Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study
Abstract
1. Introduction
2. Methods
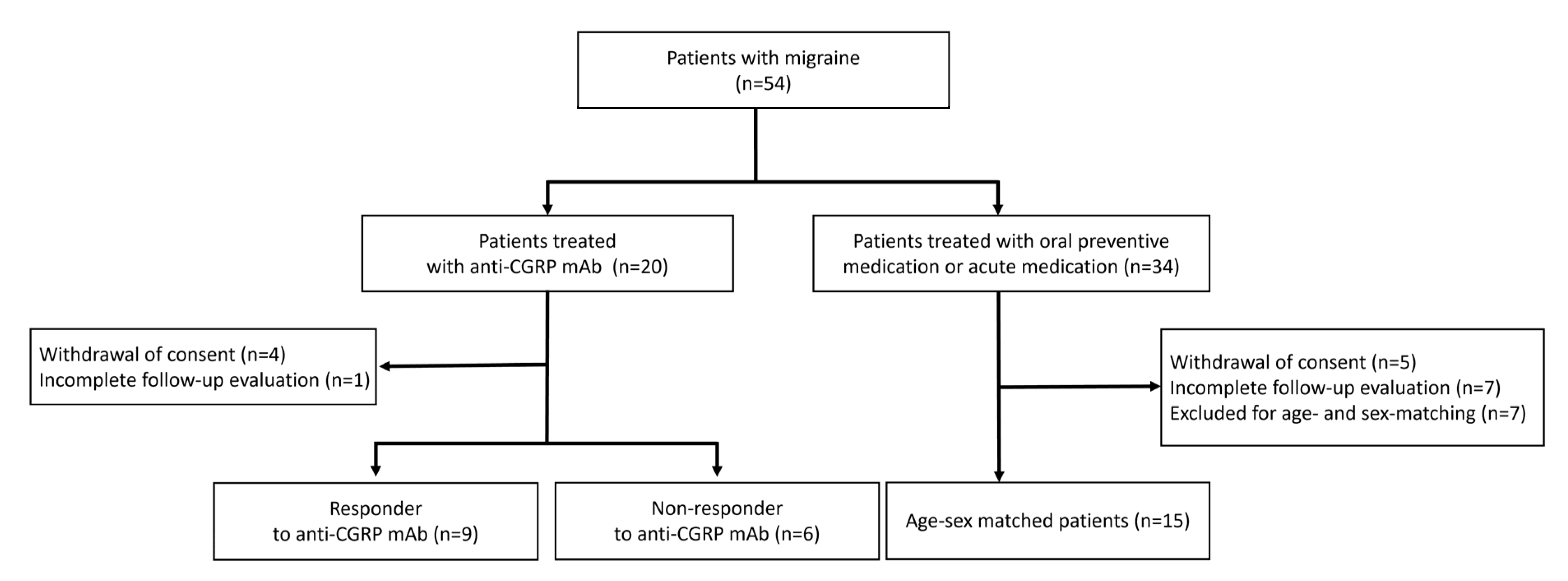
2.1. Patient Selection
2.2. Anti-CGRP Group and Oral TREATMENTGROUP
2.3. Study Protocol
2.4. MRI Acquisition
2.5. Cortical Thickness Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison Between Anti-CGRP Group and Oral Treatment Group
3.3. Comparison Between Responders and Non-Responders to Anti-CGRP mAbs
3.4. Association Between Clinical Variables and Cortical Thickness Changes
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stovner, L.J.; Nichols, E.; Steiner, T.J.; Abd-Allah, F.; Abdelalim, A.; Al-Raddadi, R.M.; Ansha, M.G.; Barac, A.; Bensenor, I.M.; Doan, L.P. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Lipton, R.B.; Ashina, S.; Ashina, M. Migraine and structural changes in the brain: A systematic review and meta-analysis. Neurology 2013, 81, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Bentivegna, E.; Martelletti, P.; Eikermann-Haerter, K. Structural and Functional Brain Changes in Migraine. Pain Ther. 2021, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F.; Yuan, H.; DePoy, E.M.G.; Silberstein, S.D. The Arrival of Anti-CGRP Monoclonal Antibodies in Migraine. Neurotherapeutics 2022, 19, 922–930. [Google Scholar] [CrossRef]
- Dodick, D.W. CGRP ligand and receptor monoclonal antibodies for migraine prevention: Evidence review and clinical implications. Cephalalgia 2019, 39, 445–458. [Google Scholar] [CrossRef]
- Kuburas, A.; Russo, A.F. Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J. Headache Pain 2023, 24, 34. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.B.; Suh, S.-i.; Seo, W.-K.; Oh, K.; Koh, S.-B. Thickening of the somatosensory cortex in migraine without aura. Cephalalgia 2014, 34, 1125–1133. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Granziera, C.; Snyder, J.; Hadjikhani, N. Thickening in the somatosensory cortex of patients with migraine. Neurology 2007, 69, 1990–1995. [Google Scholar] [CrossRef]
- Messina, R.; Rocca, M.A.; Colombo, B.; Valsasina, P.; Horsfield, M.A.; Copetti, M.; Falini, A.; Comi, G.; Filippi, M. Cortical abnormalities in patients with migraine: A surface-based analysis. Radiology 2013, 268, 170–180. [Google Scholar] [CrossRef]
- Magon, S.; May, A.; Stankewitz, A.; Goadsby, P.J.; Schankin, C.; Ashina, M.; Amin, F.M.; Seifert, C.L.; Mallar Chakravarty, M.; Müller, J. Cortical abnormalities in episodic migraine: A multi-center 3T MRI study. Cephalalgia 2019, 39, 665–673. [Google Scholar] [CrossRef]
