Kynurenine as a Predictor of Long-Term Mortality: A 10-Year Follow-Up from the KORONEF Registry
Abstract
1. Introduction
2. Materials and Methods
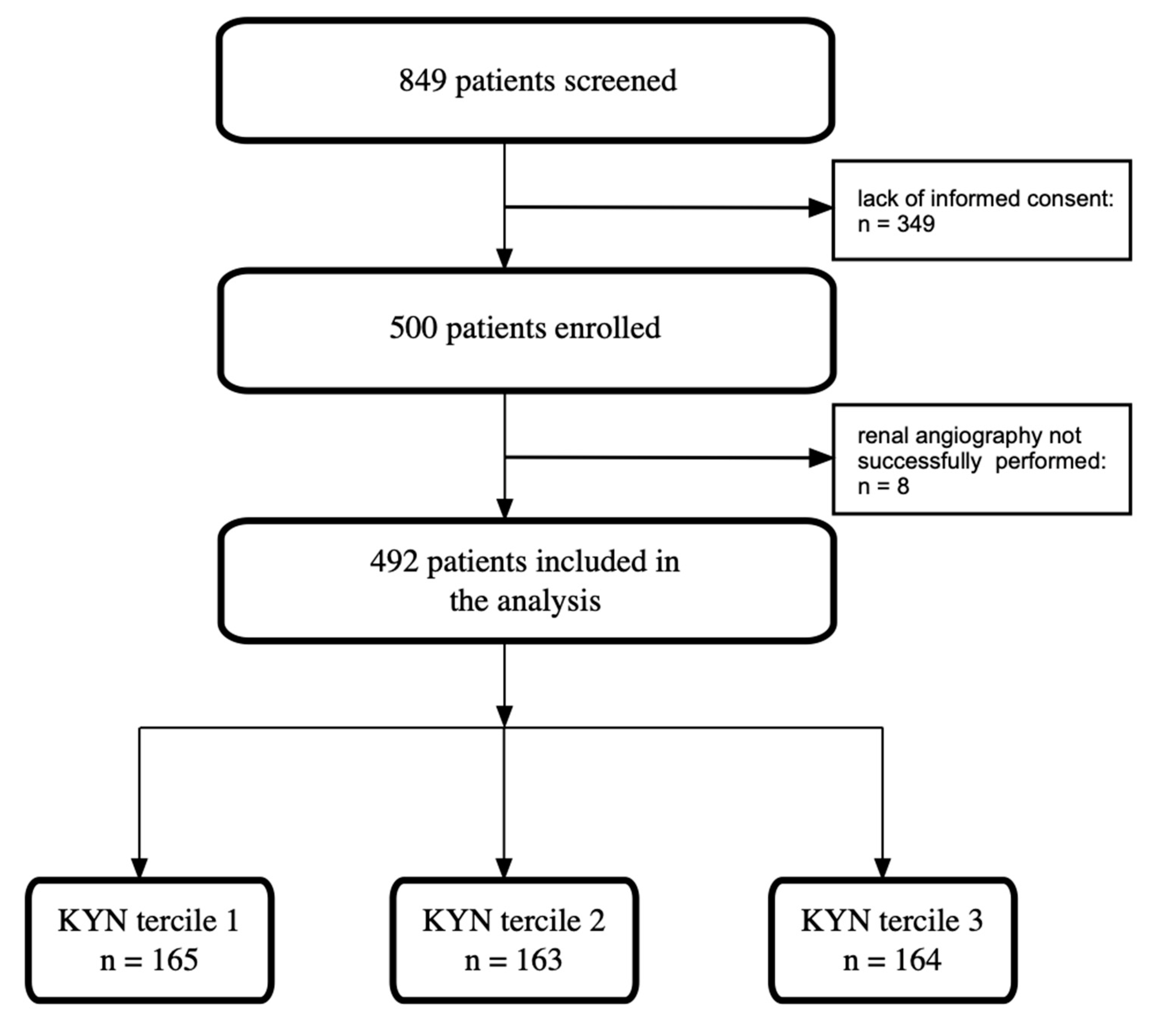
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Procedure Characteristics
2.4. Study Endpoints
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Periprocedural and Discharge Characteristics
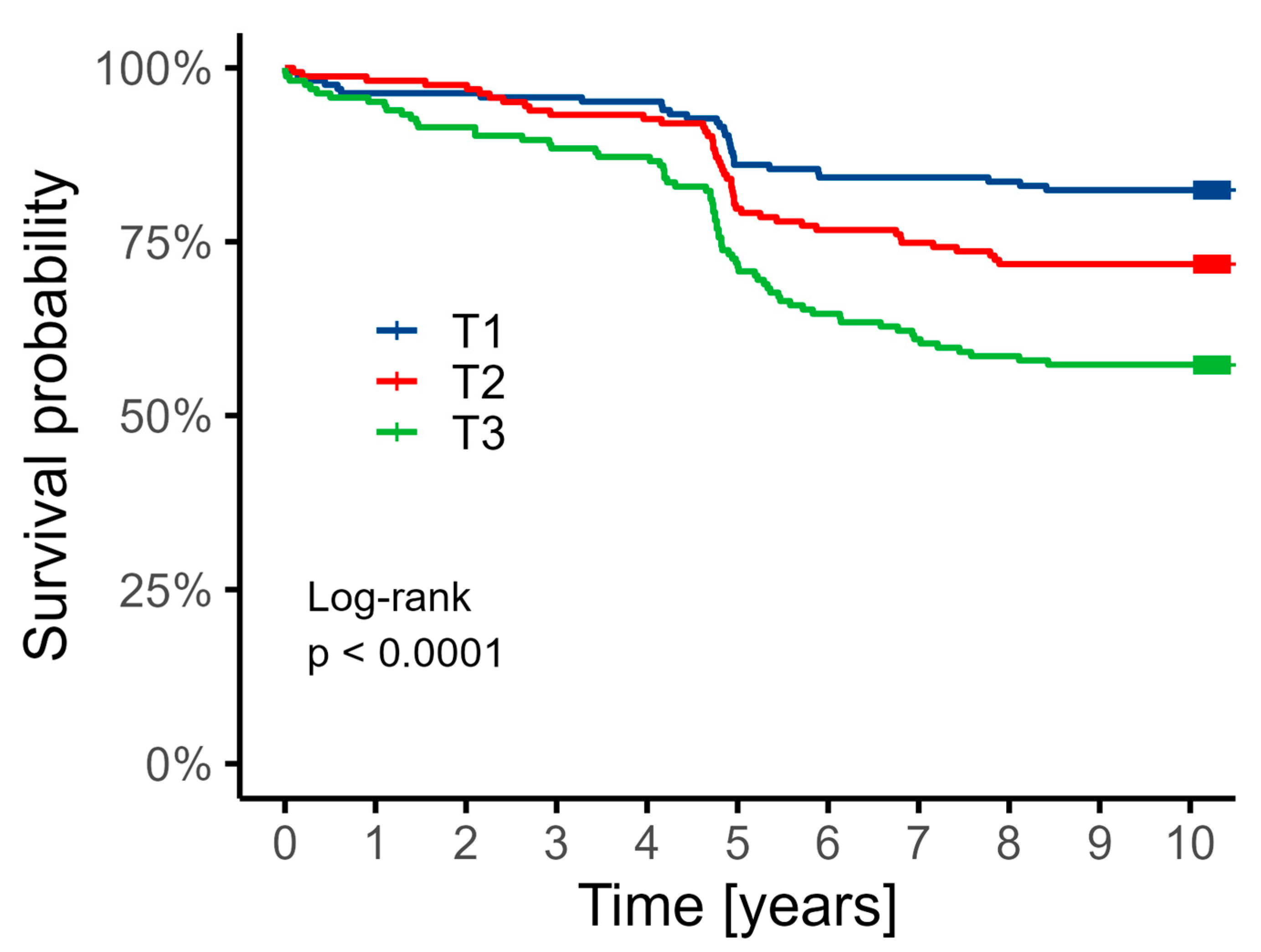
3.3. 10-Year Follow-Up Data
3.4. Cox Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-HAA | 3-Hydroxyanthranilic acid |
| 3-HK | 3-Hydroxykynurenine |
| AA | Anthranilic acid |
| ACS | Acute coronary syndrome |
| ASA | Acetylsalicylic acid |
| AhR | Aryl hydrocarbon receptor |
| BMI | Body mass index |
| CABG | Coronary artery bypass grafting |
| CAD | Coronary artery disease |
| CCS | Chronic coronary syndrome |
| CK | Creatine kinase |
| CK-MB | Creatine kinase-MB isoenzyme |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| DAPT | Dual antiplatelet therapy |
| E/e′ | Ratio of early mitral inflow velocity to mitral annular early diastolic velocity |
| ECG | Electrocardiogram |
| HDL | High-density lipoprotein |
| HPLC | High-performance liquid chromatography |
| Hgb | Hemoglobin |
| ICD | Implantable cardioverter defibrillator |
| IDO | Indoleamine 2,3-dioxygenase |
| K+ | Potassium ion |
| KP | Kynurenine pathway |
| KTR | Kynurenine/tryptophan ratio |
| KYN | Kynurenine |
| KYNA | Kynurenic acid |
| LAD | Left anterior descending artery |
| LAVI | Left atrial volume index |
| LDL | Low-density lipoprotein |
| LVEF | Left ventricular ejection fraction |
| LVMI | Left ventricular mass index |
| MI | Myocardial infarction |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| PCI | Percutaneous coronary intervention |
| PLT | Platelet |
| QA | Quinolinic acid |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| RVSP | Right ventricular systolic pressure |
| STEMI | ST-elevation myocardial infarction |
| TAPSE | Tricuspid annular plane systolic excursion |
| TG | Triglyceride |
| TIMI | Thrombolysis in myocardial infarction (flow grade) |
| TRP | Tryptophan |
| TSH | Thyroid-stimulating hormone |
| UA | Unstable angina |
| WBC | White blood cell |
| eGFR | Estimated glomerular filtration rate |
