Plasma Beta-Hydroxybutyrate and All-Cause Mortality in Patients with Liver Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Clinical Measurements
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical and Laboratory Characteristics Between Patients with Cirrhosis and PREVEND Participants
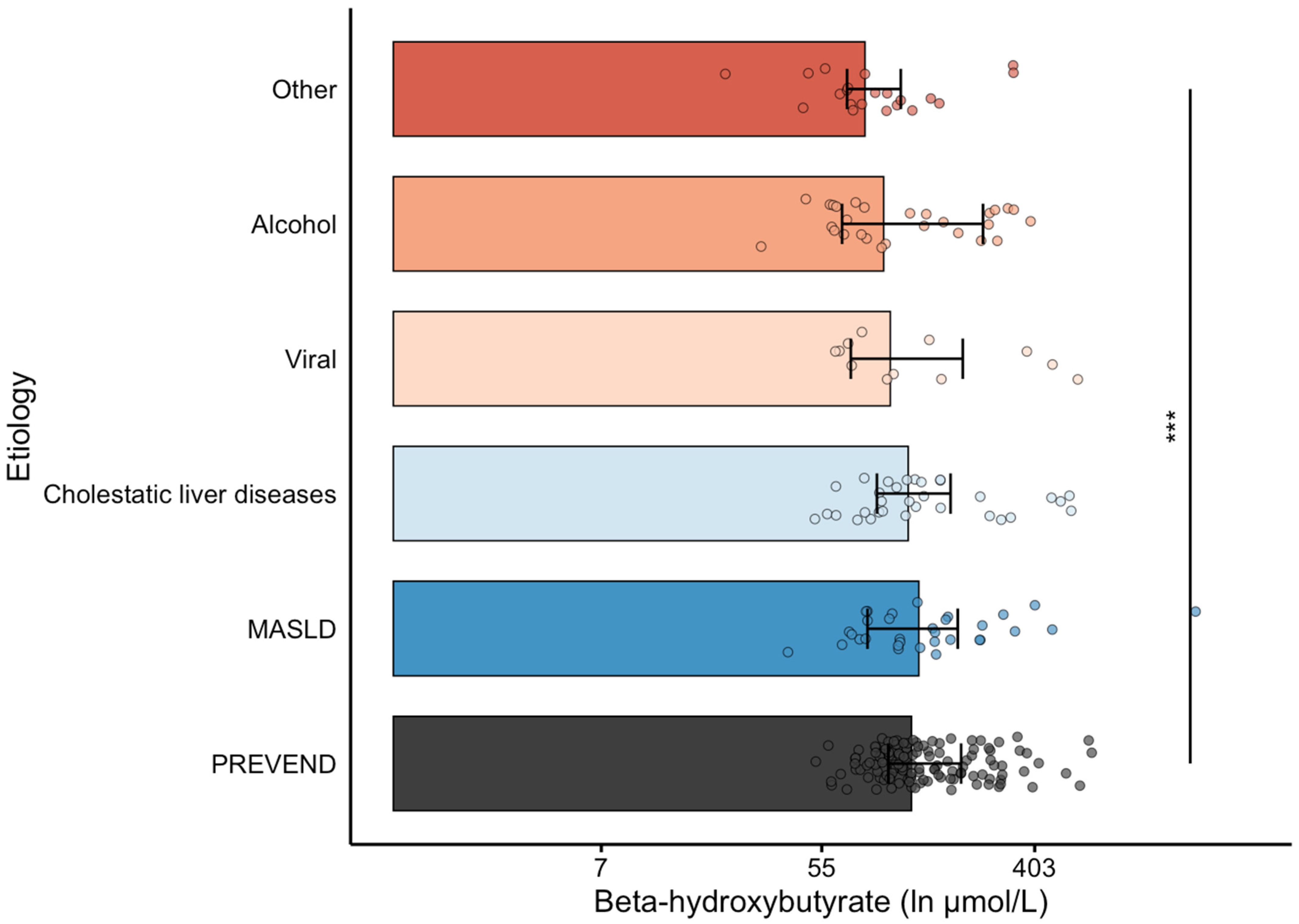
3.2. Baseline Characteristics of Patients with Cirrhosis According to β-Hydroxybutyrate Levels
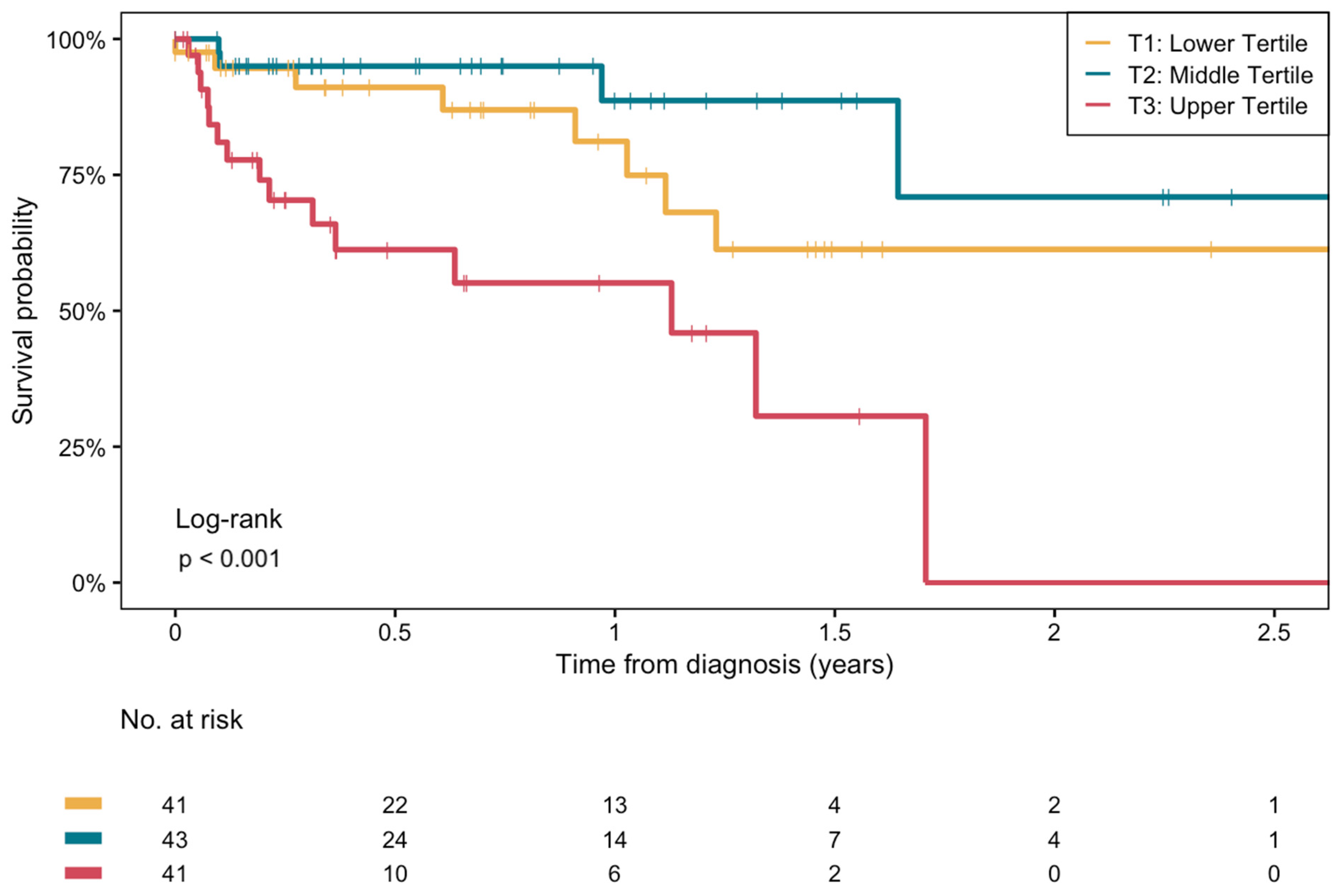
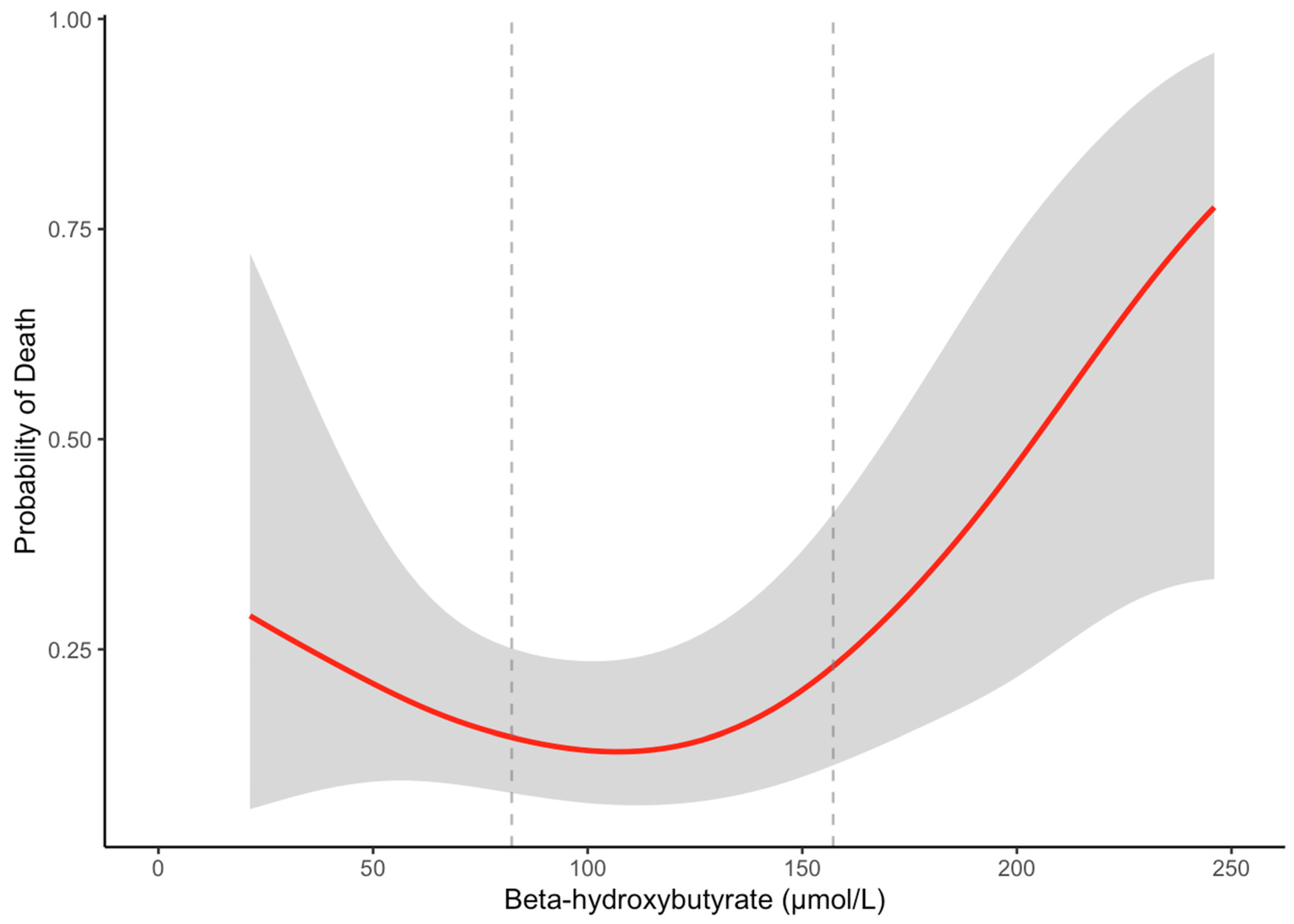
3.3. Longitudinal Analyses of β-Hydroxybutyrate with Overall Mortality in Patients with Cirrhosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BHB | Beta (β)-Hydroxybutyrate |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CI | Confidence Interval |
| CTP | Child–Turcotte–Pugh Classification |
| CVD | Cardiovascular Disease |
| eGFR | Estimated Glomerular Filtration Rate |
| GAM | Generalized Additive Model |
| GGT | Gamma-Glutamyl Transferase |
| HBA1C | Glycated Hemoglobin |
| HDL | High-Density Lipoprotein |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| LDL | Low-Density Lipoprotein |
| LT | Liver Transplantation |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MELD | Model for End-Stage Liver Disease |
| NMR | Nuclear Magnetic Resonance |
| PREVEND | Prevention of Renal and Vascular End-stage Disease |
| PSM | Propensity Score Matching |
| SD | Standard Deviation |
| UMCG | University Medical Center Groningen |
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R.L. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Lucey, M.R.; Furuya, K.N.; Foley, D.P. Liver Transplantation. N. Engl. J. Med. 2023, 389, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatol. Baltim. Md. 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Goldberg, D.; Mantero, A.; Newcomb, C.; Delgado, C.; Forde, K.; Kaplan, D.; John, B.; Nuchovich, N.; Dominguez, B.; Emanuel, E.; et al. Development and Validation of a Model to Predict Long-Term Survival After Liver Transplantation. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2021, 27, 797–807. [Google Scholar] [CrossRef]
- El-Khateeb, E.; Darwich, A.S.; Achour, B.; Athwal, V.; Rostami-Hodjegan, A. Review article: Time to revisit Child-Pugh score as the basis for predicting drug clearance in hepatic impairment. Aliment. Pharmacol. Ther. 2021, 54, 388–401. [Google Scholar] [CrossRef]
- Kumar, R.; Priyadarshi, R.N.; Anand, U. Chronic renal dysfunction in cirrhosis: A new frontier in hepatology. World J. Gastroenterol. 2021, 27, 990–1005. [Google Scholar] [CrossRef]
