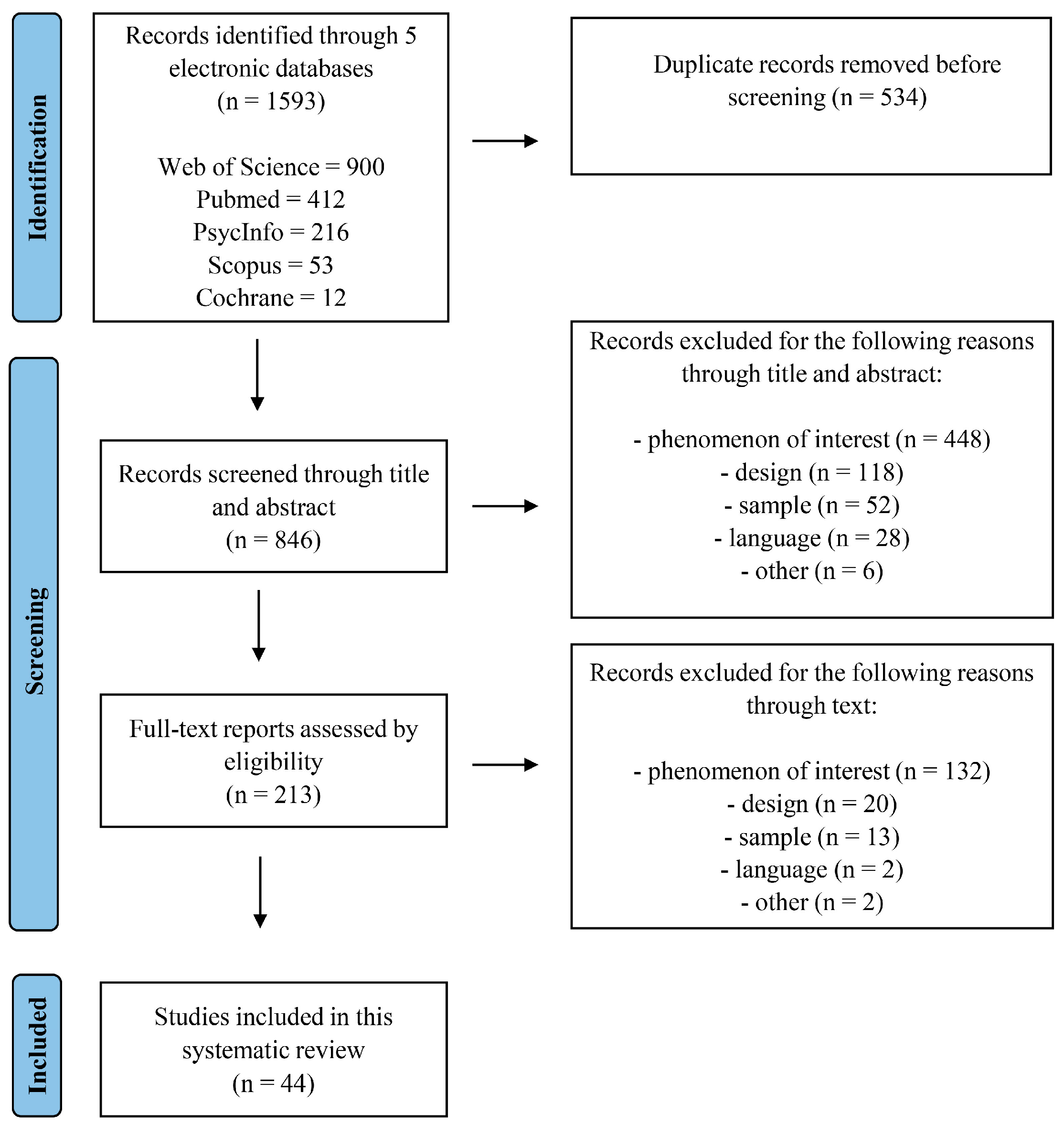
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
2.3. Study Selection
2.4. Quality Assessement
3. Results
3.1. Study Characteristics
| Authors (Year) | Sample (N) | Age Mean (sd) | Sex (F/M) | Phenomenon of Interest | MVP Assessment | Results LBD | Key Findings | Quality Index | |
|---|---|---|---|---|---|---|---|---|---|
| Statistics | Prevalence | ||||||||
| Alsemari and Boscarino (2024) [37] | DLB (94) MCI-DLB (97) HC (56) | 70.2 (7.75) 71.11(6.98) 72.11 (8.68) | 13/84 14/80 35/21 | Pareidolias | Noise pareidolia test (20 items) | 3.16 (3.85) 1.51 (2.32) | - | DLB patients exhibited more pareidolias than the HC and MCI groups. Visuospatial and visuoperceptual processes partially, but not independently, contribute to explaining noise pareidolia test scores. VH did not provide additional explanation for the variance in performance. | Strong (1) |
| Chiba et al. (2015) [43] | DLB-AD+ (12) DLB-AD− (11) AD (10) HC (11) | 77.0 (4.3) 73.0 (5.1) 74.3 (6.5) 74.6 (4.7) | 3/9 5/6 8/2 6/5 | FOP | Worksheet for patients and caregivers | - | 11 (91.7%) 3 (27.2%) | The DLB-AD+ group (with greater parietal/precuneus hypometabolism) showed higher prevalence of VH, FOP, and higher scores on the Bender-Gestalt test, compared with DLB-AD- (lower parietal/precuneus hypometabolism). | Weak (3) |
| D’Antonio et al. (2022) [15] | 35 LBD (19 DLB;16 PDD) | 76.7 (6.5) | 12/23 | MVH (illusions, FOP, feeling of passage) | NEVHI | 4.7 (8.2) severity 1.4 (1.8) duration 2.6 (3.02) frequency | Illusions: 9 (25.71%) FOP: 9 (25.71%) Passage: 12 (34.28%) | Distinct neuropsychological and functional network patterns underlie CVH and MVH. MVH were not associated with impaired visuoperceptual processing. MVH were negatively correlated with reduced FC in the left areas of the ventral visual stream, as well as between the brainstem and primary visual cortex. | Moderate (2) |
| D’Antonio et al. (2024) [17] | 28 LBD with VH (16 DLB, 12 PDD) HC (20) | 74.75 (5.76) - | 10/18 - | MVH (illusions, FOP, feeling of passage) | NEVHI | 5.43 (8.90) severity 1.57 (2.00) duration 2.93 (3.04) frequency | Illusions: 8 (28.57%); FOP: 8 (28.57%) Passage: 10 (35.71%) | MVH and CVH arose from distinct neural processes. MVH were not associated with gray matter loss. MVH were related to greater structural integrity of white matter pathways. A negative relationship was found between MVH severity and mean diffusivity, particularly for tracts linking dorsal and ventral attention networks with visual regions. | Moderate (2) |
| Ferman et al. (2013) [22] | LBD (41) AD (70) AD-amy LB (14) | 70.2 (8.8) 69.1 (9.0) 68.0 (8.7) | 17/24 36/34 10/4 | Misperceptions | Interview | - | 31 (76%) | Misperceptions and VH did not differ between groups, although these symptoms occurred earlier in LBD. The presence of LB pathology in the limbic and cortical regions has been linked to the occurrence of misperceptions in LBD patients. | Weak (3) |
| Firbank et al. (2024) [44] | LBD-VH+ (41) LBD-VH− (48) HC (60) | 75.8 (5.5) 74.2 (6.8) 74.7 (6.6) | 8/33 7/41 17/43 | Pareidolias, MVH (illusions, FOP, feeling of passage) | Noise pareidolia test (40 item), NEVHI | 5.81 (5.03) 2.66 (3.83) [subgroup: 59] | FOP: 12 (57.14%) Passage: 10 (47.62%) Illusions: 7 (33.33%) Pareidolias: 3 (14.28%) [subgroup: 21] | DLB patients with VH exhibited reduced FC within the ventral attention network and from the visual to the DMN. Significant positive association between the number of correct responses on the pareidolia task and connectivity between the visual and DMN. | Moderate (2) |
| Galvin et al. (2021) [45] | DLB (110) AD (78) HC (53) MCI DLB (22) MCI AD (79) | 77.7 (7.6) 79.7 (8.0) 67.6 (10.0) 75.3 (5.3) 73.5 (8.8) | 30/80 43/35 37/16 7/15 38/41 | Pareidolias | Noise pareidolia test (20 item) | 4.0 (3.9) 1.9 (2.2) | - | DLB (and MCI/DLB) performed significantly worse on the noise pareidolia test (with different scores computed) compared with AD and HC. Utility of the pareidolia score in discriminating between DLB and AD (and MCI-DLB and MCI-AD). | Weak (3) |
| Hamilton et al. (2021) [46] | probable MCI-LB (43) possible MCI-LB (20) MCI-AD (40) HC (34) | 74.9 (6.36) 74.1 (7.95) 76.2 (7.54) 74.2 (7.45) | 7/36 9/11 23/17 10/24 | Pareidolias | Noise pareidolia test (40 item) | 2 [0, 14] 2 [0, 20] | - | Higher rates of pareidolias were observed in MCI-LB patients. Weak association between pareidolias and VH. The noise pareidolia test had reduced predictive value for classifying LB or VH in patients with MCI. | Weak (3) |
| Heitz et al. (2015) [47] | DLB-VH+ (36) DLB-VH− (30) | 71.7 (10.2) 73.5 (6.9) | 14/19 10/18 | Illusions | Interview | - | 8 (22.22%) | Hypoperfusion in anterior and posterior brain regions was associated with VH. The occurrence of visual illusions may be specifically linked to the reduction in CBF in the cuneus. Significant association between CVH and hypoperfusion in temporal and frontal areas. | Moderate (2) |
| Hely et al. (1996) [48] | DLB (9) | 62 (4.5) | 2/7 | Illusions | Clinical description | - | 1 (11%) | Visual illusions were reported by one patient. VH emerged 2.5–9 years after symptom onset in six patients and were an initial manifestation in one case. Five individuals initially presented with symptoms resembling those of idiopathic PD and subsequently developed dementia. | Case report |