| hs-CRP | High-sensitivity C-reactive protein |
| pHPT | Primary hyperparathyroidism |
References
- Victor, G.; Shishani, K.; Vellone, E.; Froelicher, E.S. The Global Burden of Cardiovascular Disease in Adults: A Mapping Review. J. Cardiovasc. Nurs. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Jiang, X.; Chen, J.; Yuan, Y.; Li, Q.; Wu, H.; Huang, F.; Zhu, P. Global, regional and national burden of ischaemic heart disease attributable to high body mass index and low physical activity from 1990 to 2021. Diabetes Obes. Metab. 2025, 27, 2561–2572. [Google Scholar] [CrossRef]
- Bil, J.; MoZeNska, O.; Segiet-SwiEcicka, A.; Gil, R.J. Revisiting the use of the provocative acetylcholine test in patients with chest pain and nonobstructive coronary arteries: A five-year follow-up of the AChPOL registry, with special focus on patients with MINOCA. Transl. Res. 2021, 231, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Iusupova, A.O.; Pakhtusov, N.N.; Slepova, O.A.; Khabarova, N.V.; Privalova, E.V.; Bure, I.V.; Nemtsova, M.V.; Belenkov, Y.N. MiRNA-34a, miRNA-145, and miRNA-222 Expression, Matrix Metalloproteinases, TNF-alpha and VEGF in Patients with Different Phenotypes of Coronary Artery Disease. Int. J. Mol. Sci. 2024, 25, 12978. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Ford, T.J.; Galli, M.; Rinaldi, R.; Bland, A.; Morrow, A.; Angiolillo, D.J.; Berry, C.; Kaski, J.C.; Crea, F. Stratified medicine for acute and chronic coronary syndromes: A patient-tailored approach. Prog. Cardiovasc. Dis. 2024, 85, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kiluk, M.; Lewkowicz, J.; Pawlak, D.; Tankiewicz-Kwedlo, A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J. Clin. Med. 2021, 10, 2484. [Google Scholar] [CrossRef]
- Kupjetz, M.; Wences Chirino, T.Y.; Joisten, N.; Zimmer, P. Kynurenine pathway dysregulation as a mechanistic link between cognitive impairment and brain damage: Implications for multiple sclerosis. Brain Res. 2025, 1853, 149415. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wu, D.; Zhang, Z.; Zhou, Y.; Li, J. Kynurenic Acid, a Small Foodborne Molecule with the Potential to Affect Human Health. J. Agric. Food Chem. 2025, 73, 8729–8739. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, W.; Zhang, Y.; Dong, X.; Liu, Y. Diverse Physiological Roles of Kynurenine Pathway Metabolites: Updated Implications for Health and Disease. Metabolites 2025, 15, 210. [Google Scholar] [CrossRef]