- Torres-Ferrús, M.; Gallardo, V.J.; Alpuente, A.; Caronna, E.; Gine-Cipres, E.; Pozo-Rosich, P. The impact of anti-CGRP monoclonal antibodies in resistant migraine patients: A real-world evidence observational study. J. Neurol. 2021, 268, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Woldeamanuel, Y.W.; DeSouza, D.D.; Sanjanwala, B.M.; Cowan, R.P. Clinical Features Contributing to Cortical Thickness Changes in Chronic Migraine—A Pilot Study. Headache 2019, 59, 180–191. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Nikolova, S.; Dumkrieger, G.; Li, J.; Wu, T.; Chong, C.D. Longitudinal changes in functional connectivity and pain-induced brain activations in patients with migraine: A functional MRI study pre- and post- treatment with Erenumab. J. Headache Pain 2022, 23, 159. [Google Scholar] [CrossRef]
- Szabo, E.; Ashina, S.; Melo-Carrillo, A.; Bolo, N.R.; Borsook, D.; Burstein, R. Peripherally acting anti-CGRP monoclonal antibodies alter cortical gray matter thickness in migraine patients: A prospective cohort study. NeuroImage Clin. 2023, 40, 103531. [Google Scholar] [CrossRef] [PubMed]
- Newman-Norlund, R.D.; Rorden, C.; Maleki, N.; Patel, M.; Cheng, B.; Androulakis, X.M. Cortical and subcortical changes following sphenopalatine ganglion blocks in chronic migraine with medication overuse headache: A preliminary longitudinal study. Women’s Midlife Health 2020, 6, 7. [Google Scholar] [CrossRef]
- Amaral, V.C.G.; Tukamoto, G.; Kubo, T.; Luiz, R.R.; Gasparetto, E.; Vincent, M.B. Migraine improvement correlates with posterior cingulate cortical thickness reduction. Arq. Neuro-Psiquiatr. 2018, 76, 150–157. [Google Scholar] [CrossRef]
- Lee, S.; Chung, H.; Park, M.; Park, J.; Ryu, W.-S.; Ye, J.C. Improving 3D imaging with pre-trained perpendicular 2D diffusion models. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Paris, France, 2–6 October 2023; IEEE: New York, NY, USA, 2023; pp. 10710–10720. [Google Scholar]
- Seminowicz, D.A.; Shpaner, M.; Keaser, M.L.; Krauthamer, G.M.; Mantegna, J.; Dumas, J.A.; Newhouse, P.A.; Filippi, C.G.; Keefe, F.J.; Naylor, M.R. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 2013, 14, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Ofoghi, Z.; Rohr, C.S.; Dewey, D.; Bray, S.; Yeates, K.O.; Noel, M.; Barlow, K.M. Functional connectivity of the anterior cingulate cortex with pain-related regions in children with post-traumatic headache. Cephalalgia Rep. 2021, 4, 25158163211009477. [Google Scholar] [CrossRef]
- Dai, W.; Liu, R.-H.; Qiu, E.; Liu, Y.; Chen, Z.; Chen, X.; Ao, R.; Zhuo, M.; Yu, S. Cortical mechanisms in migraine. Mol. Pain 2021, 17, 17448069211050246. [Google Scholar] [CrossRef]
- Ou, Y.; Ni, X.; Gao, X.; Yu, Y.; Zhang, Y.; Wang, Y.; Liu, J.; Yin, Z.; Rong, J.; Sun, M.; et al. Structural and functional changes of anterior cingulate cortex subregions in migraine without aura: Relationships with pain sensation and pain emotion. Cereb. Cortex 2024, 34, bhae040. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Chen, K.; Cao, Y.; Liu, K.; Zhang, X.; Wu, X.; Wang, Z.; Liu, X.; Li, L. Aberrant functional connectivity in anterior cingulate gyrus subregions in migraine without aura patients. Front. Neurol. 