| Inagawa et al. (2020) [49] | probable DLB (24) AD (22) | 82.4 (5.0) 80.0 (5.6) | 14/10 11/11 | Pareidolias | Noise pareidolia test (40 item) | 10.6 (11.7) | - | Combined together, the pareidolia test (rate), odor stick identification test (OSIT-J), DaT-SPECT, and MIBG. Significant statistical differences were observed between DLB and AD groups, with DLB patients exhibiting higher rates of pareidolic illusions and more severe hyposmia. The pareidolia test and OSIT-J were useful in differentiating DLB from AD. The ROC curves did not show high sensitivity and specificity (compared to MIBG and DaT-SPECT). | Moderate (2) |
| Iseki et al. (2002) [20] | probable DLB (8) | 74.5 (3.94) | 2/6 | Illusions, FOP | Clinical description | Illusions: 4 (50%) Presence: 4 (50%) | Patients with DLB experienced psychiatric symptoms similar to those caused by levodopa (these symptoms manifested before any medication was administered). Hallucinations and cognitive decline occurred first compared with other symptoms (such as depression and delusions). Visual illusions and feelings of presence were characteristic of DLB primarily due to visual misidentification. | Case report | |
| Ishimaru et al. (2024) [50] | DLB (2) | 83.5 (7.78) | 2/0 | Illusions | PA-LE, interview | - | 2 (100%) | Some VH were induced by specific visual illusions (misidentification of common household objects). An individualized non-pharmacological strategy was developed for each patient by eliminating environmental triggers and improving the occurrence of VH. | Case report |
| Mamiya et al. (2016) [12] | probable DLB (52) AD (52) HC (20) | 79.5 (7.2) 79.8 (6.2) 78.8 (5.0) | 31/21 39/13 15/5 | Pareidolias | Noise pareidolia test (32 items), Scene pareidolia test (10 items) | 7.3 (8.4) 3.9 (1.9) 11.1 (8.6) * * Pareidolia score as the sum of images from the scene and the noise version | 31 scored above the cut-off (both in the scene and noise pareidolia test) | DLB exhibited higher pareidolia responses and worse performance on the visuospatial and visuoperceptual tests. A significant correlation was observed between the scene pareidolia test score and a visuospatial component of the ACE-R. The pareidolia score (combination of scene and noise pareidolia tests) significantly correlated with clinical VH and demonstrated excellent inter-rater/test–retest reliability and better sensitivity and specificity. | Weak (3) |
| Matar et al. (2020) [51] | DLB (27) HC (25) | 73 (63–86) 73 (55–88) median | 31/21 7/18 | Misperceptions | Interview, MDS-UPDRS, SCOPA-PC | - | 4 (14.80%) | Factor analysis revealed six factors accounting for 81% of the total symptom variance. Misperceptions were independent of VH. VH were prominently represented in the first factor, explaining 18% of the variance along with cognitive fluctuations. The fifth factor encompassed misperceptions, apathy, and delusions. | Moderate (2) |
| McCann et al. (2023) [33] | DLB-PDD (13) PD (13) AD (12) PCA (5) HC (32) | 75.7 (5.4) 67.5 (8.2) 66.8 (6.5) 67.8 (4.5) 67.3 (7.1) | 3/10 5/8 6/6 1/4 15/17 | Pareidolias | Noise pareidolia test (40 item) | 7.1 (9.4) | - | No significant correlation between pareidolic responses and history of VH in LBD. LBD patients who had experienced VH showed more visuoperceptual deficits than those without VH. Across all patient groups, impaired visuoperception, rather than VH, was predictive of pareidolic responses. The PCA group showed the highest prevalence of pareidolic responses. | Moderate (2) |
| Morenas-Rodríguez et al. (2018) [52] | probable DLB (81) | 79.8 (5.5) | 48/33 | FOP and feeling of passage | Structured questionnaire and retrospective review of medical records | - | FOP: 20 (25.3%) Passage: 24 (30.4%) | Cluster analysis of symptom presentation during prodromal phases of the disease. This study identified a neuropsychiatric cluster characterized by VH as the initial early symptom. In this group, patients were older, and their hallucinations were subsequently accompanied by misidentification, passage, and presence hallucinations, which emerged earlier than in clusters I and III. | Weak (3) |
| Mori et al. (2000) [53] | probable DLB (24) probable AD (48) | 74.0 (5.8) 74.0 (7.2) | 11/13 22/26 | FOP | Interview | - | 19 (79%) | DLB performed worse on simple and complex visuoperceptual tasks than AD. These deficits in visual perception may play a role in the onset of visual-related symptoms (VH and illusions). | Weak (3) |
| Moylett et al. (2019) [54] | DLB (251) | 78.8 (7.7) | 129/122 | Illusions | Medical records | - | 1 (0.2%) | Among the earliest reported complaints, memory loss and VH were the most prevalent. A wide range of other symptoms occurred, albeit less frequently, including those aligned with DLB criteria, such as hallucinations and related phenomena (e.g., illusions). | Weak (3) |
| Nagahama et al. (2007) [30] | DLB (100) | 77.2 (6.5) | 69/31 | FOP | Semi-structured interview (caregivers, patients) | - | 23 (23%) | Factor analysis of the relationships among psychotic symptoms. The study revealed that feelings of presence and VH were clustered together. This factor accounted for 8.2% of the variance in the data. | Weak (3) |
| Nakata et al. (2022) [55] | DLB (147) MCI DLB (15) | 78.9 (6.1) | 91/56 | Pareidolias | Noise pareidolia test (40 item) | 7.1 (7) | 90 (61.22%) Cut off ≥ 3 | Weak correlation between the noise pareidolia scores and CBF in frontal, cingulate gyrus, and left parietal cortex (supramarginal gyrus). No correlation with the CBF of occipital regions was reported. Pareidolias might also be affected by attentional deficits. | Weak (3) |
