Anti-Inflammatory Properties of Yellow Passion Fruit Bagasse Extract and Its Potential Role in Periodontal Wound Healing In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
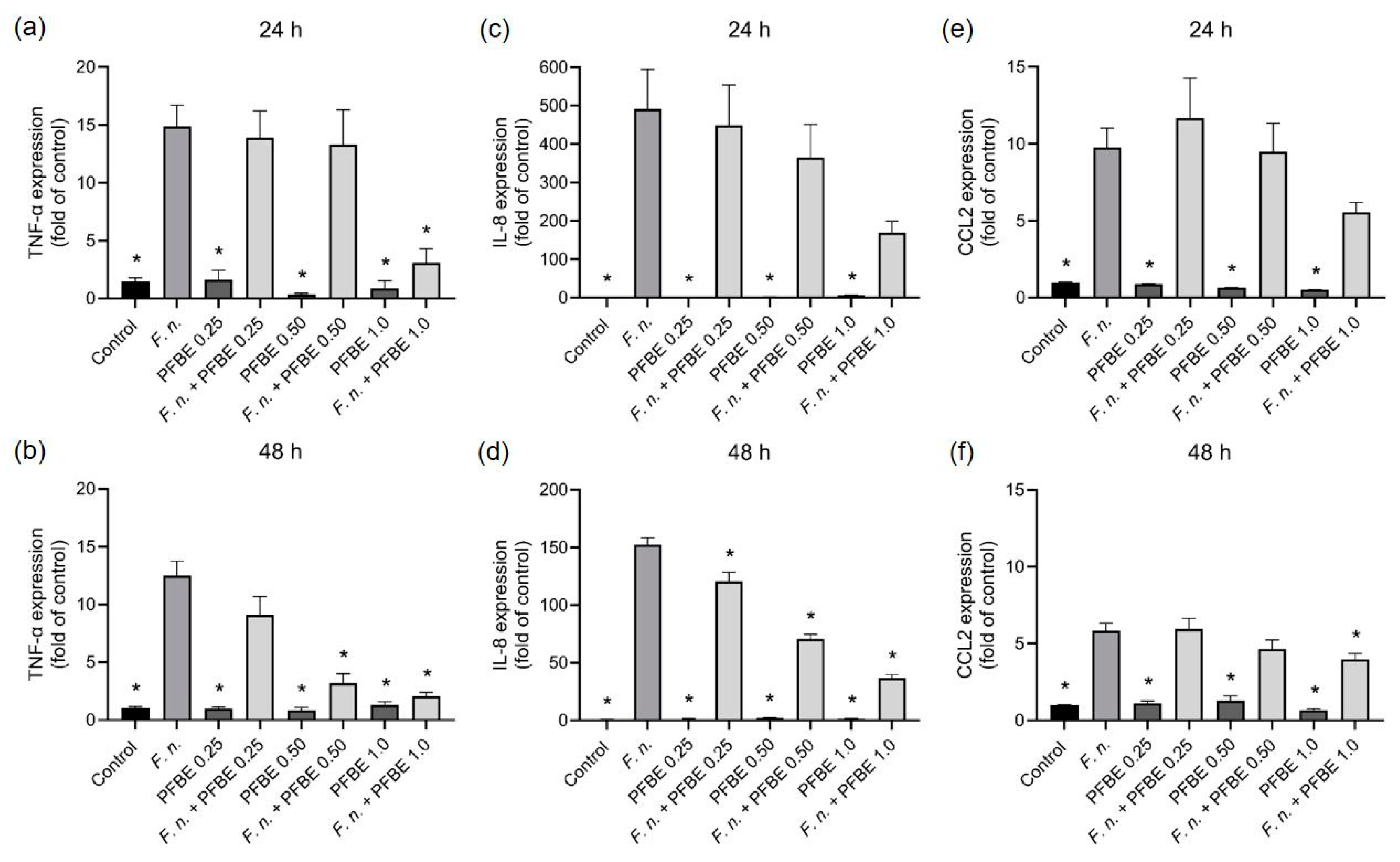
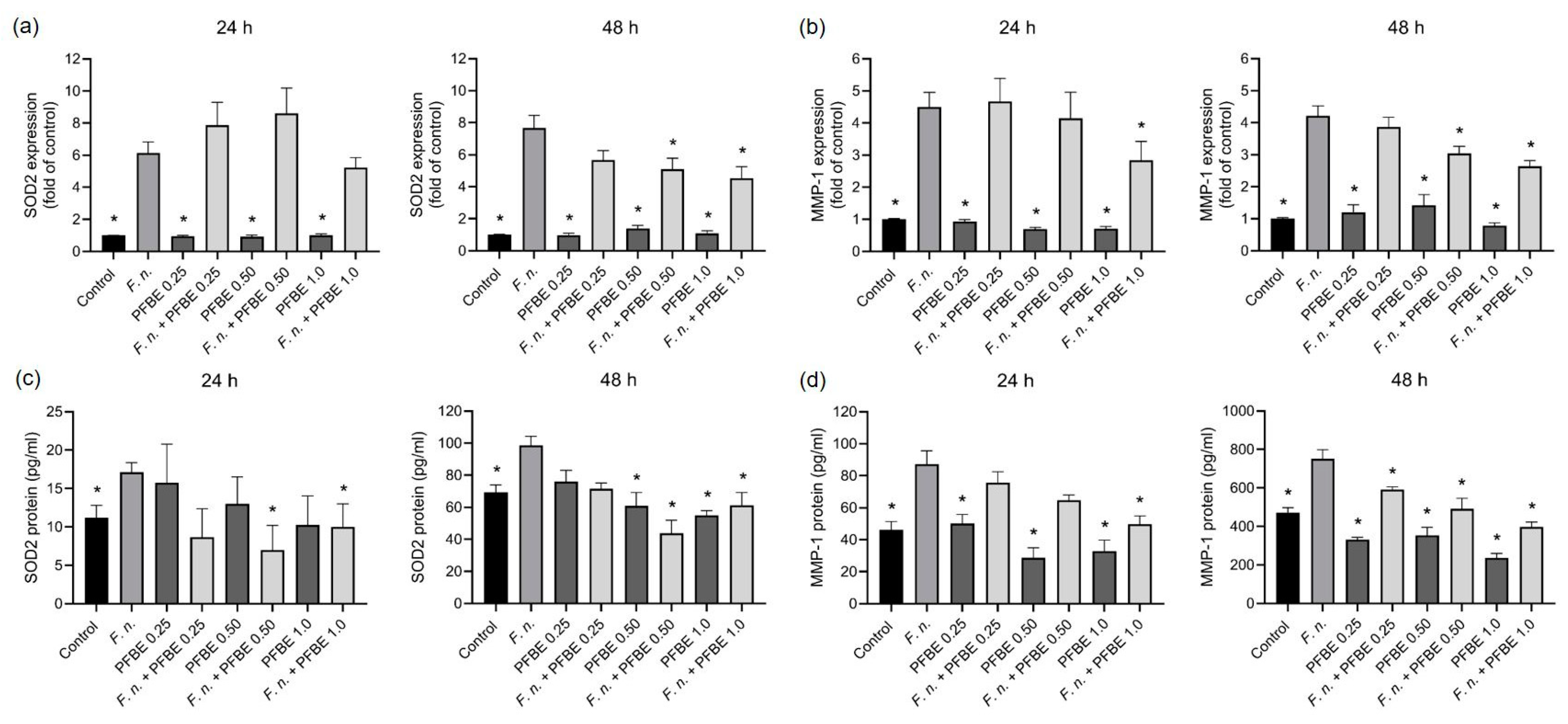
2.2. Gene Expressions
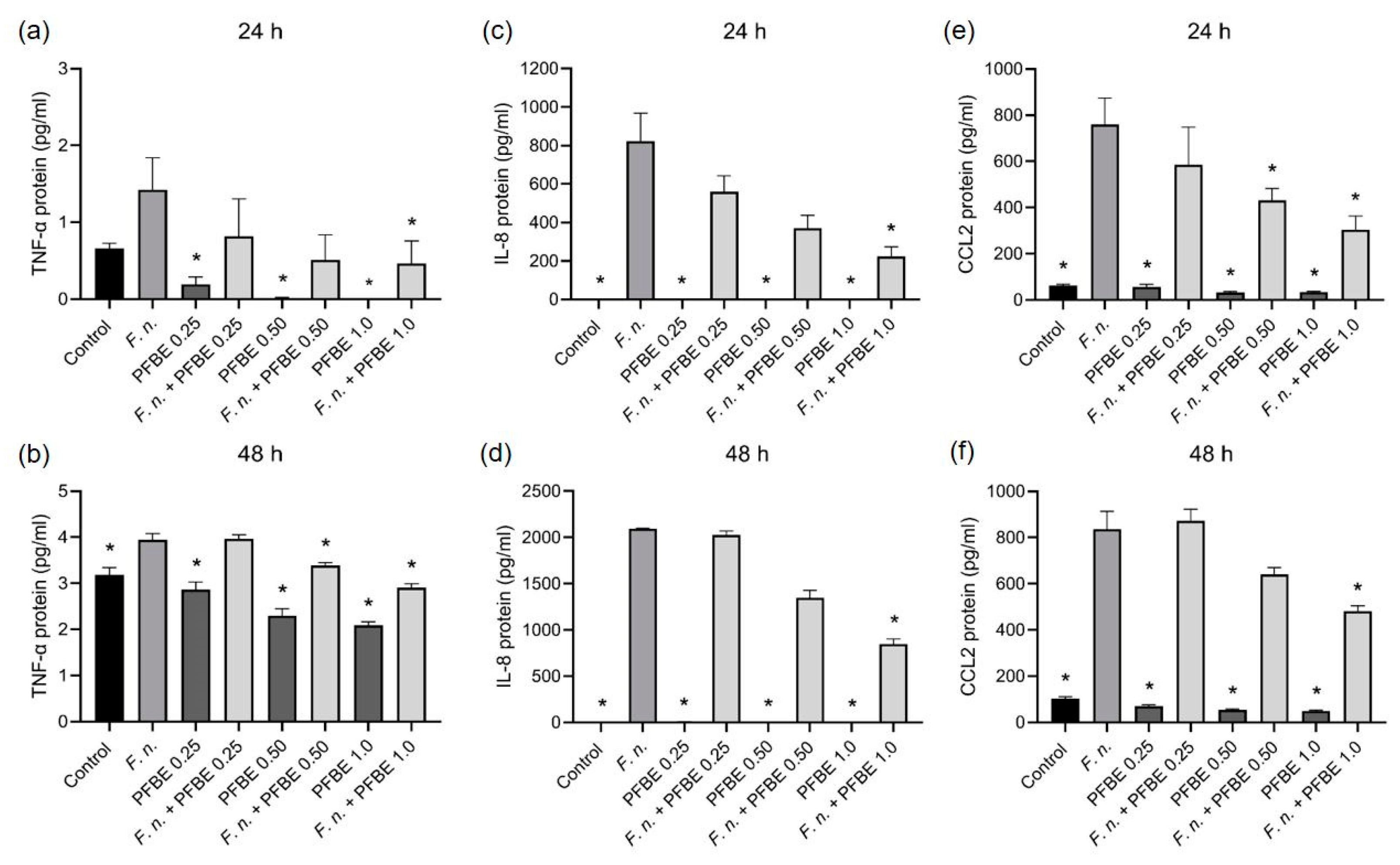
2.3. Protein Levels
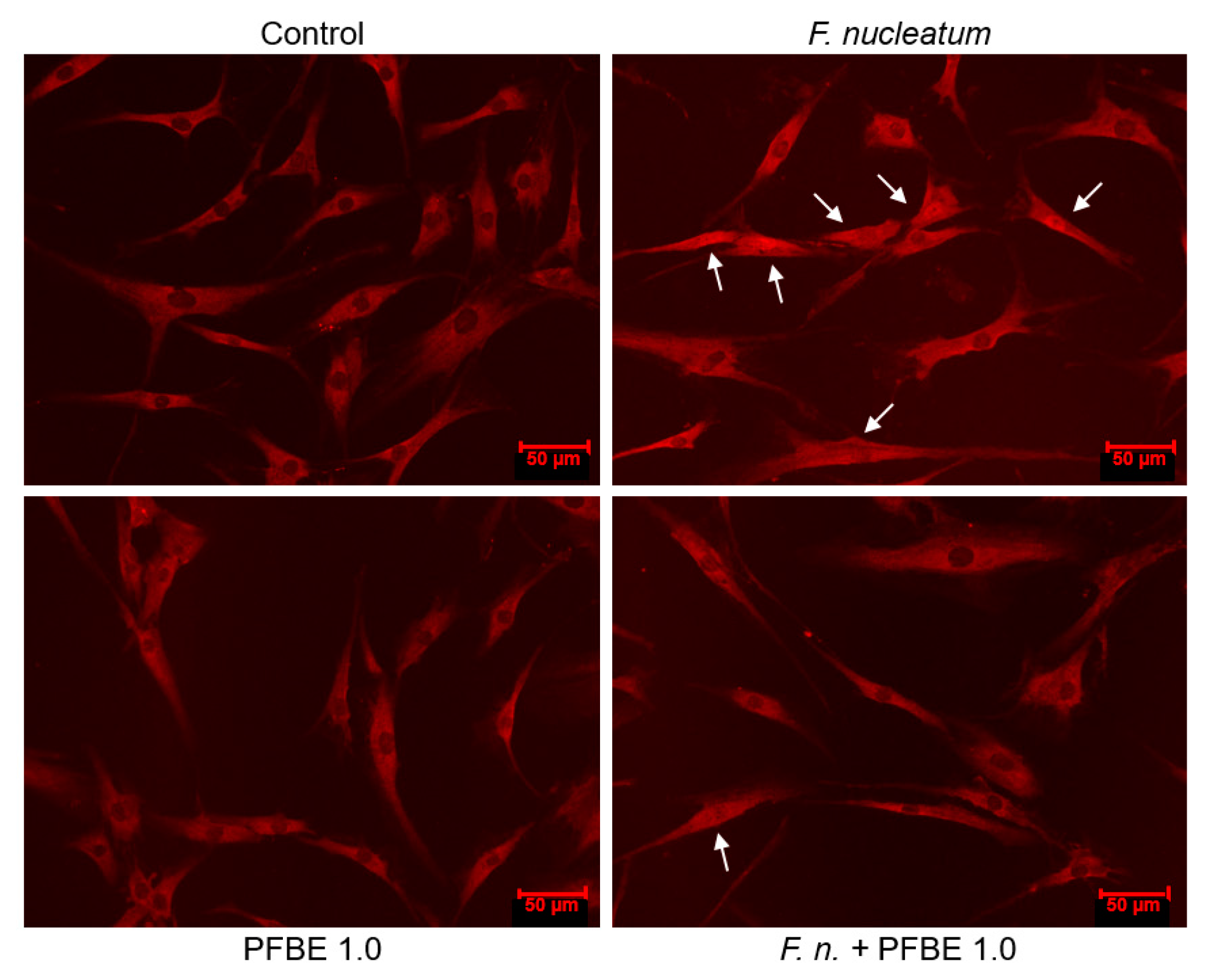
2.4. Immunofluorescence
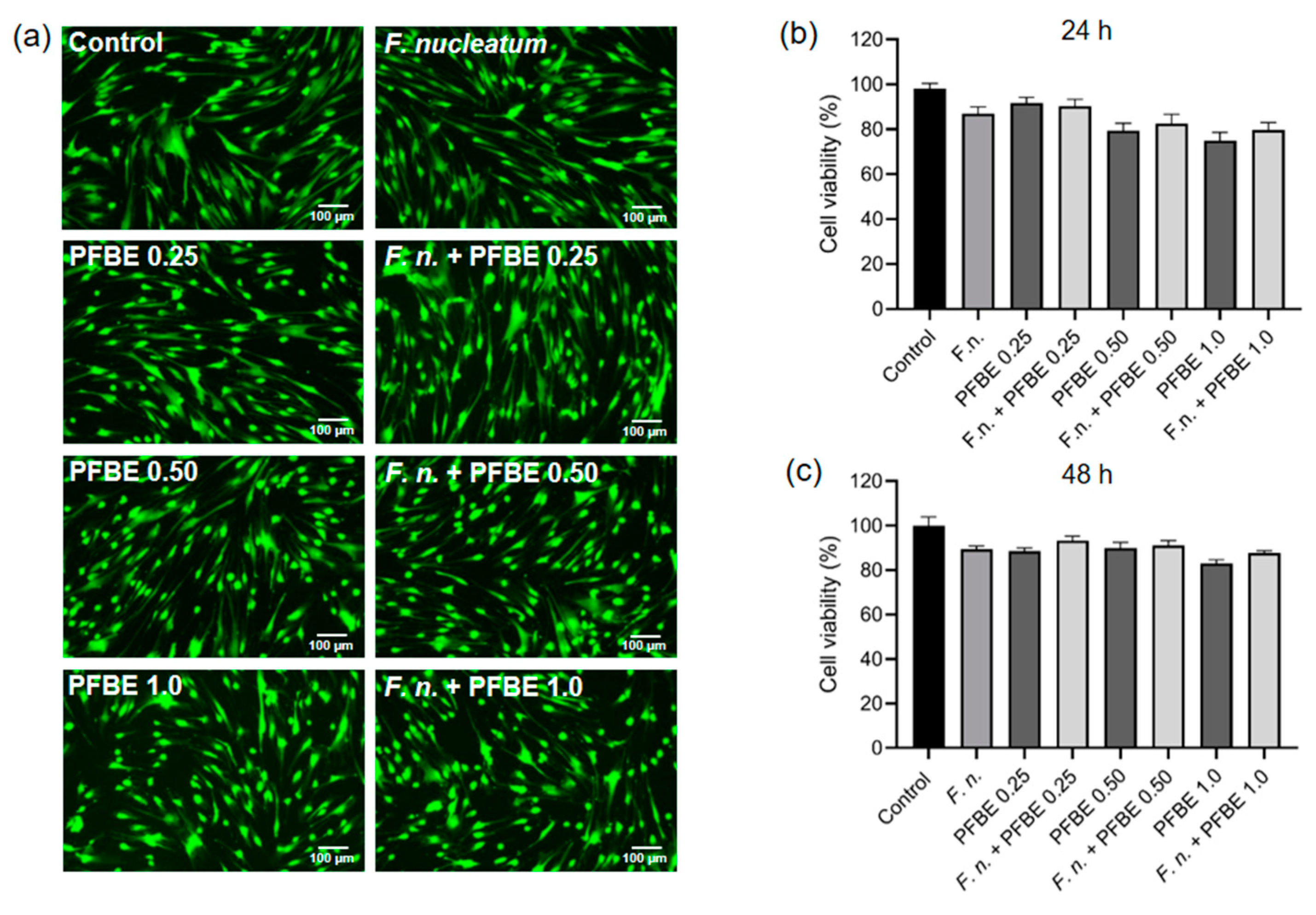
2.5. Cell Viability Assay
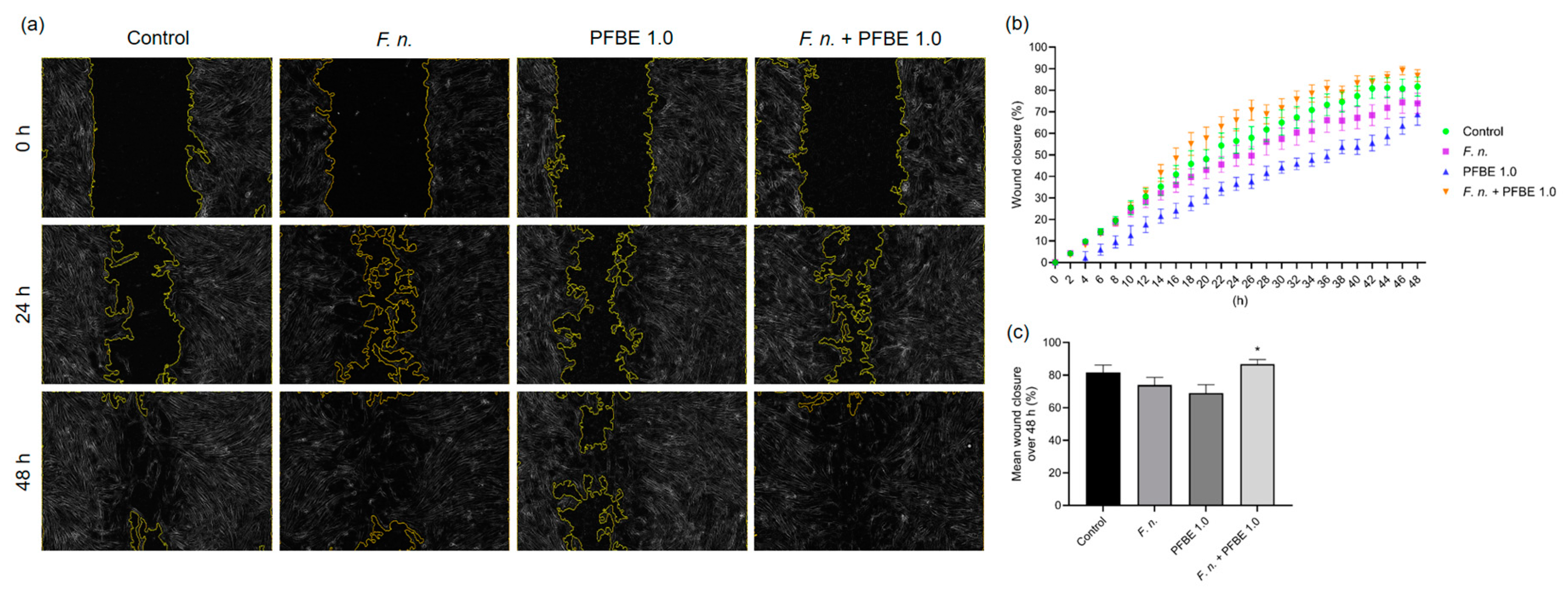
2.6. Wound Healing Assay
2.7. Statistical Analysis
3. Results
3.1. Modulatory Effects of PFBE on Pro-Inflammatory Markers
3.2. Modulatory Effects of PFBE on Markers Involved in Oxidative Stress and Proteolytic Conditions
3.3. Regulation of F. nucleatum-Induced NF-κB Nuclear Translocation by PFBE
3.4. Effects of F. nucleatum and/or PFBE on Cell Viability
3.5. Effects of F. nucleatum and/or PFBE on In Vitro Wound Closure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDL | Periodontal ligament |
| PFBE | Passion fruit bagasse extract |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| RNA | Ribonucleic acid |
| cDNA | Complementary deoxyribonucleic acid |
| TNF-α | Tumor necrosis factor-alpha |
| IL-8 | Interleukin-8 |
| CCL2 | C-C motif chemokine ligand 2 |
| SOD2 | Superoxide dismutase 2 |
| MMP-1 | Matrix metalloproteinase-1 |
| PCR | Polymerase chain reaction |
| ELISA | Enzyme-linked immunosorbent assay |
| RT | Room temperature |
| NF-κB | Nuclear factor-kappa B |
| SEM | Standarde Errors of the means |
| ANOVA | Analysis of variance |
| TRAMP | Transgenic adenocarcinoma of mouse prostrate |
| UVB | Ultraviolet B |
