Perma Curette and Hysteroscopy: An Observational Study About Endometrial Sampling
Abstract
1. Introduction
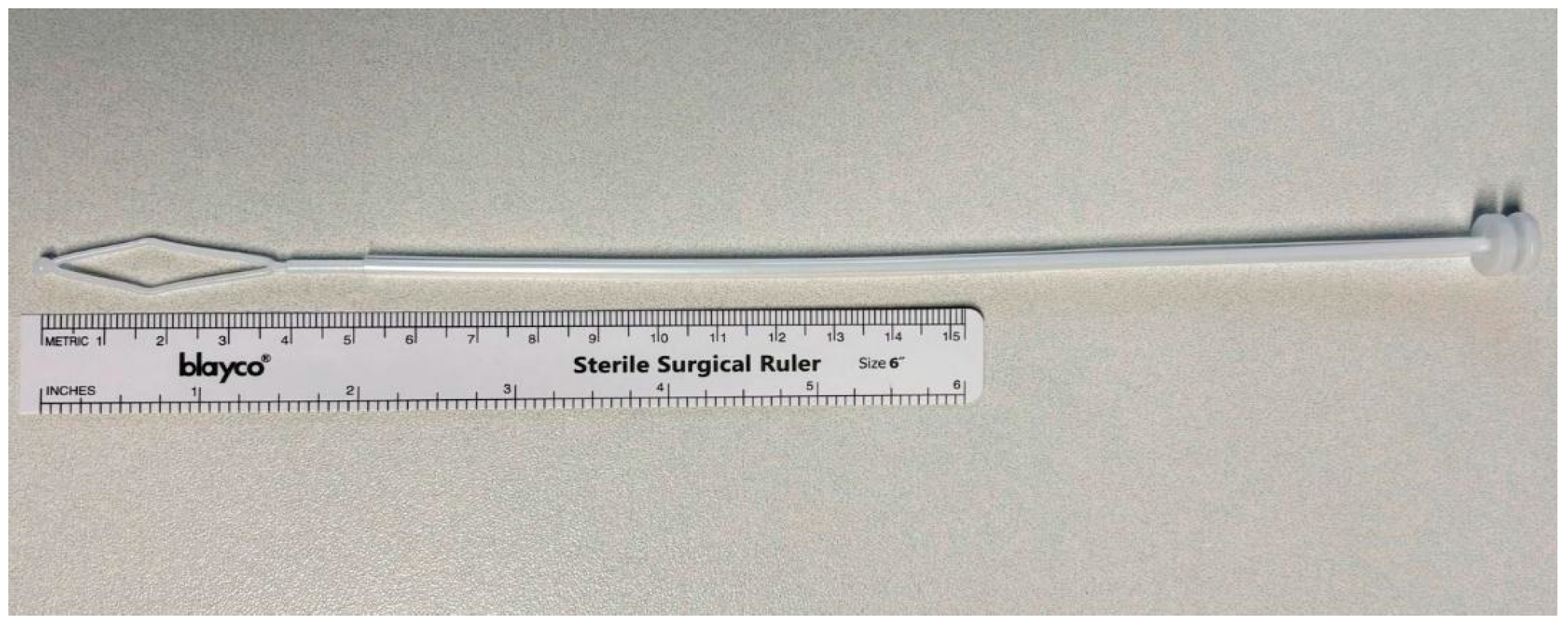
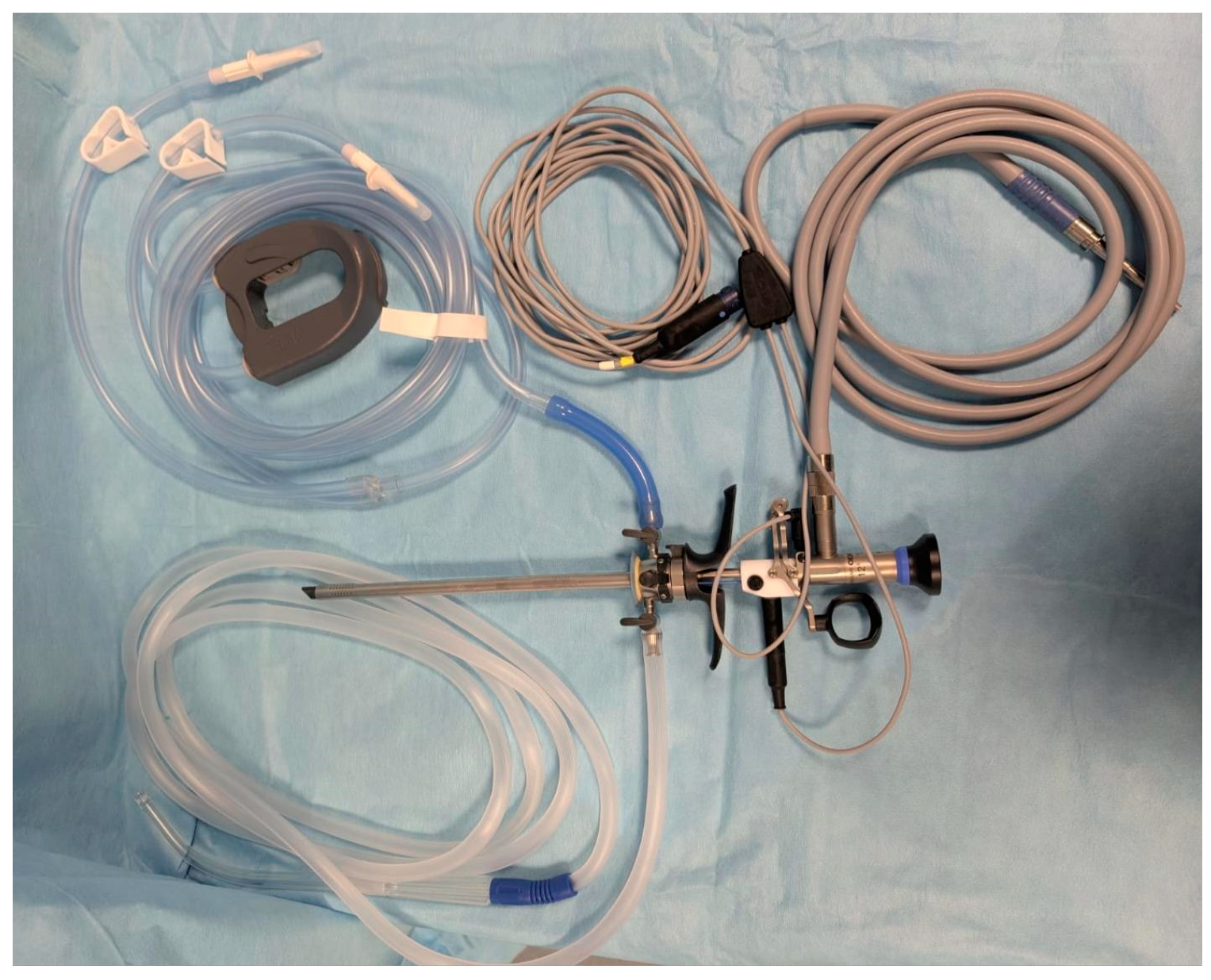
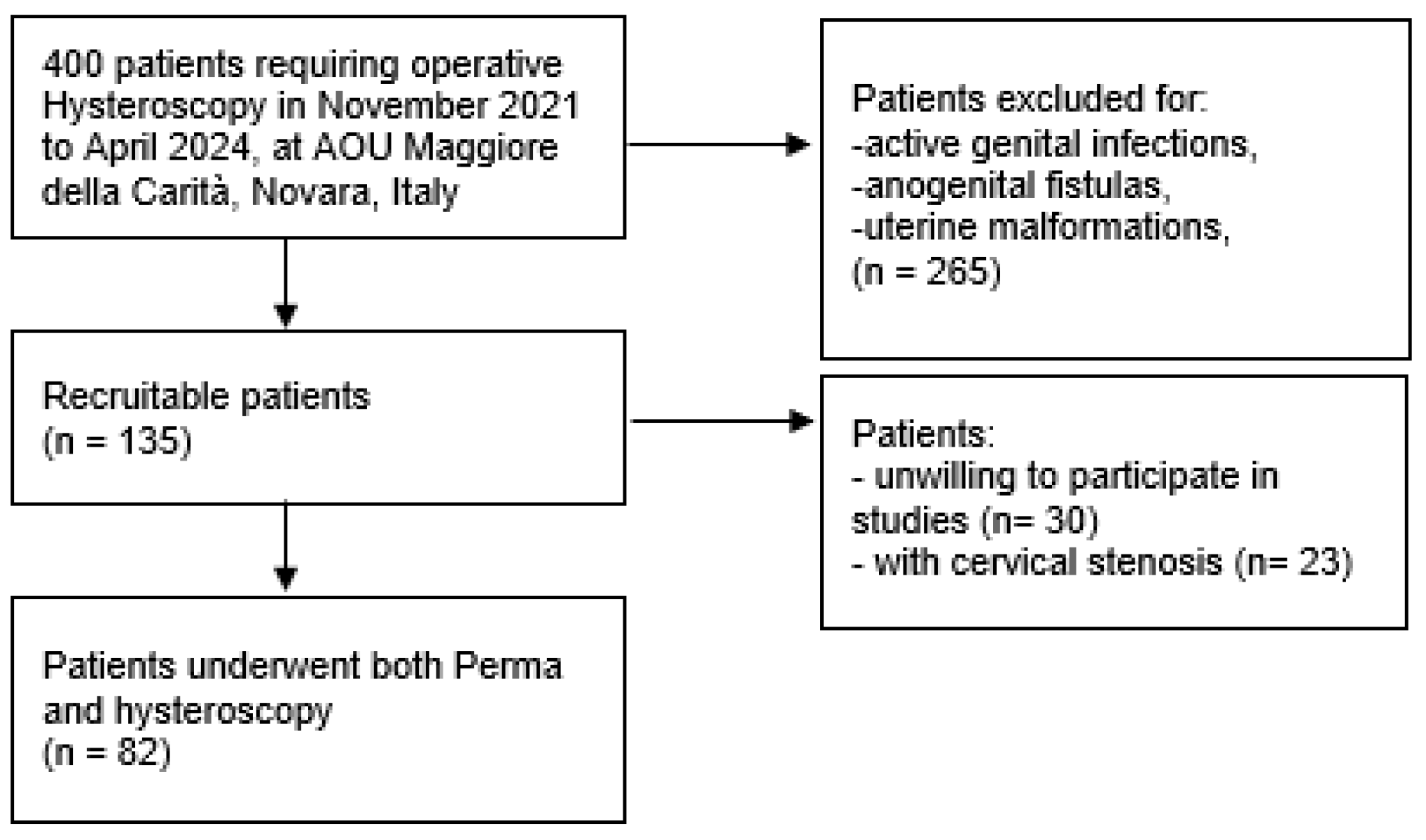
2. Materials and Methods
Statistical Analysis
- -
- <0, no agreement;
- -
- 0–0.4, poor agreement;
- -
- 0.4–0.6, fair;
- -
- 0.6–0.8, good;
- -
- 0.8–1, excellent agreement [22].
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valle, R.F. MD development of hysteroscopy: From a dream to a reality, and its linkage to the present and future. J. Minim. Invasive Gynecol. 2007, 14, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Siegler, A.M. The eary history of hysteroscopy. J. Am. Assoc. Gynecol. Laparosc. 1998, 5, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Narice, B.F.; Delaney, B.; Dickson, J.M. Endometrial sampling in low-risk patients with abnormal uterine bleeding: A systematic review and meta-synthesis. BMC Fam. Pract. 2018, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Kasiewicz, J.L.; Desai, V.B. Office versus institutional operative hysteroscopy: An economic model. J. Minim. Invasive Gynecol. 2022, 29, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Haimovich, S.; Riemma, G.; Ludwin, A.; Zizolfi, B.; De Angelis, M.C.; Carugno, J. Innovations in hysteroscopic surgery: Expanding the meaning of “in-office”. Minim. Invasive Ther. Allied Technol. 2021, 30, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Naim, N.M.; Mahdy, Z.A.; Ahmad, S.; Razi, Z.R. The Vabra aspirator versus the Pipelle device for outpatient endometrial sampling. Aust. N. Z. J. Obs. Gynaecol. 2007, 47, 132–136. [Google Scholar] [CrossRef]
- Gungorduk, K.; Asicioglu, O.; Ertas, I.E.; Ozdemir, I.A.; Ulker, M.M.; Yildirim, G.; Ataser, G.; Sanci, M. Comparison of the histopathological diagnoses of preoperative dilatation and curettage and Pipelle biopsy. Eur. J. Gynaecol. Oncol. 2014, 35, 539–543. [Google Scholar]