2024, 15, 1412117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.L.; Su, J.; Yao, Q.; Wang, M.; Li, G.F.; Zhao, R.; Shi, Y.H.; Zhao, Y.; Zhang, Q.; et al. Assessment of gray and white matter structural alterations in migraineurs without aura. J. Headache Pain 2017, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- DeSouza, D.D.; Krimmel, S.R.; Sanjanwala, B.M.; Peretz, A.; Menon, V.; Seminowicz, D.A.; Cowan, R.P. Increased amygdala volume and functional connectivity with cognitive control networks in chronic migraine. medRxiv, 2020. [Google Scholar] [CrossRef]
- Pan, X.; Ren, H.; Xie, L.; Zou, Y.; Li, F.; Sui, X.; Cui, L.; Cheng, Z.; Wu, J.; Shi, F.; et al. Analysis of the relationships between the degree of migraine with right-to-left shunts and changes in white matter lesions and brain structural volume. Sci. Rep. 2025, 15, 1145. [Google Scholar] [CrossRef]
- Hubbard, C.S.; Becerra, L.; Smith, J.H.; DeLange, J.M.; Smith, R.M.; Black, D.F.; Welker, K.M.; Burstein, R.; Cutrer, F.M.; Borsook, D. Brain Changes in Responders vs. Non-Responders in Chronic Migraine: Markers of Disease Reversal. Front. Hum. Neurosci. 2016, 10, 497. [Google Scholar] [CrossRef] [PubMed]

| Anti-CGRP Group (n = 15) | Oral Treatment Group * (n = 15) | p-Value | |
|---|---|---|---|
| Age, years | 47.0 (34.0–52.0) | 44.0 (34.0–51.0) | 0.645 |
| Female sex | 15 (100.0%) | 15 (100.0%) | >0.999 |
| BMI, kg/m2 | 23.4 (22.4–25.1) | 21.5 (20.6–23.8) | 0.021 |
| Disease duration, months | 120 (96.0–240.0) | 120 (48.0–180.0) | 0.451 |
| Aura, n | 2 (13.3%) | 3 (20.0%) | 0.165 |
| Prodromal symptoms, n | 14(93.3%) | 11 (73.3%) | 0.464 |
| Chronic migraine, n | 8 (53.3%) | 6 (40.0%) | 0.330 |
| Baseline MHDs, days | 21.0 (8.0–30.0) | 12.0 (8.0–30.0) | 0.312 |
| Headache severity, NRS | 8.0 (7.0–9.0) | 7.0 (5.0–8.0) | 0.007 |
| Oral preventive medication use, n | |||
| Amitriptyline | 4 (26.7%) | 1 (6.7%) | 0.330 |
| Topiramate | 4 (26.7%) | 11 (73.3%) | 0.027 |
| Beta blocker | 1 (6.7%) | 7 (46.7%) | 0.035 |
| Calcium channel blocker | 0 (0.0%) | 0 (0.0%) | |
| Divalproex sodium | 0 (0.0%) | 0 (0.0%) |
| Anti-CGRP Group (n = 15) | Oral Treatment Group (n = 15) | p-Value | |
|---|---|---|---|
| Baseline MHDs, days | 21.0 (8.0–30.0) | 12.0 (8.0–30.0) | 0.312 |
| MHDs after 3 months, days | 4.0 (3.0–14.0) | 9.0 (3.0–22.0) | 0.453 |
| Response rate to treatment | 57.1% (0.0–84.6) | 12.5% (0.0–40.0) | 0.035 |
| ≥50% responder to treatment | 9 (60.0%) | 2 (13.3%) | 0.021 |
| MRI follow-up duration, days | 91.0 (91.0–96.0) | 91.0 (91.0–92.0) | 0.430 |
| Cortical thickness of right caudal anterior cingulate cortex, mm | |||
| Baseline | 3.240 (3.080–3.310) | 3.150 (3.010–3.500) | 0.830 |
| After 3 months treatment | 3.140 (2.920–3.440) | 3.230 (3.090–3.370) | 0.645 |