- Fede, G.; Privitera, G.; Tomaselli, T.; Spadaro, L.; Purrello, F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 31–40. [Google Scholar]
- van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-Alcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: Role of Circulating Branched-Chain Amino Acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef]
- Cao, L.; An, Y.; Liu, H.; Jiang, J.; Liu, W.; Zhou, Y.; Shi, M.; Dai, W.; Lv, Y.; Zhao, Y.; et al. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: A systematic review and meta-analysis. BMC Med. 2024, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Wan, Q.S.; Wang, T.; Zhang, K.H. Fluid Biomarkers for Predicting the Prognosis of Liver Cirrhosis. Biomed. Res. Int. 2020, 2020, 7170457. [Google Scholar] [CrossRef] [PubMed]
- Thiele, M.; Villesen, I.F.; Niu, L.; Johansen, S.; Sulek, K.; Nishijima, S.; Van Espen, L.; Keller, M.; Israelsen, M.; Suvitaival, T.; et al. Opportunities and barriers in omics-based biomarker discovery for steatotic liver diseases. J. Hepatol. 2024, 81, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology. 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Zhang, I.W.; Curto, A.; López-Vicario, C.; Casulleras, M.; Duran-Güell, M.; Flores-Costa, R.; Colsch, B.; Aguilar, F.; Aransay, A.M.; Lozano, J.J.; et al. Mitochondrial dysfunction governs immunometabolism in leukocytes of patients with acute-on-chronic liver failure. J. Hepatol. 2022, 76, 93–106. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Bae, J.; Lee, B.W. Association between Impaired Ketogenesis and Metabolic-Associated Fatty Liver Disease. Biomolecules 2023, 13, 1506. [Google Scholar] [CrossRef]
- Lee, S.; Bae, J.; Jo, D.R.; Lee, M.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. Impaired ketogenesis is associated with metabolic-associated fatty liver disease in subjects with type 2 diabetes. Front. Endocrinol. 2023, 14, 1124576. [Google Scholar] [CrossRef]
- Post, A.; Garcia, E.; van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; Groothof, D.; Daan Westenbrink, B.; Connelly, M.A.; Bakker, S.J.l.; Dullaart, R.P.F. Nonalcoholic fatty liver disease, circulating ketone bodies and all-cause mortality in a general population-based cohort. Eur. J. Clin. Investig. 2021, 51, e13627. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, Y.; Kwon, M.J.; Hong, Y.S.; Kim, M.K.; Sohn, W.; Cho, Y.K.; Shin, H.; Wild, S.H.; Byrne, C.D.; et al. Fasting Ketonuria and the Risk of Incident Nonalcoholic Fatty Liver Disease With and Without Liver Fibrosis in Nondiabetic Adults. Am. J. Gastroenterol. 