- Gaspar, R.; Halmi, D.; Demjan, V.; Berkecz, R.; Pipicz, M.; Csont, T. Kynurenine Pathway Metabolites as Potential Clinical Biomarkers in Coronary Artery Disease. Front. Immunol. 2021, 12, 768560. [Google Scholar]
- Eussen, S.J.; Ueland, P.M.; Vollset, S.E.; Nygard, O.; Midttun, O.; Sulo, G.; Ulvik, A.; Meyer, K.; Pedersen, E.R.; Tell, G.S. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int. J. Cardiol. 2015, 189, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Grishanova, A.Y.; Perepechaeva, M.L. Kynurenic Acid/AhR Signaling at the Junction of Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 6933. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Stompor, T.; Bojko, K.; Sienkiewicz, E.; Pawlak, S.; Pawlak, D.; Poskrobko, G.; Andrasz, E.; Gromadzinski, L.; Jalali, R.; et al. Ten-Year Outcomes in Patients Undergoing Simultaneous Coronary and Renal Angiography-Does Renal Artery Stenosis Matter? J. Clin. Med. 2024, 13, 3374. [Google Scholar] [CrossRef]
- Kern, A.; Stompor, T.; Bojko, K.; Sienkiewicz, E.; Pawlak, S.; Pawlak, K.; Pawlak, D.; Poskrobko, G.; Andrasz, E.; Gromadzinski, L.; et al. Ten-year outcomes of patients undergoing simultaneous coronary and renal angiography: Insights from the KORONEF Registry based on chronic kidney disease and eGFR stratification. Kardiol. Pol. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.W. Determination of serum kynurenine and hepatic tryptophan dioxygenase activity by high-performance liquid chromatography. Anal. Biochem. 1988, 172, 518–525. [Google Scholar] [CrossRef]
- Herve, C.; Beyne, P.; Jamault, H.; Delacoux, E. Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J. Chromatogr. B Biomed. Appl. 1996, 675, 157–161. [Google Scholar] [CrossRef]
- Heyes, M.P.; Quearry, B.J. Quantification of 3-hydroxykynurenine in brain by high-performance liquid chromatography and electrochemical detection. J. Chromatogr. 1988, 428, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Zamorano, J.; Habib, G.; Badano, L. The EACVI Textbook of Echocardiography; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell. Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Liu, X.; Xie, C.; Shi, J. The role of the kynurenine pathway in cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1406856. [Google Scholar] [CrossRef]