- Du, J.; Li, Y.; Lv, S.; Wang, Q.; Sun, C.; Dong, X.; He, M.; Ulain, Q.; Yuan, Y.; Tuo, X.; et al. Endometrial sampling devices for early diagnosis of endometrial lesions. J. Cancer Res. Clin. Oncol. 2016, 142, 2515–2522. [Google Scholar] [CrossRef]
- Ejzenberg, D.; Simoes, M.J.; Pinheiro, W.; Soares, J.M., Jr.; Serafini, P.C.; Baracat, E.C. Blind aspiration biopsy versus a guided hysteroscopic technique for investigation of the endometrium in infertile women. Histol. Histopathol. 2016, 31, 981–986. [Google Scholar]
- Arumaikannu, J.; Priyadarsene, P.; Rani, U. Accuracy of pipelle aspiration versus office hysteroscope in diagnosing endometrial pathology in perimenopausal women with abnormal uterine bleeding. Indian J. Res. 2016, 5, 12. [Google Scholar]
- Bertelli, G.; Venturini, M.; Mastro, L.D.; Garrone, O.; Cosso, M.; Gustavino, C.; Cusimano, E.; Guido, T.; Nicolò, G.; Rosso, R. Tamoxifen and the endometrium: Findings of pelvic ultrasound examination and endometrial biopsy in asymptomatic breast cancer patients. Breast Cancer Res. Treat. 1998, 47, 41–46. [Google Scholar] [CrossRef]
- Carneiro, M.C.; Ferreira, P.G.; Saraiva, S.M.; Rodrigues, C.D.; Leitão, S.; Costa, C.M.; Ferreira, M.D.S. Office endometrial sampling: Effectiveness and predictive factors of success in Novak versus Endosampler devices. Minerva Obs. Gynecol. 2024, 76, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fogliettoillustrativo.net/bugiardino/curette-perma-endometriali-908291800 (accessed on 1 January 2025).
- Noci, I.; Dubini, V.; Piacentini, R.; Colafranceschi, M.; Taddei, G.; Scarselli, G. Correlation between endometrial biopsy and luteal phase plasma progesterone in women complaining for couple infertility. J. Endocrinol. Investig. 1988, 11, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Iossa, A.; Cianferoni, L.; Ciatto, S.; Cecchini, S.; Campatelli, C.; Stumbo, F.L. Hysteroscopy and endometrial cancer diagnosis: A review of 2007 consecutive examinations in self-referred patients. Tumori J. 1991, 77, 479–483. [Google Scholar]