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Villoria, G.E.M.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal disease: A systemic condition. Periodontol 2000 2024, 96, 7–19. [Google Scholar] [CrossRef]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Tomasi, C.; Abrahamsson, K.H.; Apatzidou, D. Subgingival instrumentation. Periodontol 2000 2023. [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Ding, Y.; Yao, H.; Yao, Y.; Fai, L.Y.; Zhang, Z. Protection of dietary polyphenols against oral cancer. Nutrients 2013, 5, 2173–2191. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Medori, M.C.; Dhuli, K.; Donato, K.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; Bertelli, M. Polyphenols and Lactobacillus reuteri in oral health. J. Prev. Med. Hyg. 2022, 63, E246–E254. [Google Scholar] [PubMed]
- Lagha, A.B.; Grenier, D. Tea polyphenols protect gingival keratinocytes against TNF-α-induced tight junction barrier dysfunction and attenuate the inflammatory response of monocytes/macrophages. Cytokine 2019, 115, 64–75. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Howell, A.; Grenier, D. Highbush blueberry proanthocyanidins alleviate Porphyromonas gingivalis-induced deleterious effects on oral mucosal cells. Anaerobe 2020, 65, 102266. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Z.; Chen, F.; Chai, Y. Polyphenols in oral health: Homeostasis maintenance, disease prevention, and therapeutic applications. Nutrients 2023, 15, 4384. [Google Scholar] [CrossRef]