- Zuo, H.; Ueland, P.M.; Ulvik, A.; Eussen, S.J.; Vollset, S.E.; Nygard, O.; Midttun, O.; Theofylaktopoulou, D.; Meyer, K.; Tell, G.S. Plasma Biomarkers of Inflammation, the Kynurenine Pathway, and Risks of All-Cause, Cancer, and Cardiovascular Disease Mortality: The Hordaland Health Study. Am. J. Epidemiol. 2016, 183, 249–258. [Google Scholar] [CrossRef]
- Verheyen, N.; Meinitzer, A.; Grubler, M.R.; Ablasser, K.; Kolesnik, E.; Fahrleitner-Pammer, A.; Belyavskiy, E.; Trummer, C.; Schwetz, V.; Pieske-Kraigher, E.; et al. Low-grade inflammation and tryptophan-kynurenine pathway activation are associated with adverse cardiac remodeling in primary hyperparathyroidism: The EPATH trial. Clin. Chem. Lab. Med. 2017, 55, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Nordrehaug, J.E.; Slettom, G.; Solvang, S.H.; Pedersen, E.K.; Midttun, O.; Ulvik, A.; Ueland, P.M.; Nygard, O.; Giil, L.M. Plasma kynurenines and prognosis in patients with heart failure. PLoS ONE 2020, 15, e0227365. [Google Scholar]
- El Chamieh, C.; Larabi, I.A.; Alencar De Pinho, N.; Lambert, O.; Combe, C.; Fouque, D.; Frimat, L.; Jacquelinet, C.; Laville, M.; Laville, S.; et al. Study of the association between serum levels of kynurenine and cardiovascular outcomes and overall mortality in chronic kidney disease. Clin. Kidney J. 2024, 17, sfad248. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.J.; Stroes, E.S.G.; Boekholdt, S.M.; Wareham, N.J.; Murphy, A.J.; Nieuwdorp, M.; Hazen, S.L.; Hanssen, N.M.J. Tryptophan metabolites and incident cardiovascular disease: The EPIC-Norfolk prospective population study. Atherosclerosis 2023, 387, 117344. [Google Scholar] [CrossRef]
- Ala, M.; Eftekhar, S.P. The Footprint of Kynurenine Pathway in Cardiovascular Diseases. Int. J. Tryptophan Res. 2022, 15, 11786469221096643. [Google Scholar] [CrossRef]