| Change in cortical thickness | −0.120 (−0.290–0.180) | 0.400 (−0.220–0.150) | 0.560 |
| Cortical thickness of left rostral middle frontal cortex, mm | |||
| Baseline | 3.830 (3.750–4.010) | 3.810 (3.710–3.930) | 0.372 |
| After 3 months treatment | 3.860 (3.610–3.960) | 3.770 (3.640–3.860) | 0.372 |
| Change in cortical thickness | −0.120 (−0.210–0.120) | −0.300 (−0.150–0.050) | 0.675 |
| Responders (n = 9) | Non-Responders (n = 6) | p-Value | |
|---|---|---|---|
| Baseline MHDs, days | 26.0 (14.0–30.0) | 17.5 (5.3–28.5) | 0.227 |
| MHDs after 3 months, days | 4.0 (3.0–5.5) | 14.5 (6.8–28.5) | 0.017 |
| Response rate to treatment | 81.0% (58.6–88.3) | 0.0% (0.0–34.2) | <0.001 |
| MRI follow-up duration, days | 91.0 (91.0–97.0) | 91.0 (87.5–95.3) | 0.405 |
| Cortical thickness of right caudal anterior cingulate cortex, mm | |||
| Baseline | 3.260 (3.100–3.380) | 3.160 (2.900–3.398) | 0.438 |
| After 3 months treatment | 2.990 (2.865–3.175) | 3.450 (3.198–3.615) | 0.012 |
| Change in cortical thickness | −0.170 (−0.335–−0.110) | 0.130 (−0.005–0.460) | 0.026 |
| Cortical thickness of left rostral middle frontal cortex, mm | |||
| Baseline | 3.830 (3.760–4.035) | 3.880 (3.610–4.028) | 0.887 |
| After 3 months treatment | 3.730 (3.595–3.885) | 3.965 (3.793–4.100) | 0.082 |
| Change in cortical thickness | −0.170 (−0.220–−0.100) | 0.055 (−0.068–0.283) | 0.007 |
| Right Caudal Anterior Cingulate Cortex | Left Rostral Middle Frontal Cortex | |||||
|---|---|---|---|---|---|---|
| B (95% CI) | SE | P | B (95% CI) | SE | P | |
| Intercept | 0.061 (−0.986–1.108) | 0.534 | 0.909 | 0.075 (−0.425–0.575) | 0.255 | 0.769 |
| Age | −0.002 (−0.013–0.009) | 0.006 | 0.739 | −0.002 (−0.007–0.004) | 0.003 | 0.550 |
| BMI | −0.003 (−0.041–0.034) | 0.019 | 0.855 | −0.003 (−0.021–0.015) | 0.009 | 0.750 |
| Chronic migraine | −0.134 (−0.457–0.189) | 0.165 | 0.416 | −0.002 (−0.156–0.152) | 0.079 | 0.977 |
| Baseline MHDs | 0.007 (−0.009–0.024) | 0.008 | 0.374 | 0.001 (−0.007–0.009) | 0.004 | 0.808 |
| Anti-CGRP treatment | 0.259 (−0.045–0.563) | 0.155 | 0.095 | 0.134 (−0.011–0.279) | 0.074 | 0.070 |
| Response to anti-CGRP mAbs | −0.429 (−0.777–−0.081) | 0.178 | 0.016 | −0.224 (−0.390–−0.057) | 0.085 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S. Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study. Biomedicines 2025, 13, 1150. https://doi.org/10.3390/biomedicines13051150
Cho S. Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study. Biomedicines. 2025; 13(5):1150. https://doi.org/10.3390/biomedicines13051150
Chicago/Turabian StyleCho, Soohyun. 2025. "Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study" Biomedicines 13, no. 5: 1150. https://doi.org/10.3390/biomedicines13051150
APA StyleCho, S. (2025). Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study. Biomedicines, 13(5), 1150. https://doi.org/10.3390/biomedicines13051150