| Nicastro et al. (2020) [19] | DLB (25) | 71.9 (6.7) | 8/17 | FOP | Medical records | - | 9 (36%) | Subjects with FOP showed hypometabolism in left frontoparietal areas, including the superior parietal lobule and precuneus. The presence of VH was not associated with FOP. | Weak (3) |
| Oishi et al. (2020) [56] | DLB (37) AD (58) HC (32) | 81.2 (6.7) 80.2 (5.9) 79.4 (4.1) | 26/11 42/16 16/16 | Pareidolias | Noise pareidolia test (40 items), object-identifying test | - 2.0 (2.4) | 29 (78.3%) (object identification test). 23 pareidolias (noise), 21 pareidolia-like responses (object identifying test) | Visual texture agnosia was associated with the impairment of object recognition and visual misidentification (pareidolias) in DLB. Pareidolia-like responses were likely to occur when patients could not use texture as a supporting cue with ambiguous shape information for object recognition. | Weak (3) |
| Phillips et al. (2021) [57] | DLB (23) HC (20) | 73.91 69.95 | Misperceptions | Bistable percept paradigm (BPP), PsycH-Q | 5.83 misperceptions | - | DLB showed more misperceptions and misses than HC. Significant correlation between the Visual Misperception subscale of PsycH-Q and the number of misperceptions in BPP. DLB with VH had more misperceptions. | Moderate (2) | |
| Phillips et al. (2022) [23] | iRBD-MCI probable prodromal DLB (12) DLB (1) iRBD-CN (34) | 70.5 (7.7) 84 69.3 (7.4) | 0/1 | Misperceptions | BPP | 1.25 (1.3) [Only 4 completed the BPP] | - | Group differences between iRBD-CN (19) and iRBD-MCI (4) in the BPP (for misses, no misperceptions or error rate). No differences on SART and mental rotation tests. One subject who converted to DLB had 11 misses (and no misperceptions) in the BPP test at baseline. | Weak (3) |
| Posner et al. (2001) [58] | DLB (1) | 64 | 0/1 | Illusions | Clinical description | - | 1 (100%) | Development of visual symptoms such as visual illusions and parkinsonism occurred very early in the course of the disease, along with progressive cognitive impairment (e.g., in visuospatial skills). | Case report |
| Rahman-Filipiak et al. (2022) [59] | LBD (56) AD (44) aMCI (96) non-aMCI (61) HC (202) | 72.49 (6.75) 75.86 (8.43) 74.35 (8.84) 71.02 (7.35) 72.52 (6.88) | 8/48 20/24 45/51 31/30 142/60 | Pareidolias | Noise pareidolia task (20 items) (NACC Version- short version with faces) | 3.44 (4.04) | - | LBD (especially those with VH) performed worse on the noise pareidolia task (on correct faces and in pareidolic errors). VH might represent misperceptions of real visual stimuli due to poor visual integration. The speeded attention and noise pareidolia task showed good convergent and discriminant validity, hence showing promising clinical utility. | Weak (3) |
| Reckner et al. (2020) [32] | 25 LBD with FOP (7 probable DLB, 18 PD) PD without FOP (25) | 67.0 (7.1) 67.7 (5.2) 64.4 (5.7) | 1/6 4/14 9/16 | FOP | Semi-structured interview | - | FOP: 7 (100%) Passage: 4 (57.1%) | FOP may occur in DLB and PD patients. Patients with FOP showed more impairment in visual processing skills, more visual hallucinations, and feeling of passage phenomena. | Moderate (2) |
| Revankar et al. (2024) [60] | DLB (25) AD (29) PD (5) HC (11) | 73.7 (5.5) 73.4 (6.4) 66.6 (11.3) 62.1 (9.5) | 6/19 18/11 1/4 3/8 | Pareidolias | Noise pareidolia test digital and paper (40 items) | 8.1 (10.3) | 35 (DLB + AD + PD) | Pareidolias were captured on both versions of the pareidolia noise test (paper and digital). DLB patients had more pareidolias compared with the other groups, especially in those experiencing VH. | Weak (3) |
| Rothenberg et al. (2023) [61] | DLB (4) | 70 (8.28) | 0/4 | Illusions | Clinical description | - | 2 (50%) | Case description of 4 male DLB patients with visual illusions, hallucinations, and paranoid delusions. Typical medications prescribed for DLB with psychosis did not improve the symptoms; however, only pimavanserin seemed to be useful and tolerable by patients. | Case report |
| Stavitsky et al. (2006) [62] | DLB (28) AD (55) | 73.46 (7.56) 73.09 (8.26) | 9/19 34/21 | Illusions | CUSPAD | - | 8 (32%) [subgroup: 25 patients] | At baseline, DLB patients experienced more visual illusions, VH, and impairment in the visuoconstructional domain than AD patients. These symptoms tend to be relatively stable in DLB over the course of the disease, whereas they appear later in AD patients. | Moderate (2) |
| Suárez-González et al. (2014) [25] | probable DLB (80) probable AD (85) | 75.9 (13.0) 74.0 (7.0) | 36/44 55/30 | Illusions, FOP | CUSPAD | - | Illusions: 10 (12.5%) FOP: 18 (22.5%) | DLB patients showed significantly more VH, visual illusions, and feeling of presence than AD patients. Illusions were observed only in DLB patients. | Weak (3) |
| Sumi et al. (2022) [18] | DLB (3) [converted from iRBD (36)] | 76 (1.73) | 1/2 | FOP, feeling of passage, illusions, pareidolias | Semi-structured interview, noise pairedolia test (32 items) | - | Baseline: Illusions: 2 (66.66%), FOP: 2 (66.66%), Passage: 1 (33.33%) Follow-up: Illusions: 2 (66.66%), FOP: 3 (100%), Passage: 1 (33.33%) Pareidolias not reported | Two iRBD patients who initially experienced MVP progressed to DLB during follow-up and developed more severe VH. Another patient who did not initially report minor visual phenomena later experienced a feeling of presence. The progression rate was notably higher in individuals with minor visual phenomena. | Strong (1) |
| Suzuki et al. (2017) [63] | DLB (8) HC (9) | 77.4 (6.9) 71.4 (6.7) | 5/3 1/8 | Pareidolias | Scene pareidolia test (25 items) | - | 123 pareidolias responses | Changes in pupil diameter were observed in DLB patients prior to the occurrence of pareidolias. | Moderate (2) |