- Paz, G.A.; Rangel, M.V.; Farias, C.L.; Soares, A.L.C.; Langner, E.; Teixeira, T.; Dutra, P.M.L.; Bouskela, E.; Farinatti, P.; Souza, M.; et al. Acute and chronic effects of physical exercise on atherosclerosis, kynurenine pathway, endothelial function and inflammation in patients with coronary artery disease: A clinical trial protocol. BMJ Open Sport. Exerc. Med. 2025, 11, e002432. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Rohrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
| Parameter | Total N = 492 | Tertile 1 N = 165 | Tertile 2 N = 163 | Tertile 3 N = 164 | p |
|---|---|---|---|---|---|
| Females | 184 (37.4%) | 66 (40.0%) | 50 (30.7%) | 68 (41.5%) | 0.092 |
| Age [years] | 64.4 (9.8) | 62.17 (9.45) | 64.98 (9.34) | 66.04 (10.37) | <0.001 |
| BMI [kg/m2] | 27.9 (4.3) | 27.45 (4.57) | 28.00 (3.84) | 28.28 (4.34) | 0.126 |
| Arterial hypertension | 366 (74.8%) | 115 (69.7%) | 123 (76.4%) | 128 (78.5%) | 0.157 |
| Dyslipidemia | 225 (46.0%) | 78 (47.3%) | 68 (42.2%) | 79 (48.5%) | 0.490 |
| Diabetes | 126 (25.8%) | 42 (25.5%) | 35 (21.7%) | 49 (30.1%) | 0.229 |
| Obesity | 147 (30.1%) | 44 (26.7%) | 53 (32.9%) | 50 (30.7%) | 0.459 |
| Previous MI | 154 (31.5%) | 37 (22.4%) | 54 (33.5%) | 63 (38.7%) | 0.005 |
| Previous stroke | 32 (6.6%) | 5 (3.0%) | 13 (8.1%) | 14 (8.6%) | 0.078 |
| Peripheral artery disease | 24 (4.9%) | 4 (2.4%) | 11 (6.8%) | 9 (5.5%) | 0.166 |
| Chronic kidney disease | 47 (9.6%) | 6 (3.6%) | 11 (6.8%) | 30 (18.4%) | <0.001 |
| Previous CABG | 21 (4.3%) | 2 (1.2%) | 9 (5.6%) | 10 (6.1%) | 0.055 |
| Previous PCI | 103 (21.1%) | 27 (16.4%) | 32 (19.9%) | 44 (27.0%) | 0.056 |
| Echocardiography results | |||||
| LV ejection fraction [%] | 52.5 (11.5) | 54.33 (10.3) | 52.30 (11.56) | 50.97 (12.49) | 0.096 |
| RVSP [mmHg] | 38.9 (10.3) | 39.14 (7.36) | 36.22 (11.37) | 40.38 (10.96) | 0.558 |
| TAPSE [mm] | 17.4 (4.2) | 21.00 (0.21) | 22.50 (0.71) | 14.50 (2.43) | 0.029 |
| Parameter | Total N = 492 | Tertile 1 N = 165 | Tertile 2 N = 163 | Tertile 3 N = 164 | p |
|---|---|---|---|---|---|
| Hemoglobin [g/dL] | 13.87 (1.39) | 13.87 (1.40) | 13.92 (1.32) | 13.82 (1.45) | 0.854 |
| Glucose [mg/dL] | 116.42 (39.56) | 116.69 (34.69) | 111.38 (34.65) | 121.05 (47.58) | 0.105 |
| Creatinine [mg/dL] | 0.99 (0.57) | 0.85 (0.20) | 0.93 (0.19) | 1.19 (0.91) | <0.001 |
| eGFR [mL/min] | 81.68 (24.22) | 90.37 (23.26) | 83.77 (21.81) | 70.76 (23.41) | <0.001 |