- Zutshi, V.; Saran, A.; Ahluwalia, C. Comparison of adequacy of endometrial biopsy using endosampler and hysteroscope guided sampling. Int. J. Reprod. Contracept. Obstet. Gynecol. 2021, 10, 2291–2297. [Google Scholar] [CrossRef]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Linee guida AIOM 2022, Neoplasie Dell’utero, Endometrio e Cervice. Available online: https://www.aiom.it/wp-content/uploads/2021/10/2021_LGAIOM_Utero_endometrio_cervice.pdf (accessed on 1 January 2025).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Soeters, R.; Whittaker, J.; Dehaeck, K. Endometrial sampling: A comparison between the Pipelle endometrium sampler and the Endosampler. S. Afr. J. Gynaecol. Oncol. 2011, 3, 34–38. [Google Scholar] [CrossRef]
- Litta, P.; Merlin, F.; Saccardi, C.; Pozzan, C.; Sacco, G.; Fracas, M.; Capobianco, G.; Dessole, S. Role of hysteroscopy with endometrial biopsy to rule out endometrial cancer in postmenopausal women with abnormal uterine bleeding. Maturitas 2005, 50, 117–123. [Google Scholar] [CrossRef]
- Więckowska, B.; Kubiak, K.B.; Jóźwiak, P. Cohen’s Kappa Coefficient as a Measure to Assess Classification Improvement following the Addition of a New Marker to a Regression Model. Int. J. Environ. Res. Public Health 2022, 19, 10213. [Google Scholar] [CrossRef]
- Van Stralen, K.J.; Stel, V.S.; Reitsma, J.B.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney Int. 2009, 75, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.T.; Chambers, S.K. Endometrial sampling: When? Where? Why? With what? Clin. Obs. Gynecol. 1992, 35, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Gambadauro, P.; Milenkovic, M.; Hadlaczky, G. Simulation for Training and Assessment in Hysteroscopy: A Systematic Review. J. Minim. Invasive Gynecol. 2018, 25, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.W.; Vredevoogd, C.B.; Van Ulzen, K.; Hermans, J.O.; Trimbos, J.B.; Trimbos-Kemper, T.C. Complications of hysteroscopy: A prospective multicenter study. Obs. Gynecol. 2000, 96, 266–270. [Google Scholar] [CrossRef]