2021, 116, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Kang, M.; Park, J. Association between Fasting Ketonuria and Advanced Liver Fibrosis in Non-Alcoholic Fatty Liver Disease Patients without Prediabetes and Diabetes Mellitus. Nutrients 2021, 13, 3400. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; Londen Mvan Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and design of TransplantLines: A prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018, 8, e024502. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Diercks, G.F.H.; van Boven, A.J.; Hillege, H.L.; Janssen, W.M.T.; Kors, J.A.; de Jong, P.E.; Grobbee, D.E.; Crijns, H.J.; van Gilst, W.H. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur. Heart J. 2000, 21, 1922–1927. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F.; on behalf of the PREVEND study Group. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chvatal-Medina, M.; Trillos-Almanza, M.C.; Bourgonje, A.R.; Connelly, M.A.; Moshage, H.; Bakker, S.J.L.; de Meijer, V.E.; Blokzijl, H.; Dullaart, R.P.F. Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality. Int. J. Mol. Sci. 2024, 25, 12806. [Google Scholar] [CrossRef]
- Trillos-Almanza, M.C.; Chvatal-Medina, M.; Connelly, M.A.; Moshage, H.; TransplantLines Investigators; Bakker, S.J.L.; de Meijder, V.E.; Blokzijl, H.; Dullaart, R.P.F. Circulating Trimethylamine-N-Oxide Is Elevated in Liver Transplant Recipients. Int. J. Mol. Sci. 2024, 25, 6031. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Gruppen, E.G.; Garcia, E.; Connelly, M.A.; Jeyarajah, E.J.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. TMAO is Associated with Mortality: Impact of Modestly Impaired Renal Function. Sci. Rep. 2017, 7, 13781. [Google Scholar] [CrossRef]
- Garcia, E.; Shalaurova, I.; Matyus, S.P.; Oskardmay, D.N.; Otvos, J.D.; Dullaart, R.P.F.; Connelly, M.A. Ketone Bodies Are Mildly Elevated in Subjects with Type 2 Diabetes Mellitus and Are Inversely Associated with Insulin Resistance as Measured by the Lipoprotein Insulin Resistance Index. J. Clin. Med. 2020, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. HDL Particle Subspecies and Their Association With Incident Type 2 Diabetes: The PREVEND Study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, A.; Knol, M.G.E.; de Borst, M.H.; Bakker, S.J.L.; Connelly, M.A.; Garcia, E.; Bilo, H.J.G.; van Dijk, P.R.; Dullaart, R.P.F. The Paradoxical Role of Circulating Ketone Bodies in Glycemic Control of Individuals with Type 2 Diabetes: High Risk, High Reward? Biomolecules 2022, 12, 1318. [Google Scholar] [CrossRef]
- Giaccari, A.; Solini, A.; Frontoni, S.; Del Prato, S. Metformin Benefits: Another Example for Alternative Energy Substrate Mechanism? Diabetes Care 2021, 44, 647–654. [Google Scholar] [CrossRef]