| K+ [mmol/L] | 4.37 (0.46) | 4.28 (0.44) | 4.36 (0.40) | 4.48 (0.51) | 0.001 |
| Na+ [mmol/L] | 140.72 (2.84) | 140.88 (2.84) | 141.07 (2.63) | 140.20 (2.99) | 0.027 |
| Total cholesterol [mg/dL] | 183.23 (51.13) | 197.28 (59.51) | 177.36 (45.80) | 174.62 (43.51) | <0.001 |
| LDL [mg/dL] | 109.60 (44.49) | 118.87 (51.67) | 104.93 (41.39) | 104.79 (37.81) | 0.020 |
| HDL [mg/dL] | 53.47 (17.45) | 55.70 (18.84) | 53.73 (16.94) | 50.89 (16.17) | 0.036 |
| Triglycerides [mg/dL] | 315.01 (39.53) | 138.91 (86.85) | 667.98 (77.78) | 139.23 (77.43) | 0.234 |
| TSH [μIU/mL] | 2.14 (4.84) | 2.68 (8.23) | 1.86 (2.01) | 1.91 (1.51) | 0.282 |
| hs-CRP [mg/L] | 1.16 (4.94) | 0.93 (3.58) | 0.92 (2.50) | 1.62 (7.35) | 0.004 |
| Parameter | Total N = 492 | Tertile 1 N = 165 | Tertile 2 N = 163 | Tertile 3 N = 164 | p |
|---|---|---|---|---|---|
| Coronary angiography indications | |||||
| Planned coronary artery disease diagnostics | 290 (59.4%) | 78 (47.3%) | 106 (66.2%) | 106 (65.0%) | 0.021 |
| Acute coronary syndrome | 170 (34.6%) | 78 (47.3%) | 45 (27.6%) | 47 (28.7%) | |
| Heart failure diagnostics | 4 (0.8%) | 2 (1.2%) | 0 (0.0%) | 2 (1.2%) | |
| Pacemaker/ICD qualification | 4 (0.8%) | 2 (1.2%) | 0 (0.0%) | 2 (1.2%) | |
| Cardiovascular surgery (heart valve defect, ascending aorta aneurysm) | 24 (4.9%) | 7 (4.2%) | 9 (5.6%) | 8 (4.9%) | |
| Coronary angiography results | |||||
| No significant atherosclerotic lesions * | 122 (25.0%) | 38 (23.0%) | 41 (25.6%) | 43 (26.4%) | 0.022 |
| One-vessel disease | 140 (28.7%) | 60 (36.4%) | 45 (28.1%) | 35 (21.5%) | |
| Two-vessel disease | 137 (28.1%) | 40 (24.2%) | 45 (28.1%) | 52 (31.9%) | |
| Three-vessel disease | 67 (13.7%) | 20 (12.1%) | 23 (14.4%) | 24 (14.7%) | |
| Left main stem | 22 (4.5%) | 7 (4.2%) | 6 (3.8%) | 9 (5.5%) | |
| Qualification for revascularization | |||||
| Pharmacological treatment | 156 (34.3%) | 43 (27.4%) | 58 (39.7%) | 55 (36.2%) | 0.021 |
| PCI | 208 (45.7%) | 87 (55.4%) | 53 (36.3%) | 68 (44.7%) | |
| CABG | 91 (20.0%) | 27 (17.2%) | 35 (24.0%) | 29 (19.1%) | |
| Location of lesions treated by PCI | |||||
| Left main stem | 1 (0.4%) | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) | 0.034 |