| Taomoto et al. (2022) [64] | prodromal DLB (2) | 76 (9.90) | 1/1 | Illusions | Clinical description | - | 1 (50%) | Two years before the onset of delusional infestation, a patient with prodromal DLB (with SAH sequelae) began complaining of visual illusions, mistaking lint for insects. | Case report |
| Taomoto et al. (2024) [65] | prodromal DLB (5) | 64 (8) | 2/3 | Illusion, feeling of passage, FOP | Clinical description | - | Illusions: 1 (20%) Passage: 1 (20%), FOP: 1 (20%) | Two of the five patients with delirium-onset prodromal DLB experienced (among other symptoms) visual illusions, and feeling of passage months before the onset of delirium. | Case report |
| Uchiyama et al. (2012) [27] | probable DLB (34) AD (34) HC (26) | 81.0 (3.9) 80.0 (3.6) 79.2 (4.9) | 19/15 10/24 8/18 | Pareidolias | Scene pareidolia test (25 item) | 15.5 (median) 11.0 (IQR) | - | DLB patients displayed a higher number of pareidolia responses than AD patients and HC. Positive association between scene pareidolia scores and VH only in those who did not take donepezil. Negative relationship between scene pareidolia scores and face recognition tasks in participants using donepezil. | Moderate (2) |
| Urwyler et al. (2016) [66] | LBD (79) * PDD (48); DLB (31) ED (135), PD (156) HC (164) | 74.8 (7.4) 79.8 (8.3) 70.9 (9.4) 72.9 (8.2) | 23/56 93/42 64/92 92/72 | Illusions, FOP/feeling of passage | NEVHI | - | Illusions: 34 (43%) FOP/passage: 52 (66%) | LBD patients primarily experienced complex VH, illusions, and feeling of passage/FOP compared with PD and ED. ED patients predominantly encountered simple VH. Simple VH may be associated with pathology in the primary retinocortical pathway, and complex VH were likely associated with higher-level cortical dysfunction in the context of LB pathology. | Moderate (2) |
| van de Beek et al. (2021) [67] | DLB (100) probable DLB (73) MCI (27) | 69 (6) 70 (5) 67 (7) | 10/90 8/65 2/25 | MVH (illusions, feeling of passage, FOP) | QPE | - | Ilusions: 27 (27%), Passage: 25 (25%), FOP: 23 (23%) | VH were less prevalent, occurring in less than 50% of patients. Some patients also experienced illusions, feelings of passage, and presence. Discrepancy between NPI and QPE assessments regarding the presence of VH. | Moderate (2) |
| Watanabe et al. (2018) [68] | DLB (36) AD (12) | 79.8 (7.4) 78.8 (6.2) | 22/14 10/2 | Pareidolias | Noise pareidolia test (80 item) | 11.2 (16.1) | 27 (75%) at least one pareidolia, 24 (66.66%) at least two pareidolias | Negative mood led to a twofold increase in pareidolic illusions among DLB patients compared to neutral mood. AD patients exhibited no notable differences in pareidolic responses between negative and neutral emotional conditions. | Moderate (2) |
| Watanabe et al. (2023) [36] | DLB (43) | 78.3 (5.6) | 26/17 | Pareidolias | Scene pareidolia test (25 item) | 13.5 (7.6) | 43 (100%) | Pareidolia responses contributed to both VH and visual processing impairment. Hypoperfusion in the occipitotemporal and posterior parietal regions was correlated with higher scores on Factor 1 (hallucinations) and lower scores on Factor 3 (visual processing). | Weak (3) |
| Watanabe et al. (2020) [69] | probable DLB (1) | 71 | 1/0 | Pareidolias | Noise pareidolia test (32 item) | 1 (100%) with 3 pareidolic responses | 4.5 years after the onset of language impairment, a patient with PPA exhibited pareidolic responses, visual hallucinations, and additional symptoms associated with DLB diagnosis. These observations indicated the potential existence of a prodromal DLB phase characterized by PPA. | Case report | |
| Yokoi et al. (2014) [26] | probable DLB (34) AD (34) HC (28) | 79.4 (0.9) 77.7 (0.8) 78.0 (0.5) | 21/13 25/9 16/12 | Pareidolias | Noise pareidolia test (40 item object and 40 item face), Scene pareidolia test (25 item) [subgroup: 11 patients] | 12.6 (3.3) object, 15.4 (3.2) face | - | The noise pareidolia test (face version) demonstrated a stronger association with VH and better differentiation between DLB and AD groups than the object version. DLB exhibited more illusory responses than those with AD and HC. Significant positive relationship between pareidolias and VH. Significant negative association between VH and visuospatial tests (shape detection and spatial span assessments). | Moderate (2) |
| Yoshizawa et al. (2013) [70] | pure DLB (12) DLB + AD (23) pure AD (89) | 68.5 (6.2) 66.0 (8.6) 68.3 (9.8) | 3/9 8/15 47/42 | Illusions | CUSPAD | 33.3% illusions 5% illusions [subgroups: 9 pure DLB and 20 DLB + AD patients ] | Patients with pure DLB experienced a higher occurrence of visual illusions and VH at the initial assessment than those with pure AD. Patients with pure DLB demonstrated greater impairment in visuospatial functions but less severe memory recognition deficits than those with pure AD and DLB + AD. | Moderate (2) | |
3.2. Minor Visual Phenomena
3.2.1. Pareidolias
3.2.2. Illusions and Misperceptions
3.2.3. Presence Hallucinations and Passage Hallucinations
3.3. Associations Between Minor Visual Phenomena and Visual Hallucinations
3.4. Associations Between Minor Visual Phenomena and Visuoperceptual/Visuospatial Impairment
3.5. Neural Correlates Underlying Minor Visual Phenomena
3.6. Case Report
3.7. Quality Assessment Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBD | Lewy body disease |
| AD | Alzheimer’s disease |
| DLB | Dementia with Lewy bodies |
| PDD | Parkinson’s disease dementia |