- Zhang, I.W.; López-Vicario, C.; Duran-Güell, M.; Clària, J. Mitochondrial Dysfunction in Advanced Liver Disease: Emerging Concepts. Front. Mol. Biosci. 2021, 8, 772174. [Google Scholar] [CrossRef]
- Glass, C.; Hipskind, P.; Tsien, C.; Malin, S.K.; Kasumov, T.; Shah, S.N.; Kirwan, J.P.; Dasarathy, S. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: A prospective controlled study. J. Appl. Physiol. 2013, 114, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.; Paternostro, R.; Schwabl, P.; Scheiner, B.; Stättermayer, A.F.; Pinter, M.; et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 2021, 74, 819–828. [Google Scholar] [CrossRef]
- Berg EHvan den Flores-Guerrero, J.L.; Dullaart, R.P.F. Lipoprotein Z, an abnormal LDL-like lipoprotein, independently predicts mortality in cirrhosis. Eur. J. Intern. Med. 2022, 101, 128–129. [Google Scholar]
- Li, S.; Niu, M.; Jing, J.; Huang, Y.; Zhang, Z.; Chen, S.; Shi, G.; He, X.; Zhang, H.; Xiao, X.; et al. Metabolomic Signatures of Autoimmune Hepatitis in the Development of Cirrhosis. Front. Med. 2021, 8, 644376. [Google Scholar] [CrossRef]
- Gümüş, E.; Özen, H. Glycogen storage diseases: An update. World J. Gastroenterol. 2023, 29, 3932–3963. [Google Scholar] [CrossRef]
- Oo, Y.H.; Hubscher, S.G.; Adams, D.H. Autoimmune hepatitis: New paradigms in the pathogenesis, diagnosis, and management. Hepatol. Int. 2010, 4, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Cazals-Hatem, D.; Vilgrain, V.; Genin, P.; Denninger, M.H.; Durand, F.; Belghiti, J.; Valla, D.; Degott, C. Arterial and portal circulation and parenchymal changes in Budd–Chiari syndrome: A study in 17 explanted livers. Hepatol. Baltim. Md. 2003, 37, 510–519. [Google Scholar] [CrossRef]
- Senzolo, M.; Garcia-Tsao, G.; García-Pagán, J.C. Current knowledge and management of portal vein thrombosis in cirrhosis. J. Hepatol. 2021, 75, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Männistö, V.T.; Simonen, M.; Hyysalo, J.; Soininen, P.; Kangas, A.J.; Kaminska, D.; Matte, A.K.; Venesmaa, S.; Käkelä, P.; Kärjä, V.; et al. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver Int. Off. J. Int. Assoc. Study Liver. 2015, 35, 1853–1861. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Dyck, J.R.B. Ketones and the cardiovascular system. Nat. Cardiovasc. Res. 2023, 2, 425–437. [Google Scholar] [CrossRef]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone Bodies in Neurological Diseases: Focus on Neuroprotection and Underlying Mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef]