| Left anterior descending artery/ diagonal branches | 90 (38.3%) | 38 (41.3%) | 24 (36.4%) | 28 (36.4%) | |
| Left circumflex artery/marginal branches | 58 (24.7%) | ||||
| Intermediate artery | 4 (1.7%) | 2 (2.2%) | 1 (1.5%) | 1 (1.3%) | |
| Right coronary artery | 80 (34.0%) | 33 (35.9%) | 22 (33.3%) | 25 (32.5%) | |
| Venous graft | 2 (0.9%) | 0 (0.0%) | 1 (1.5%) | 1 (1.3%) | |
| TIMI after PCI | |||||
| 0 | 10 (4.2%) | 0 (0.0%) | 6 (9.0%) | 4 (5.3%) | 0.012 |
| 1 | 2 (0.8%) | 1 (1.1%) | 1 (1.5%) | 0 (0.0%) | |
| 2 | 1 (0.4%) | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | |
| 3 | 223 (94.5%) | 91 (97.8%) | 60 (89.6%) | 72 (94.7%) | |
| Periprocedural complications (PCI) | |||||
| No reflow/slow reflow | 8 (3.8%) | 0 (0.0%) | 4 (2.8%) | 4 (2.5%) | 0.116 |
| Stent thrombosis | 1 (0.5%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0.998 |
| Parameter | Total N = 492 | Tertile 1 N = 165 | Tertile 2 N = 163 | Tertile 3 N = 164 | p |
|---|---|---|---|---|---|
| Acetylsalicylic acid | 442 (90.0%) | 155 (93.9%) | 147 (90.7%) | 140 (85.4%) | 0.032 |
| Clopidogrel | 301 (61.3%) | 111 (67.3%) | 91 (56.2%) | 99 (60.4%) | 0.114 |
| ACE inhibitor | 426 (86.8%) | 143 (86.7%) | 144 (88.9%) | 139 (84.8%) | 0.545 |
| Angiotensin antagonist | 21 (4.3%) | 10 (6.1%) | 6 (3.7%) | 5 (3.0%) | 0.365 |
| Beta-blocker | 452 (92.1%) | 152 (92.1%) | 154 (95.1%) | 146 (89.0%) | 0.131 |
| Ca-blocker | 124 (25.3%) | 39 (23.6%) | 43 (26.5%) | 42 (25.6%) | 0.826 |
| Statin | 457 (93.1%) | 157 (95.2%) | 154 (95.1%) | 146 (89.0%) | 0.043 |
| Fibrate | 17 (3.5%) | 5 (3.0%) | 3 (1.9%) | 9 (5.5%) | 0.186 |
| Loop diuretic | 89 (18.1%) | 16 (9.7%) | 23 (14.2%) | 50 (30.5%) | <0.001 |
| Thiazide | 50 (10.2%) | 13 (7.9%) | 19 (11.7%) | 18 (11.0%) | 0.474 |
| Mineralocorticoid receptor antagonist | 59 (12.0%) | 10 (6.1%) | 18 (11.1%) | 31 (18.9%) | 0.001 |
| Vitamin K antagonist | 37 (7.5%) | 7 (4.2%) | 9 (5.6%) | 21 (12.8%) | 0.007 |
| Insulin | 44 (9.0%) | 17 (10.3%) | 10 (6.2%) | 17 (10.4%) | 0.316 |
| Endpoint | Total N = 492 | Tertile 1 N = 165 | Tertile 2 N = 163 | Tertile 3 N = 164 | p |
|---|---|---|---|---|---|
| Death | 145 (29.5%) | 29 (17.6%) | 46 (28.2%) | 70 (42.9%) | <0.001 |
| MI | 56 (11.4%) | 17 (10.3%) | 21 (12.9%) | 18 (11.0%) | 0.751 |