| VH | Visual hallucinations |
| RBD | Rapid eye movement sleep behavior disorder |
| MVP | Minor visual phenomena |
| MCI | Mild cognitive impairment |
| NEVHI | North-East Visual Hallucinations Interview |
| CUSPAD | Columbia University Scale for Psychopathology in Alzheimer’s disease |
| QPE | Questionnaire for Psychotic Experiences |
| PD | Parkinson’s disease |
| NPI | Neuropsychiatric Inventory |
References
- Kane, J.P.M.; Surendranathan, A.; Bentley, A.; Barker, S.A.H.; Taylor, J.-P.; Thomas, A.J.; Allan, L.M.; McNally, R.J.; James, P.W.; McKeith, I.G.; et al. Clinical Prevalence of Lewy Body Dementia. Alzheimer’s Res. Ther. 2018, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- van de Beek, M.; van Steenoven, I.; Ramakers, I.H.G.B.; Aalten, P.; Koek, H.L.; Olde Rikkert, M.G.M.; Manniën, J.; Papma, J.M.; de Jong, F.J.; Lemstra, A.W.; et al. Trajectories and Determinants of Quality of Life in Dementia with Lewy Bodies and Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 70, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zweig, Y.R.; Galvin, J.E. Lewy Body Dementia: The Impact on Patients and Caregivers. Alzheimer’s Res. Ther. 2014, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Ballard, C.G.; Halliday, G. Are Parkinson’s Disease with Dementia and Dementia with Lewy Bodies the Same Entity? J. Geriatr. Psychiatry Neurol. 2004, 17, 137–145. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Korczyn, A.D. Are Dementia with Lewy Bodies and Parkinson’s Disease Dementia the Same Disease? BMC Med. 2018, 16, 34. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and Management of Dementia with Lewy Bodies: Fourth Consensus Report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical Diagnostic Criteria for Dementia Associated with Parkinson’s Disease. Mov. Disord. 2007, 22, 1689–1707; quiz 1837. [Google Scholar] [CrossRef]
- Yumoto, A.; Suwa, S. Difficulties and Associated Coping Methods Regarding Visual Hallucinations Caused by Dementia with Lewy Bodies. Dementia 2021, 20, 291–307. [Google Scholar] [CrossRef]
- Collerton, D.; Barnes, J.; Diederich, N.J.; Dudley, R.; Ffytche, D.; Friston, K.; Goetz, C.G.; Goldman, J.G.; Jardri, R.; Kulisevsky, J.; et al. Understanding Visual Hallucinations: A New Synthesis. Neurosci. Biobehav. Rev. 2023, 150, 105208. [Google Scholar] [CrossRef]
- Burghaus, L.; Eggers, C.; Timmermann, L.; Fink, G.R.; Diederich, N.J. Hallucinations in Neurodegenerative Diseases. CNS Neurosci. Ther. 2012, 18, 149–159. [Google Scholar] [CrossRef]
- Ey, H. Traité des Hallucinations—Lot de 2 Tomes; Crehey: Perpignan, France, 2012; ISBN 978-2-9527859-3-8. [Google Scholar]
- Mamiya, Y.; Nishio, Y.; Watanabe, H.; Yokoi, K.; Uchiyama, M.; Baba, T.; Iizuka, O.; Kanno, S.; Kamimura, N.; Kazui, H.; et al. The Pareidolia Test: A Simple Neuropsychological Test Measuring Visual Hallucination-Like Illusions. PLoS ONE 2016, 11, e0154713. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.; Aynsworth, C.; Mosimann, U.; Taylor, J.-P.; Smailes, D.; Collerton, D.; McCarthy-Jones, S.; Urwyler, P. A Comparison of Visual Hallucinations across Disorders. Psychiatry Res. 2019, 272, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Fénelon, G. Psychosis in Parkinson’s Disease: Phenomenology, Frequency, Risk Factors, and Current Understanding of Pathophysiologic Mechanisms. CNS Spectr. 2008, 13, 18–25. [Google Scholar] [CrossRef]
- D’Antonio, F.; Boccia, M.; Di Vita, A.; Suppa, A.; Fabbrini, A.; Canevelli, M.; Caramia, F.; Fiorelli, M.; Guariglia, C.; Ferracuti, S.; et al. Visual Hallucinations in Lewy Body Disease: Pathophysiological Insights from Phenomenology. J. Neurol. 2022, 269, 3636–3652. [Google Scholar] [CrossRef]
- Shahid, M.; Rawls, A.; Ramirez, V.; Ryman, S.; Santini, V.E.; Yang, L.; Sha, S.J.; Hall, J.N.; Montine, T.J.; Lin, A.; et al. Illusory Responses across the Lewy Body Disease Spectrum. Ann. Neurol. 2023, 93, 702–714. [Google Scholar] [CrossRef]
- D’Antonio, F.; Teghil, A.; Boccia, M.; Bechi Gabrielli, G.; Giulietti, G.; Conti, D.; Suppa, A.; Fabbrini, A.; Fiorelli, M.; Caramia, F.; et al. Distinct Grey and White Matter Changes Are Associated with the Phenomenology of Visual Hallucinations in Lewy Body Disease. Sci. Rep. 2024, 14, 14748. [Google Scholar] [CrossRef]
- Sumi, Y.; Ubara, A.; Ozeki, Y.; Kadotani, H. Minor Hallucinations in Isolated Rapid Eye Movement Sleep Behavior Disorder Indicative of Early Phenoconversion: A Preliminary Study. Acta Neurol. Scand. 2022, 145, 348–359. [Google Scholar] [CrossRef]
- Nicastro, N.; Eger, A.F.; Assal, F.; Garibotto, V. Feeling of Presence in Dementia with Lewy Bodies Is Related to Reduced Left Frontoparietal Metabolism. Brain Imaging Behav. 2020, 14, 1199–1207. [Google Scholar] [CrossRef]
- Iseki, E.; Marui, W.; Nihashi, N.; Kosaka, K. Psychiatric Symptoms Typical of Patients with Dementia with Lewy Bodies—Similarity to Those of Levodopa-Induced Psychosis. Acta Neuropsychiatr. 2002, 14, 237–241. [Google Scholar] [CrossRef]
- Hwang, J.-P.; Yang, C.-H.; Tsai, S.-J. Phantom Boarder Symptom in Dementia. Int. J. Geriatr. Psychiatry 2003, 18, 417–420. [Google Scholar] [CrossRef]