- Akbari, E.; Epstein, J.B.; Samim, F. Unveiling the hidden links: Periodontal disease, Fusobacterium nucleatum, and cancers. Curr. Oncol. Rep. 2024, 26, 1388–1397. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Ben Lagha, A.; Grenier, D. Effects of a berry polyphenolic fraction on the pathogenic properties of Porphyromonas gingivalis. Front. Oral Health 2022, 3, 923663. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit seeds as sources of bioactive compounds: Sustainable production of high value-added ingredients from by-products within circular economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Son, P.S.; Park, S.A.; Na, H.K.; Jue, D.M.; Kim, S.; Surh, Y.J. Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-κB activation and cyclooxygenase-2 expression in human breast epithelial cells: Cysteine 179 of IKKβ as a potential target. Carcinogenesis 2010, 31, 1442–1449. [Google Scholar] [CrossRef]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.; Vijayaraghavan, K. A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sato, A.; Takai, Y.; Yoshimori, A.; Umehara, M.; Ogino, Y.; Inada, M.; Shimada, N.; Nishida, A.; Ichida, R.; et al. Effect of piceatannol-rich passion fruit seed extract on human glyoxalase I-mediated cancer cell growth. Biochem. Biophys. Rep. 2019, 20, 100684. [Google Scholar] [CrossRef]
- Costa, F.P.D.; Puty, B.; Nogueira, L.S.; Mitre, G.P.; Santos, S.M.D.; Teixeira, B.J.B.; Kataoka, M.S.D.S.; Martins, M.D.; Barboza, C.A.G.; Monteiro, M.C.; et al. Piceatannol increases antioxidant defense and reduces cell death in human periodontal ligament fibroblast under oxidative stress. Antioxidants 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Damasceno, S.R.; Brito, T.V.; Dias, J.M.; Fontenele, A.M.; Braúna, I.S.; Júnior, J.S.; Maciel, J.S.; de Paula, R.C.; Ribeiro, R.A.; et al. Polysaccharide fraction isolated from Passiflora edulis inhibits the inflammatory response and oxidative stress in mice. J. Pharm. Pharmacol. 