| Stroke | 24 (4.9%) | 11 (6.7%) | 9 (5.5%) | 4 (2.5%) | 0.188 |
| CABG | 37 (7.5%) | 12 (7.3%) | 14 (8.6%) | 11 (6.7%) | 0.810 |
| PCI | 81 (16.5%) | 24 (14.5%) | 29 (17.8%) | 28 (17.2%) | 0.701 |
| Study Population N = 492 (%) | |||
|---|---|---|---|
| Parameter | HR | 95% CI | p-Value |
| Age: | |||
| 65–75 years | 3.54 | 1.51, 8.12 | 0.004 |
| 75–90 years | 9.62 | 4.21, 21.8 | <0.001 |
| Diabetes | 1.62 | 1.01, 2.44 | 0.025 |
| Previous myocardial infarction | 1.61 | 1.03, 2.45 | 0.017 |
| Chronic kidney disease | 2.34 | 1.44, 3.81 | <0.001 |
| Coronary angiography indications | |||
| STEMI | 1.74 | 1.05, 3.03 | 0.043 |
| Left ventricular ejection fraction | |||
| [40, 50] | 0.41 | 0.29, 0.74 | 0.004 |
| [50, 60] | 0.52 | 0.30, 0.95 | 0.031 |
| >60 | 0.34 | 0.24, 0.72 | 0.003 |
| KYN | 1.79 | 1.15, 2.44 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kern, A.; Stompór, T.; Bojko, K.; Sienkiewicz, E.; Pawlak, S.; Pawlak, K.; Pawlak, D.; Poskrobko, G.; Andrasz, E.; Gromadziński, L.; et al. Kynurenine as a Predictor of Long-Term Mortality: A 10-Year Follow-Up from the KORONEF Registry. Biomedicines 2025, 13, 1123. https://doi.org/10.3390/biomedicines13051123
Kern A, Stompór T, Bojko K, Sienkiewicz E, Pawlak S, Pawlak K, Pawlak D, Poskrobko G, Andrasz E, Gromadziński L, et al. Kynurenine as a Predictor of Long-Term Mortality: A 10-Year Follow-Up from the KORONEF Registry. Biomedicines. 2025; 13(5):1123. https://doi.org/10.3390/biomedicines13051123
Chicago/Turabian StyleKern, Adam, Tomasz Stompór, Krystian Bojko, Ewa Sienkiewicz, Sebastian Pawlak, Krystyna Pawlak, Dariusz Pawlak, Grzegorz Poskrobko, Ewa Andrasz, Leszek Gromadziński, and et al. 2025. "Kynurenine as a Predictor of Long-Term Mortality: A 10-Year Follow-Up from the KORONEF Registry" Biomedicines 13, no. 5: 1123. https://doi.org/10.3390/biomedicines13051123
APA StyleKern, A., Stompór, T., Bojko, K., Sienkiewicz, E., Pawlak, S., Pawlak, K., Pawlak, D., Poskrobko, G., Andrasz, E., Gromadziński, L., Jalali, R., Onichimowski, D., Piwko, G., Zalewski, A., & Bil, J. (2025). Kynurenine as a Predictor of Long-Term Mortality: A 10-Year Follow-Up from the KORONEF Registry. Biomedicines, 13(5), 1123. https://doi.org/10.3390/biomedicines13051123