- McGurgan, P.M.; McIlwaine, P. Complications of hysteroscopy and how to avoid them. Best Pract. Res. Clin. Obs. Gynaecol. 2015, 29, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Suh-Burgmann, E.J.; Alavi, M.; Schmittdiel, J. Endometrial cancer detection during the coronavirus disease 2019 (COVID-19) pandemic. Obstet. Gynecol. 2020, 136, 842–843. [Google Scholar] [CrossRef]
- Seay, K.; Katcher, A.; Hare, M.; Kohn, N.; Juhel, H.; Goldberg, G.L.; Frimer, M. The Impact of Surgical Delay: A Single Institutional Experience at the Epicenter of the COVID Pandemic Treatment Delays in Women with Endometrial Cancer and Endometrial Intraepithelial Hyperplasia. COVID 2023, 4, 38–43. [Google Scholar] [CrossRef]
- Garrett, A.P.; Seidman, B.C. The impact of the COVID-19 pandemic on the stage of endometrial cancer at diagnosis. Gynecol. Oncol. Rep. 2023, 47, 101191. [Google Scholar] [CrossRef]
- Aquino, C.I.; Nicosia, A.; Ligori, A.; Volpicelli, A.I.; Surico, D. Microbiota Status and Endometrial Cancer: A Narrative Review About Possible Correlations in Affected Versus Healthy Patients. Sci 2024, 6, 75. [Google Scholar] [CrossRef]
- Aquino, C.I.; Troisi, J.; D’Antonio, A.; Giugliano, L.; Raffone, A.; Sarno, L.; Saccone, G.; Scala, G.; Guida, M. Endometrial carcinoma and bisphenol A: A pilot case-control study. Biomed. J. Sci. Tech. Res. 2019, 21, 16073–16079. [Google Scholar]
- Anca-Stanciu, M.B.; Manu, A.; Olinca, M.V.; Coroleucă, C.; Comandașu, D.E.; Coroleuca, C.A.; Maier, C.; Bratila, E. Comprehensive Review of Endometrial Cancer: New Molecular and FIGO Classification and Recent Treatment Changes. J. Clin. Med. 2025, 14, 1385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neveu, M.E.; Debras, E.; Niro, J.; Fernandez, H.; Panel, P. Standardizing hysteroscopy teaching: Development of a curriculum using the Delphi method. Surg. Endosc. 2017, 31, 5389–5398. [Google Scholar] [CrossRef]