2015, 67, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Nogueira, A.V.B.; Memmert, S.; Nokhbehsaim, M.; Cirelli, J.A.; Eick, S.; Miosge, N.; Kirschneck, C.; Kesting, M.; Deschner, J.; et al. Regulation of anti-apoptotic SOD2 and BIRC3 in periodontal cells and tissues. Int. J. Mol. Sci. 2021, 22, 591. [Google Scholar] [CrossRef]
- Cores Ziskoven, P.; Nogueira, A.V.B.; Gutierrez, L.S.; Weusmann, J.; Eick, S.; Buduneli, N.; Deschner, J. Apelin enhances the effects of Fusobacterium nucleatum on periodontal ligament cells in vitro. Int. J. Mol. Sci. 2023, 24, 4733. [Google Scholar] [CrossRef]
- Viganó, J.; Aguiar, A.C.; Moraes, D.R.; Jara, J.L.P.; Eberlin, M.N.; Cazarin, C.B.B.; Maróstica, M.R., Jr.; Martínez, J. Sequential high-pressure extractions applied to recover piceatannol and scirpusin B from passion fruit bagasse. Food Res. Int. 2016, 85, 51–58. [Google Scholar] [CrossRef]
- Viganó, J.; Assis, B.F.P.; Náthia-Neves, G.; Dos Santos, P.; Meireles, M.A.A.; Veggi, P.C.; Martínez, J. Extraction of bioactive compounds from defatted passion fruit bagasse (Passiflora edulis sp.) applying pressurized liquids assisted by ultrasound. Ultrason. Sonochem. 2020, 64, 104999. [Google Scholar] [CrossRef]
- Maruki-Uchida, H.; Kurita, I.; Sugiyama, K.; Sai, M.; Maeda, K.; Ito, T. The protective effects of piceatannol from passion fruit (Passiflora edulis) seeds in UVB-irradiated keratinocytes. Biol. Pharm. Bull. 2013, 36, 845–849. [Google Scholar] [CrossRef]
- Milovanova-Palmer, J.; Pendry, B. Is there a role for herbal medicine in the treatment and management of periodontal disease? J. Herb. Med. 2018, 12, 33–48. [Google Scholar] [CrossRef]