- Ferman, T.J.; Arvanitakis, Z.; Fujishiro, H.; Duara, R.; Parfitt, F.; Purdy, M.; Waters, C.; Barker, W.; Graff-Radford, N.R.; Dickson, D.W. Pathology and Temporal Onset of Visual Hallucinations, Misperceptions and Family Misidentification Distinguishes Dementia with Lewy Bodies from Alzheimer’s Disease. Park. Relat. Disord. 2013, 19, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.R.; Matar, E.; Ehgoetz Martens, K.A.; Moustafa, A.A.; Halliday, G.M.; Lewis, S.J.G. Exploring the Sensitivity of Prodromal Dementia with Lewy Bodies Research Criteria. Brain Sci. 2022, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, J. Über Leibhaftige Bewusstheiten (Bewusstheitstaüschungen), Ein Psychopathologisches Elementarsymptom. Z. Pathopsychol. 1913, 2, 150. [Google Scholar]
- Suárez-González, A.; Serrano-Pozo, A.; Arroyo-Anlló, E.M.; Franco-Macías, E.; Polo, J.; García-Solís, D.; Gil-Néciga, E. Utility of Neuropsychiatric Tools in the Differential Diagnosis of Dementia with Lewy Bodies and Alzheimer’s Disease: Quantitative and Qualitative Findings. Int. Psychogeriatr. 2014, 26, 453–461. [Google Scholar] [CrossRef]
- Yokoi, K.; Nishio, Y.; Uchiyama, M.; Shimomura, T.; Iizuka, O.; Mori, E. Hallucinators Find Meaning in Noises: Pareidolic Illusions in Dementia with Lewy Bodies. Neuropsychologia 2014, 56, 245–254. [Google Scholar] [CrossRef]
- Uchiyama, M.; Nishio, Y.; Yokoi, K.; Hirayama, K.; Imamura, T.; Shimomura, T.; Mori, E. Pareidolias: Complex Visual Illusions in Dementia with Lewy Bodies. Brain 2012, 135, 2458–2469. [Google Scholar] [CrossRef]
- Fénelon, G.; Soulas, T.; Cleret de Langavant, L.; Trinkler, I.; Bachoud-Lévi, A.-C. Feeling of Presence in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1219–1224. [Google Scholar] [CrossRef]
- Lenka, A.; Pagonabarraga, J.; Pal, P.K.; Bejr-Kasem, H.; Kulisvesky, J. Minor Hallucinations in Parkinson Disease. Neurology 2019, 93, 259–266. [Google Scholar] [CrossRef]
- Nagahama, Y.; Okina, T.; Suzuki, N.; Matsuda, M.; Fukao, K.; Murai, T. Classification of Psychotic Symptoms in Dementia with Lewy Bodies. Am. J. Geriatr. Psychiatry 2007, 15, 961–967. [Google Scholar] [CrossRef]
- Ffytche, D.H.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Weintraub, D.; Ballard, C.; Aarsland, D. The Psychosis Spectrum in Parkinson Disease. Nat. Rev. Neurol. 2017, 13, 81–95. [Google Scholar] [CrossRef]
- Reckner, E.; Cipolotti, L.; Foley, J.A. Presence Phenomena in Parkinsonian Disorders: Phenomenology and Neuropsychological Correlates. Int. J. Geriatr. Psychiatry 2020, 35, 785–793. [Google Scholar] [CrossRef] [PubMed]
- McCann, E.; Lee, S.; Coleman, F.; O’Sullivan, J.D.; Nestor, P.J. Pareidolias Are a Function of Visuoperceptual Impairment. PLoS ONE 2023, 18, e0293942. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, U.P.; Collerton, D.; Dudley, R.; Meyer, T.D.; Graham, G.; Dean, J.L.; Bearn, D.; Killen, A.; Dickinson, L.; Clarke, M.P.; et al. A Semi-Structured Interview to Assess Visual Hallucinations in Older People. Int. J. Geriatr. Psychiatry 2008, 23, 712–718. [Google Scholar] [CrossRef]
- Rossell, S.L.; Schutte, M.J.L.; Toh, W.L.; Thomas, N.; Strauss, C.; Linszen, M.M.J.; van Dellen, E.; Heringa, S.M.; Teunisse, R.; Slotema, C.W.; et al. The Questionnaire for Psychotic Experiences: An Examination of the Validity and Reliability. Schizophr. Bull. 2019, 45, S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Uchiyama, M.; Yokoi, K.; Mamiya, Y.; Narita, W.; Iizuka, O.; Baba, T.; Suzuki, K.; Mori, E.; Nishio, Y. Behavioral and Neural Correlates of Pareidolic Illusions in Dementia with Lewy Bodies. Park. Relat. Disord. 2023, 113, 105513. [Google Scholar] [CrossRef]
- Alsemari, A.; Boscarino, J.J. Neuropsychological and Neuroanatomical Underpinnings of the Face Pareidolia Errors on the Noise Pareidolia Test in Patients with Mild Cognitive Impairment and Dementia Due to Lewy Bodies. J. Clin. Exp. Neuropsychol. 2024, 46, 588–598. [Google Scholar] [CrossRef]
- Collerton, D.; Perry, E.; McKeith, I. Why People See Things That Are Not There: A Novel Perception and Attention Deficit Model for Recurrent Complex Visual Hallucinations. Behav. Brain Sci. 2005, 28, 737–757; discussion 757–794. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. Glob. Adv. Health Med. 2013, 2, 38–43. [Google Scholar] [CrossRef]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A Process for Systematically Reviewing the Literature: Providing the Research Evidence for Public Health Nursing Interventions. Worldviews Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef]
- Devanand, D.P.; Miller, L.; Richards, M.; Marder, K.; Bell, K.; Mayeux, R.; Stern, Y. The Columbia University Scale for Psychopathology in Alzheimer’s Disease. Arch. Neurol. 1992, 49, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Fujishiro, H.; Ota, K.; Kasanuki, K.; Arai, H.; Hirayasu, Y.; Sato, K.; Iseki, E. Clinical Profiles of Dementia with Lewy Bodies with and without Alzheimer’s Disease-like Hypometabolism. Int. J. Geriatr. Psychiatry 2015, 30, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Firbank, M.J.; Collerton, D.; da Silva Morgan, K.; Schumacher, J.; Donaghy, P.C.; O’Brien, J.T.; Thomas, A.; Taylor, J.-P. Functional Connectivity in Lewy Body Disease with Visual Hallucinations. Eur. J. Neurol. 2023, 31, e16115. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Chrisphonte, S.; Cohen, I.; Greenfield, K.K.; Kleiman, M.J.; Moore, C.; Riccio, M.L.; Rosenfeld, A.; Shkolnik, N.; Walker, M.; et al. Characterization of Dementia with Lewy Bodies (DLB) and Mild Cognitive Impairment Using the Lewy Body Dementia Module (LBD-MOD). Alzheimer’s Dement. 2021, 17, 1675–1686. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Matthews, F.E.; Allan, L.M.; Barker, S.; Ciafone, J.; Donaghy, P.C.; Durcan, R.; Firbank, M.J.; Lawley, S.; O’Brien, J.T.; et al. Utility of the Pareidolia Test in Mild Cognitive Impairment with Lewy Bodies and Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 2021, 36, 1407–1414. [Google Scholar] [CrossRef]