- Neudorf, H.; Islam, H.; Falkenhain, K.; Oliveira, B.; Jackson, G.S.; Moreno-Cabañas, A.; Madden, K.; Singer, J.; Walsh, J.J.; Little, J.P. Effect of the ketone beta-hydroxybutyrate on markers of inflammation and immune function in adults with type 2 diabetes. Clin. Exp. Immunol. 2024, 216, 89–103. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, X.; Hoque, R.; Garcia-Martinez, I.; Yousaf, M.N.; Tonack, S.; Offermanns, S.; Dubuquoy, L.; Louvet, A.; Mathurin, P.; et al. β-Hydroxybutyrate protects from alcohol-induced liver injury via a Hcar2-cAMP dependent pathway. J. Hepatol. 2018, 69, 687–696. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Qadri, S.; Ahlholm, N.; Porthan, K.; Männistö, V.; Sammalkorpi, H.; Penttilä, A.K.; Hakkarainen, A.; Lehtimäki, T.E.; Gaggini, M.; et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 526–535. [Google Scholar] [CrossRef]
- Gambino, R.; Musso, G.; Cassader, M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: Mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1325–1365. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Koh, H.B.; Heo, G.Y.; Ko, B.; Kim, H.W.; Park, J.T.; Yoo, T.H.; Kang, S.W.; Han, S. . Association of ketone bodies with incident CKD and death: A UK Biobank study. Diabetes Metab. 2024, 50, 101527. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Westenbrink, B.D.; Connelly, M.A.; Otvos, J.D.; Groothof, D.; Shalaurova, I.; Garcia, E.; Navis, G.; de Boer, R.A.; Bakker, S.J.L.; et al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur. J. Clin. Investig. 2021, 51, e13468. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.P.; Shryack, G.; Alessi, I.; Wieschhaus, N.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ibdah, J.A.; Parks, E.J.; Rector, R.S. Relationship between serum β-hydroxybutyrate and hepatic fatty acid oxidation in individuals with obesity and NAFLD. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E493–E502. [Google Scholar] [CrossRef] [PubMed]
- Bolinas, D.K.M.; Barcena, A.J.R.; Mishra, A.; Bernardino, M.R.; Lin, V.; Heralde, F.C.; Chintalapani, G.; Fowlkes, N.W.; Huang, S.Y.; Melancon, M.P. Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats. Gels 2025, 11, 250. [Google Scholar] [CrossRef]
- Kasahara, N.; Teratani, T.; Doi, J.; Yokota, S.; Shimodaira, K.; Kaneko, Y.; Ohzawa, H.; Sakuma, Y.; Sasanuma, H.; Fujimoto, Y.; et al. Controlled release of hydrogel-encapsulated mesenchymal stem cells-conditioned medium promotes functional liver regeneration after hepatectomy in metabolic dysfunction-associated steatotic liver disease. Stem Cell Res. Ther. 2024, 15, 395. [Google Scholar] [CrossRef]
| Cirrhosis (n = 125) | PREVEND (n = 125) | p-Value | |