- Pasupuleti, M.K.; Nagate, R.R.; Alqahtani, S.M.; Penmetsa, G.S.; Gottumukkala, S.N.V.S.; Ramesh, K.S.V. Role of medicinal herbs in periodontal therapy: A systematic review. J. Int. Soc. Prev. Community Dent. 2023, 13, 9–16. [Google Scholar] [CrossRef]
- Baseggio, A.M.; Kido, L.A.; Viganó, J.; Carneiro, M.J.; Lamas, C.A.; Martínez, J.; Sawaya, A.C.H.F.; Cagnon, V.H.A.; Maróstica Júnior, M.R. Systemic antioxidant and anti-inflammatory effects of yellow passion fruit bagasse extract during prostate cancer progression. J. Food Biochem. 2022, 46, e13885. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Götz, W.; Deschner, J.; Jäger, A.; Nogueira, A.V.B. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues—In vitro and in vivo studies. Clin. Oral Investig. 2020, 24, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Kirakodu, S.; Novak, M.J.; Stromberg, A.J.; Shen, S.; Orraca, L.; Gonzalez-Martinez, J.; Burgos, A.; Gonzalez, O.A. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J. Clin. Periodontol. 2014, 41, 853–861. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Afzali, H.; Graves, D.T. An update on periodontal inflammation and bone loss. Front. Immunol. 2024, 15, 1385436. [Google Scholar] [CrossRef]
- Ashikawa, K.; Majumdar, S.; Banerjee, S.; Bharti, A.C.; Shishodia, S.; Aggarwal, B.B. Piceatannol inhibits TNF-induced NF-κB activation and NF-κB-mediated gene expression through suppression of IκBα kinase and p65 phosphorylation. J. Immunol. 2002, 169, 6490–6497. [Google Scholar] [CrossRef]