- Heitz, C.; Noblet, V.; Cretin, B.; Philippi, N.; Kremer, L.; Stackfleth, M.; Hubele, F.; Armspach, J.P.; Namer, I.; Blanc, F. Neural Correlates of Visual Hallucinations in Dementia with Lewy Bodies. Alzheimer’s Res. Ther. 2015, 7, 6. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.; Halliday, G.M.; McRitchie, D.A.; Leicester, J.; Joffe, R.; Brooks, W.; Broe, G.A.; Morris, J.G. Diffuse Lewy Body Disease: Clinical Features in Nine Cases without Coexistent Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 1996, 60, 531–538. [Google Scholar] [CrossRef]
- Inagawa, Y.; Kanetaka, H.; Tsugawa, A.; Sakurai, S.; Serisawa, S.; Shimizu, S.; Sakurai, H.; Hanyu, H. Efficacy of Olfactory and Pareidolia Tests Compared With That of Indicative Biomarkers in Diagnosis of Dementia With Lewy Bodies. Front. Neurol. 2020, 11, 540291. [Google Scholar] [CrossRef]
- Ishimaru, D.; Kanemoto, H.; Hotta, M.; Nagata, Y.; Koizumi, F.; Satake, Y.; Taomoto, D.; Ikeda, M. Case Report: Environmental Adjustment for Visual Hallucinations in Dementia with Lewy Bodies Based on Photo Assessment of the Living Environment. Front. Psychiatry 2024, 15, 1283156. [Google Scholar] [CrossRef]
- Matar, E.; Ehgoetz Martens, K.A.; Halliday, G.M.; Lewis, S.J.G. Clinical Features of Lewy Body Dementia: Insights into Diagnosis and Pathophysiology. J. Neurol. 2020, 267, 380–389. [Google Scholar] [CrossRef]
- Morenas-Rodríguez, E.; Sala, I.; Subirana, A.; Pascual-Goñi, E.; Sánchez-Saudinós, M.B.; Alcolea, D.; Illán-Gala, I.; Carmona-Iragui, M.; Ribosa-Nogué, R.; Camacho, V.; et al. Clinical Subtypes of Dementia with Lewy Bodies Based on the Initial Clinical Presentation. J. Alzheimer’s Dis. 2018, 64, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Shimomura, T.; Fujimori, M.; Hirono, N.; Imamura, T.; Hashimoto, M.; Tanimukai, S.; Kazui, H.; Hanihara, T. Visuoperceptual Impairment in Dementia with Lewy Bodies. Arch. Neurol. 2000, 57, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Moylett, S.; Price, A.; Cardinal, R.N.; Aarsland, D.; Mueller, C.; Stewart, R.; O’Brien, J.T. Clinical Presentation, Diagnostic Features, and Mortality in Dementia with Lewy Bodies. J. Alzheimer’s Dis. 2019, 67, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T.; Shimada, K.; Iba, A.; Oda, H.; Terashima, A.; Koide, Y.; Kawasaki, R.; Yamada, T.; Ishii, K. Correlation between Noise Pareidolia Test Scores for Visual Hallucinations and Regional Cerebral Blood Flow in Dementia with Lewy Bodies. Ann. Nucl. Med. 2022, 36, 384–392. [Google Scholar] [CrossRef]
- Oishi, Y.; Imamura, T.; Shimomura, T.; Suzuki, K. Visual Texture Agnosia Influences Object Identification in Dementia with Lewy Bodies and Alzheimer’s Disease. Cortex 2020, 129, 23–32. [Google Scholar] [CrossRef]
- Phillips, J.R.; Matar, E.; Martens, K.A.E.; Moustafa, A.A.; Halliday, G.M.; Lewis, S.J. Evaluating a Novel Behavioral Paradigm for Visual Hallucinations in Dementia with Lewy Bodies. Aging Brain 2021, 1, 100011. [Google Scholar] [CrossRef]
- Posner, H.; Chin, S.; Marder, K. Dementia with lewy bodies. Sci. Aging Knowl. Environ. 2001, 2001, dn3. [Google Scholar] [CrossRef] [PubMed]
- Rahman-Filipiak, A.; Sadaghiyani, S.; Davis, K.; Bhaumik, A.K.; Paulson, H.L.; Giordani, B.; Hampstead, B.M. Validation of the National Alzheimer’s Coordinating Center (NACC) Lewy Body Disease Module Neuropsychological Tests. Alzheimer’s Dement. 2022, 14, e12279. [Google Scholar] [CrossRef]
- Revankar, G.S.; Ozono, T.; Suzuki, M.; Kanemoto, H.; Furuya, K.; Shigenobu, K.; Yoshiyama, K.; Yamamoto, Y.; Ogasawara, I.; Yoshida, N.; et al. Perceptual Constancy of Pareidolias across Paper and Digital Testing Formats in Neurodegenerative Diseases. Heliyon 2024, 10, e40254. [Google Scholar] [CrossRef]
- Rothenberg, K.G.; McRae, S.G.; Dominguez-Colman, L.M.; Shutes-David, A.; Tsuang, D.W. Pimavanserin Treatment for Psychosis in Patients with Dementia with Lewy Bodies: A Case Series. Am. J. Case Rep. 2023, 24, e939806. [Google Scholar] [CrossRef]
- Stavitsky, K.; Brickman, A.M.; Scarmeas, N.; Torgan, R.L.; Tang, M.-X.; Albert, M.; Brandt, J.; Blacker, D.; Stern, Y. The Progression of Cognition, Psychiatric Symptoms, and Functional Abilities in Dementia with Lewy Bodies and Alzheimer Disease. Arch. Neurol. 2006, 63, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Hirayama, K.; Shimomura, T.; Uchiyama, M.; Fujii, H.; Mori, E.; Nishio, Y.; Iizuka, O.; Inoue, R.; Otsuki, M.; et al. Changes in Pupil Diameter Are Correlated with the Occurrence of Pareidolias in Patients with Dementia with Lewy Bodies. Neuroreport 2017, 28, 187–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taomoto, D.; Kanemoto, H.; Satake, Y.; Yoshiyama, K.; Iwase, M.; Hashimoto, M.; Ikeda, M. Case Report: Delusional Infestation in Dementia with Lewy Bodies. Front. Psychiatry 2022, 13, 1051067. [Google Scholar] [CrossRef] [PubMed]
- Taomoto, D.; Nishio, Y.; Hidaka, Y.; Kanemoto, H.; Takahashi, S.; Ikeda, M. Delirium-Onset Prodromal Lewy Body Disease: A Series of 5 Cases. Clin. Park. Relat. Disord. 2024, 11, 100289. [Google Scholar] [CrossRef]
- Urwyler, P.; Nef, T.; Müri, R.; Archibald, N.; Makin, S.M.; Collerton, D.; Taylor, J.-P.; Burn, D.; McKeith, I.; Mosimann, U.P. Visual Hallucinations in Eye Disease and Lewy Body Disease. Am. J. Geriatr. Psychiatry 2016, 24, 350–358. [Google Scholar] [CrossRef]