|---|---|---|---|
| β-hydroxybutyrate (µmol/L) | 111.5 [75.9, 178.1] | 138.4 [97.7, 197.5] | 0.02 |
| Age (years) | 60 [52, 65] | 58 [50, 66] | 0.6 |
| Sex (Female, %) | 42 (33.6) | 42 (33.6) | 1.0 |
| BMI (kg/m2) | 27.8 [24.8, 30.9] | 27.8 [25.5, 31.0] | 0.7 |
| Current smoking (N, %) | 16 (12.8) | 25 (20.0) | 0.2 |
| Alcohol consumption (g/day, %) | <0.001 | ||
| 0 | 120 (96.0) | 43 (34.4) | |
| 0.1–10 | 5 (4.0) | 27 (21.6) | |
| 10–30 | 0 (0.0) | 28 (22.4) | |
| >30 | 0 (0.0) | 27 (21.6) | |
| Systolic Blood Pressure (mmHg) | 115 [107, 130] | 132 [117, 144] | <0.001 |
| Diastolic Blood Pressure (mmHg) | 65 [59, 75] | 76 [69, 83] | <0.001 |
| History of cardiovascular disease (N, %) | 6 (4.8) | 5 (4.0) | 1.0 |
| History of diabetes (N, %) | 35 (28.0) | 33 (26.4) | 0.9 |
| Antihypertensive drugs (N, %) | 79 (63.2) | 22 (17.6) | <0.001 |
| Glucose-lowering drugs (N, %) | 34 (27.2) | 20 (16.0) | 0.05 |
| Lipid-lowering drugs (N, %) | 19 (15.2) | 20 (16.0) | 1.0 |
| Etiology (N, %) | - | NA | |
| MASLD | 32 (25.6) | ||
| Cholestatic liver disease | 32 (25.6) | ||
| Alcohol | 28 (22.4) | ||
| Viral | 12 (9.6) | ||
| Other | 21 (16.8) | ||
| CTP classification (N, %) | - | NA | |
| A | 27 (21.6) | ||
| B | 62 (49.6) | ||
| C | 36 (28.8) | ||
| Fasting glucose (mmol/L) | 6.35 [5.03, 8.00] | 5.10 [4.60, 6.20] | 0.002 |
| eGFR (mL/min/1.73 m2) | 99.5 [76.1, 109.7] | 88.3 [75.6, 99.9] | 0.01 |
| Total cholesterol (mmol/L) | 3.26 [2.61, 4.14] | 5.55 [4.95, 6.14] | <0.001 |
| HDL cholesterol (mmol/L) | 0.88 [0.59, 1.19] | 1.16 [0.92, 1.34] | <0.001 |
| LDL cholesterol (mmol/L) | 1.81 [1.29, 2.25] | 3.71 [3.09, 4.17] | <0.001 |
| Triglycerides (mmol/L) | 0.67 [0.46, 1.07] | 1.30 [0.98, 1.72] | <0.001 |
| ALT (U/L) | 39 [28, 59] | 21 [15, 28] | <0.001 |
| AST (U/L) | 54 [44, 83] | 24 [20, 30] | <0.001 |
| GGT (U/L) | 95 [49, 151] | 28 [18, 45] | <0.001 |
| AP (U/L) | 141 [99, 210] | 65 [54, 82] | <0.001 |
| Total Bilirubin (mmol/L) | 40 [23, 94] | 8 [6, 10] | <0.001 |
| Hemoglobin (mmol/L) | 6.9 [5.9, 7.8] | 8.5 [8.0, 9.0] | <0.001 |
| T1 (n = 41): ≤82.3 μmol/L | T2 (n = 43): 82.3–157.2 μmol/L | T3 (n = 41): >157.2 μmol/L | p-Value | |
|---|---|---|---|---|
| β -hydroxybutyrate (µmol/L) | 66.7 [59.4, 74.6] | 111.5 [97.7, 131.1] | 277.2 [182.4, 373.7] | |
| Age (years) | 58 [48, 62] | 61 [55, 66] | 60.00 [55, 65] | 0.1 |
| Sex (Female, %) | 13 (31.7) | 15 (34.9) | 14 (34.1) | 1.0 |
| BMI (kg/m2) | 27.8 [26.2, 30.4] | 27.8 [25.0, 31.8] | 28.1 [24.3, 30.40] | 0.5 |
| Smoking (%) | 7 (17.1) | 5 (11.6) | 4 (9.8) | 0.6 |
| Alcohol consumption (g/day, %) | 0.05 | |||
| 0 | 37 (90.2) | 43 (100) | 40 (97.6) | |