- Morotti, M.; Menada, M.V.; Moioli, M.; Sala, P.; Maffeo, I.; Abete, L.; Fulcheri, E.; Menoni, S.; Venturini, P.; Papadia, A. Frozen section pathology at time of hysterectomy accurately predicts endometrial cancer in patients with preoperative diagnosis of atypical endometrial hyperplasia. Gynecol. Oncol. 2012, 125, 536–540. [Google Scholar] [CrossRef]
- De Aloysio, D.; Rocca, G.; Miliffi, L. Cyto-histologic evaluation of the endometrium in climacteric women at risk for endometrial carcinoma. Tumori J. 1986, 72, 431–437. [Google Scholar] [CrossRef]
- Lukanović, D.; Matjašič, M.; Kobal, B. Accuracy of preoperative sampling diagnosis for predicting final pathology in patients with endometrial carcinoma: A review. Transl. Cancer Res. 2020, 9, 7785–7796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitale, S.G.; Giannini, A.; Carugno, J.; van Herendael, B.; Riemma, G.; Pacheco, L.A.; Drizi, A.; Mereu, L.; Bettocchi, S.; Angioni, S.; et al. Hysteroscopy: Where did we start, and where are we now? The compelling story of what many considered the “Cinderella” of gynecological endoscopy. Arch. Gynecol. Obstet. 2024, 310, 1877–1888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, A.R.W.; Brechin, S.; Porter, A.J.L.; Warner, P.; Critchley, H.O.D. Factors affecting adequacy of Pipelle and Tao Brush endometrial sampling. BJOG: Int. J. Obstet. Gynaecol. 2008, 115, 1028–1036. [Google Scholar] [CrossRef]
- van Hanegem, N.; Prins, M.M.; Bongers, M.Y.; Opmeer, B.C.; Sahota, D.S.; Mol, B.W.; Timmermans, A. The accuracy of endometrial sampling in women with postmenopausal bleeding: A systematic review and meta-analysis. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 197, 147–155. [Google Scholar] [CrossRef] [PubMed]
| Mean± | SD | |
|---|---|---|
| Age (years) | 55.5 | 11.3 |
| Weight (kg) * | 70.8 | 16.2 |
| Height (cm) * | 162.3 | 7.3 |
| BMI * (kg/m2) | 26.7 | 5.0 |
| Menopause (years) | 50.6 | 3.4 |
| Yes | No | |
| Smoke | 69/82 84.1% | 13/82 15.8% |
| Hypertension | 56/82 68.3% | 26/82 31.7% |
| Diabetes | 74/82 90.2% | 8/82 9.8% |