- Offenbacher, S.; Barros, S.P.; Paquette, D.W.; Winston, J.L.; Biesbrock, A.R.; Thomason, R.G.; Gibb, R.D.; Fulmer, A.W.; Tiesman, J.P.; Juhlin, K.D.; et al. Gingival transcriptome patterns during induction and resolution of experimental gingivitis in humans. J. Periodontol. 2009, 80, 1963–1982. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, T.; Lee, J.; Kim, D. SOD2 is upregulated in periodontitis to reduce further inflammation progression. Oral Dis. 2018, 24, 1572–1580. [Google Scholar] [CrossRef]
- Sukhtankar, L.; Kulloli, A.; Kathariya, R.; Shetty, S. Effect of non-surgical periodontal therapy on superoxide dismutase levels in gingival tissues of chronic periodontitis patients: A clinical and spectrophotometric analysis. Dis. Markers 2013, 34, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Milward, M.R.; Chapple, I.L.; Carter, K.; Matthews, J.B.; Cooper, P.R. Micronutrient modulation of NF-κB in oral keratinocytes exposed to periodontal bacteria. Innate Immun. 2013, 19, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sztukowska, M.; Ojo, A.; Scott, D.A.; Wang, H.; Lamont, R.J. FOXO responses to Porphyromonas gingivalis in epithelial cells. Cell. Microbiol. 2015, 17, 1605–1617. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gupta Vallur, P.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the dichotomous regulation of SOD2 in cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef]
- Zhu, T.; Fang, F.; Sun, D.; Yang, S.; Zhang, X.; Yu, X.; Yang, L. Piceatannol inhibits P. acnes-induced keratinocyte proliferation and migration by downregulating oxidative stress and the inflammatory response. Inflammation 2020, 43, 347–357. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Chang, Q.; Wang, J.; Liu, J.; Lv, Y.; Wang, T.T.Y.; Gao, B.; Zhang, Y.; Yu, L.L. Inhibitory effect of piceatannol on TNF-α-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. J. Agric. Food Chem. 2017, 65, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Sun, T.; Shen, S.; Li, Z.; Ma, X.; Gu, X.; Zhang, X.; Peng, A.; Xu, X.; et al. Study of the inflammatory activating process in the early stage of Fusobacterium nucleatum-infected PDLSCs. Int. J. Oral Sci. 2023, 15, 8. [Google Scholar] [CrossRef]
- Çınar Ayan, İ.; Güçlü, E.; Vural, H.; Dursun, H.G. Piceatannol induces apoptotic cell death through activation of the caspase-dependent pathway and upregulation of ROS-mediated mitochondrial dysfunction in pancreatic cancer cells. Mol. Biol. Rep. 2022, 49, 11947–11957. [Google Scholar] [CrossRef]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K. Antioxidants counteract nicotine and promote migration via RacGTP in oral fibroblast cells. J. Periodontol. 2010, 81, 1675–1690. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Keser, S.; Nogueira, A.V.; Cirelli, J.A.; Jepsen, S.; Jäger, A.; Eick, S.; Deschner, J. Beneficial effects of adiponectin on periodontal ligament cells under normal and regenerative conditions. J. Diabetes Res. 2014, 2014, 796565. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Keser, S.; Nogueira, A.V.; Jäger, A.; Jepsen, S.; Cirelli, J.A.; Bourauel, C.; Eick, S.; Deschner, J. Leptin effects on the regenerative capacity of human periodontal cells. Int. J. Endocrinol. 2014, 2014, 180304. [Google Scholar] [CrossRef] [PubMed]
- Damanaki, A.; Nokhbehsaim, M.; Eick, S.; Götz, W.; Winter, J.; Wahl, G.; Jäger, A.; Jepsen, S.; Deschner, J. Regulation of NAMPT in human gingival fibroblasts and biopsies. Mediat. Inflamm. 2014, 2014, 912821. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Andrian, E.; Grenier, D. Resveratrol attenuates the pathogenic and inflammatory properties of Porphyromonas gingivalis. Mol. Oral Microbiol. 2019, 34, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, T.; Li, Y.; Huang, L.; Yin, D. Fusobacterium nucleatum: The opportunistic pathogen of periodontal and peri-implant diseases. Front. Microbiol. 2022, 13, 860149. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, A.V.B.; Faria, L.V.; Lopes, M.E.S.; Viganó, J.; Martínez, J.; Eick, S.; Cirelli, J.A.; Deschner, J. Anti-Inflammatory Properties of Yellow Passion Fruit Bagasse Extract and Its Potential Role in Periodontal Wound Healing In Vitro. Biomedicines 2025, 13, 1134. https://doi.org/10.3390/biomedicines13051134
Nogueira AVB, Faria LV, Lopes MES, Viganó J, Martínez J, Eick S, Cirelli JA, Deschner J. Anti-Inflammatory Properties of Yellow Passion Fruit Bagasse Extract and Its Potential Role in Periodontal Wound Healing In Vitro. Biomedicines. 2025; 13(5):1134. https://doi.org/10.3390/biomedicines13051134
Chicago/Turabian StyleNogueira, Andressa V. B., Luan V. Faria, Maria Eduarda S. Lopes, Juliane Viganó, Julian Martínez, Sigrun Eick, Joni A. Cirelli, and James Deschner. 2025. "Anti-Inflammatory Properties of Yellow Passion Fruit Bagasse Extract and Its Potential Role in Periodontal Wound Healing In Vitro" Biomedicines 13, no. 5: 1134. https://doi.org/10.3390/biomedicines13051134
APA StyleNogueira, A. V. B., Faria, L. V., Lopes, M. E. S., Viganó, J., Martínez, J., Eick, S., Cirelli, J. A., & Deschner, J. (2025). Anti-Inflammatory Properties of Yellow Passion Fruit Bagasse Extract and Its Potential Role in Periodontal Wound Healing In Vitro. Biomedicines, 13(5), 1134. https://doi.org/10.3390/biomedicines13051134