| 0.1–10 | 4 (9.8) | 0 (0.0) | 1 (2.4) | |
| 10–30 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| >30 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Systolic Blood Pressure (mmHg) | 115 [108, 128] | 119 [108, 134] | 111 [104, 122] | 0.3 |
| Diastolic Blood Pressure (mmHg) | 65 [58, 75] | 66 [61, 75] | 63 [57, 71] | 0.4 |
| History of cardiovascular disease (N, %) | 1 (2.4) | 3 (7.0) | 2 (4.9) | 0.6 |
| History of diabetes (N, %) | 10 (24.4) | 14 (32.6) | 11 (26.8) | 0.7 |
| Antihypertensive drugs (N, %) | 23 (56.1) | 29 (67.4) | 27 (65.9) | 0.5 |
| Glucose-lowering drugs (N, %) | 7 (17.1) | 17 (39.5) | 10 (24.4) | 0.06 |
| Lipid-lowering drugs (N, %) | 4 (9.8) | 11 (25.6) | 4 (9.8) | 0.07 |
| Etiology (N, %) | 0.3 | |||
| MASLD | 6 (14.6) | 13 (30.2) | 13 (31.7) | |
| Cholestatic liver disease | 7 (17.1) | 14 (32.6) | 11 (26.8) | |
| Alcohol | 12 (29.3) | 6 (14.0) | 10 (24.4) | |
| Viral | 5 (12.2) | 3 (7.0) | 4 (9.8) | |
| Other | 11 (26.8) | 7 (16.2) | 3 (7.3) | |
| CTP classification (N, %) | 0.9 | |||
| A | 9 (22.0) | 10 (23.3) | 8 (19.5) | |
| B | 22 (53.7) | 19 (44.2) | 21 (51.2) | |
| C | 10 (24.4) | 14 (32.6) | 12 (29.3) | |
| MELD score | 14 [10, 18] | 14 [10, 17] | 17 [11, 21] | 0.07 |
| HbA1c (%) | 5.0 [4.7, 5.4] | 5.5 [4.8, 6.3] | 4.6 [4.0, 5.3] | 0.3 |
| Fasting glucose (mmol/L) | 6.2 [5.5, 6.7] | 6.7 [5.4, 8.0] | 7.0 [4.6, 8.8] | 0.8 |
| eGFR (mL/min/1.73 m2) | 101.8 [76.4, 113.8] | 100.3 [85.3, 112.2] | 97.6 [69.5, 102.9] | 0.05 |
| Total cholesterol (mmol/L) | 3.28 [2.79, 4.14] | 3.21 [2.73, 4.01] | 2.95 [2.33, 3.98] | 0.6 |
| HDL cholesterol (mmol/L) | 1.11 [0.80, 1.27] | 0.83 [0.63, 1.12] | 0.67 [0.16, 1.06] | 0.004 |
| LDL cholesterol (mmol/L) | 1.99 [1.42, 2.33] | 1.81 [1.24, 2.37] | 1.76 [1.19, 2.22] | 0.6 |
| Triglycerides (mmol/L) | 0.56 [0.37, 0.71] | 0.85 [0.54, 1.17] | 0.79 [0.50, 1.08] | 0.02 |
| ALT (U/L) | 35 [25, 56] | 39 [30, 47] | 44 [32, 78] | 0.2 |
| AST (U/L) | 50 [41, 59] | 53 [37, 70] | 81 [44, 113] | 0.06 |
| GGT (U/L) | 90 [47, 142] | 101 [52, 214] | 96 [60, 132] | 0.7 |
| AP (U/L) | 136 [109, 177] | 142 [101, 217] | 153 [87, 234] | 0.9 |
| Total Bilirubin (mmol/L) | 30 [17, 53] | 50 [26, 93] | 63 [24, 229] | 0.02 |
| Albumin (g/L) | 31 [27, 36] | 30 [27, 35] | 32 [28, 37] | 0.9 |
| Hemoglobin (mmol/L) | 7.2 [6.2, 8.1] | 6.8 [6.1, 7.2] | 6.4 [5.4, 8.1] | 0.4 |
| T1 HR [95% CI] | T2 (Reference) | T3 HR [95% CI] | |
|---|---|---|---|
| All patients with cirrhosis (n = 125, deaths = 27) | |||
| Model 1 | 2.3 [0.7–7.5] p = 0.2 | Reference | 6.6 [2.2–20.2] p < 0.001 |
| Model 2 | 2.5 [0.7–8.2] p = 0.1 | Reference | 6.6 [2.2–20.3] p < 0.001 |