- Van de beek, M.; van Steenoven, I.; van der Zande, J.J.; Porcelijn, I.; Barkhof, F.; Stam, C.J.; Raijmakers, P.G.H.M.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M.; et al. Characterization of Symptoms and Determinants of Disease Burden in Dementia with Lewy Bodies: DEvELOP Design and Baseline Results. Alzheimer’s Res. Ther. 2021, 13, 53. [Google Scholar] [CrossRef]
- Watanabe, H.; Nishio, Y.; Mamiya, Y.; Narita, W.; Iizuka, O.; Baba, T.; Takeda, A.; Shimomura, T.; Mori, E. Negative Mood Invites Psychotic False Perception in Dementia. PLoS ONE 2018, 13, e0197968. [Google Scholar] [CrossRef]
- Watanabe, H.; Ikeda, M.; Mori, E. Primary Progressive Aphasia as a Prodromal State of Dementia With Lewy Bodies: A Case Report. Front. Neurol. 2020, 11, 49. [Google Scholar] [CrossRef]
- Yoshizawa, H.; Vonsattel, J.P.G.; Honig, L.S. Early Neuropsychological Discriminants for Lewy Body Disease: An Autopsy Series. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1326–1330. [Google Scholar] [CrossRef]
- Cummings, J.L. The Neuropsychiatric Inventory. Neurology 1997, 48, 10S–16S. [Google Scholar] [CrossRef]
- Warrington, E.K.; James, M. The Visual Object and Space Perception Battery: VOSP; Pearson: London, UK, 1991; ISBN 978-0-7491-3303-0. [Google Scholar]
- Possin, K.L.; Laluz, V.R.; Alcantar, O.Z.; Miller, B.L.; Kramer, J.H. Distinct Neuroanatomical Substrates and Cognitive Mechanisms of Figure Copy Performance in Alzheimer’s Disease and Behavioral Variant Frontotemporal Dementia. Neuropsychologia 2011, 49, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A Brief Cognitive Test Battery for Dementia Screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Shiraishi, T.; Murakami, H.; Yoshimaru, D.; Onda, A.; Matsuno, H.; Komatsu, T.; Sakuta, K.; Sakai, K.; Umehara, T.; et al. Structural MRI Study of Pareidolia and Visual Hallucinations in Drug–Naïve Parkinson’s Disease. Sci. Rep. 2024, 14, 31293. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Halliday, G.M.; Gilat, M.; Matar, E.; Bolitho, S.J.; Carlos, M.; Naismith, S.L.; Lewis, S.J.G. The Role of Dysfunctional Attentional Control Networks in Visual Misperceptions in Parkinson’s Disease. Hum. Brain Mapp. 2014, 35, 2206–2219. [Google Scholar] [CrossRef]
- Shine, J.M.; Halliday, G.M.; Naismith, S.L.; Lewis, S.J.G. Visual Misperceptions and Hallucinations in Parkinson’s Disease: Dysfunction of Attentional Control Networks? Mov. Disord. 2011, 26, 2154–2159. [Google Scholar] [CrossRef]
- Beretta, L.; Caminiti, S.P.; Santangelo, R.; Magnani, G.; Ferrari-Pellegrini, F.; Caffarra, P.; Perani, D. Two Distinct Pathological Substrates Associated with MMSE-Pentagons Item Deficit in DLB and AD. Neuropsychologia 2019, 133, 107174. [Google Scholar] [CrossRef]
- Ffytche, D.H.; Howard, R.J.; Brammer, M.J.; David, A.; Woodruff, P.; Williams, S. The Anatomy of Conscious Vision: An fMRI Study of Visual Hallucinations. Nat. Neurosci. 1998, 1, 738–742. [Google Scholar] [CrossRef]
- Moskovitz, C.; Moses, H.; Klawans, H.L. Levodopa-Induced Psychosis: A Kindling Phenomenon. Am. J. Psychiatry 1978, 135, 669–675. [Google Scholar] [CrossRef]
- Poewe, W. Psychosis in Parkinson’s Disease. Mov. Disord. 2003, 18 (Suppl. 6), S80–S87. [Google Scholar] [CrossRef]
- Abadir, A.; Dalton, R.; Zheng, W.; Pincavitch, J.; Tripathi, R. Neuroleptic Sensitivity in Dementia with Lewy Body and Use of Pimavanserin in an Inpatient Setting: A Case Report. Am. J. Case Rep. 2022, 23, e937397. [Google Scholar] [CrossRef]
- Honeycutt, L.; Gagnon, J.-F.; Pelletier, A.; De Roy, J.; Montplaisir, J.Y.; Postuma, R.B. Pareidolias and Cognition in Isolated REM Sleep Behavior Disorder. Park. Relat. Disord. 2020, 75, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Sasai-Sakuma, T.; Nishio, Y.; Yokoi, K.; Mori, E.; Inoue, Y. Pareidolias in REM Sleep Behavior Disorder: A Possible Predictive Marker of Lewy Body Diseases? Sleep 2017, 40, zsw045. [Google Scholar] [CrossRef] [PubMed]
- Collerton, D.; Mosimann, U.P. Visual Hallucinations. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 1, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.C. Hallucinations in the General Population. Curr. Psychiatry Rep. 2005, 7, 162–167. [Google Scholar] [CrossRef]
- Mocellin, R.; Walterfang, M.; Velakoulis, D. Neuropsychiatry of Complex Visual Hallucinations. Aust. N. Z. J. Psychiatry 2006, 40, 742–751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capogna, E.; Pollarini, V.; Quinzi, A.; Guidi, L.; Sambati, L.; Criante, M.S.; Mengoli, E.; Venneri, A.; Lodi, R.; Tonon, C.; et al. Minor Visual Phenomena in Lewy Body Disease: A Systematic Review. Biomedicines 2025, 13, 1152. https://doi.org/10.3390/biomedicines13051152
Capogna E, Pollarini V, Quinzi A, Guidi L, Sambati L, Criante MS, Mengoli E, Venneri A, Lodi R, Tonon C, et al. Minor Visual Phenomena in Lewy Body Disease: A Systematic Review. Biomedicines. 2025; 13(5):1152. https://doi.org/10.3390/biomedicines13051152
Chicago/Turabian StyleCapogna, Elettra, Virginia Pollarini, Alessia Quinzi, Lucia Guidi, Luisa Sambati, Maria Sasca Criante, Elena Mengoli, Annalena Venneri, Raffaele Lodi, Caterina Tonon, and et al. 2025. "Minor Visual Phenomena in Lewy Body Disease: A Systematic Review" Biomedicines 13, no. 5: 1152. https://doi.org/10.3390/biomedicines13051152
APA StyleCapogna, E., Pollarini, V., Quinzi, A., Guidi, L., Sambati, L., Criante, M. S., Mengoli, E., Venneri, A., Lodi, R., Tonon, C., & Mitolo, M. (2025). Minor Visual Phenomena in Lewy Body Disease: A Systematic Review. Biomedicines, 13(5), 1152. https://doi.org/10.3390/biomedicines13051152