| Model 3 | 2.9 [0.8–9.8] p = 0.1 | Reference | 6.5 [2.1–20.1] p = 0.001 |
| Model 4 | 2.9 [0.8–10.0] p = 0.1 | Reference | 8.3 [2.5–27.6] p < 0.001 |
| Model 5 | 3.3 [0.9–11.7] p = 0.06 | Reference | 7.6 [2.3–25.6] p = 0.001 |
| Sensitivity analysis: Excluding patients with diabetes (n = 90, deaths = 21) | |||
| Model 1 | 1.5 [0.4–5.4] p = 0.5 | Reference | 7.3 [2.2–24.3] p = 0.001 |
| Model 2 | 1.5 [0.4–5.3] p = 0.6 | Reference | 6.5 [1.9–21.7] p = 0.002 |
| Model 3 | 2.2 [0.6–8.3] p = 0.3 | Reference | 6.5 [1.8–23.0] p = 0.004 |
| Model 4′ | 2.5 [0.6–9.4] p = 0.2 | Reference | 5.4 [1.5–20.0] p = 0.01 |
| Sensitivity analysis: Excluding patients with MASLD (n = 93, deaths = 19) | |||
| Model 1 | 1.3 [0.4–5.0] p = 0.7 | Reference | 5.4 [1.6–18.1] p = 0.007 |
| Model 2 | 1.5 [0.4–5.7] p = 0.6 | Reference | 5.6 [1.6–19.0] p = 0.006 |
| Model 3 | 2.2 [0.5–9.1] p = 0.3 | Reference | 5.3 [1.5–18.3] p = 0.008 |
| Model 4 | 1.9 [0.5–8.2] p = 0.4 | Reference | 7.3 [1.9–27.6] p = 0.004 |
| Model 5 | 2.1 [0.5–8.8] p = 0.3 | Reference | 6.4 [1.7–24.8] p = 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chvatal-Medina, M.; Li, Y.; Trillos-Almanza, M.C.; Post, A.; Connelly, M.A.; Moshage, H.; Bakker, S.J.L.; Meijer, V.E.d.; Blokzijl, H.; Dullaart, R.P.F., on behalf of TransplantLines Investigators. Plasma Beta-Hydroxybutyrate and All-Cause Mortality in Patients with Liver Cirrhosis. Biomedicines 2025, 13, 1120. https://doi.org/10.3390/biomedicines13051120
Chvatal-Medina M, Li Y, Trillos-Almanza MC, Post A, Connelly MA, Moshage H, Bakker SJL, Meijer VEd, Blokzijl H, Dullaart RPF on behalf of TransplantLines Investigators. Plasma Beta-Hydroxybutyrate and All-Cause Mortality in Patients with Liver Cirrhosis. Biomedicines. 2025; 13(5):1120. https://doi.org/10.3390/biomedicines13051120
Chicago/Turabian StyleChvatal-Medina, Mateo, Yakun Li, María Camila Trillos-Almanza, Adrian Post, Margery A. Connelly, Han Moshage, Stephan J. L. Bakker, Vincent E. de Meijer, Hans Blokzijl, and Robin P. F. Dullaart on behalf of TransplantLines Investigators. 2025. "Plasma Beta-Hydroxybutyrate and All-Cause Mortality in Patients with Liver Cirrhosis" Biomedicines 13, no. 5: 1120. https://doi.org/10.3390/biomedicines13051120
APA StyleChvatal-Medina, M., Li, Y., Trillos-Almanza, M. C., Post, A., Connelly, M. A., Moshage, H., Bakker, S. J. L., Meijer, V. E. d., Blokzijl, H., & Dullaart, R. P. F., on behalf of TransplantLines Investigators. (2025). Plasma Beta-Hydroxybutyrate and All-Cause Mortality in Patients with Liver Cirrhosis. Biomedicines, 13(5), 1120. https://doi.org/10.3390/biomedicines13051120