| Tumors | 72/82 87.8% | 10/8 12.2% |
| Parity * | G0 30.5% G1 24.4% G2 32.9% G3 9.8% G4 2.4% | |
| N | % | |
|---|---|---|
| Endometrial polyp | 32 | 39.0 |
| Endometrial thickening | 31 | 37.8 |
| Endometrial hyperplasia, dysplasia | 4 | 4.9 |
| Genital bleeding | 19 | 23.2 |
| Myoma | 8 | 9.8 |
| Adhesions | 1 | 1.2 |
| Cervical polyp | 3 | 3.7 |
| Other | 4 | 4.9 |
| Perma | Tumor 10.9% vs. Negative 89.1% |
| Hysteroscopy | Tumor 13.4% vs. Negative 86.6% |
| Percentage of outcome concordance—Expert 1 | 52/82 (63.4%) |
| Percentage of outcome concordance—Expert 2 | 50/82 (60.9%) |
| Percentage of outcome concordance—Non-expert 1 | 58/82 (70.7%) |
| Percentage of outcome concordance—Non-expert 2 | 72/82 (87.8%) |
| Percentage of outcome concordance between Experts | A: 65.8% EA: 52.9% K = 0.2743 |
| Percentage of outcome concordance between Non-experts | A: 80.5% EA: 65.7% K = 0.4315 |
| Expert 1 and Non-expert 1 | A: 65.8% EA: 55.6% K = 0.2316 |
| Expert 1 and Non-expert 2 | A: 63.4% EA: 60.1% K = 0.0821 |
| Expert 2 and Non-expert 1 | A: 85.4% EA: 54.5% K = 0.6780 |
| Expert 2 and Non-expert 2 | A: 70.7% EA: 58.3% K = 0.2981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, C.I.; Surico, D.; Miglino, F.; Ligori, A.; Ferrante, D.; Remorgida, V. Perma Curette and Hysteroscopy: An Observational Study About Endometrial Sampling. Biomedicines 2025, 13, 1113. https://doi.org/10.3390/biomedicines13051113
Aquino CI, Surico D, Miglino F, Ligori A, Ferrante D, Remorgida V. Perma Curette and Hysteroscopy: An Observational Study About Endometrial Sampling. Biomedicines. 2025; 13(5):1113. https://doi.org/10.3390/biomedicines13051113
Chicago/Turabian StyleAquino, Carmen Imma, Daniela Surico, Francesca Miglino, Arianna Ligori, Daniela Ferrante, and Valentino Remorgida. 2025. "Perma Curette and Hysteroscopy: An Observational Study About Endometrial Sampling" Biomedicines 13, no. 5: 1113. https://doi.org/10.3390/biomedicines13051113
APA StyleAquino, C. I., Surico, D., Miglino, F., Ligori, A., Ferrante, D., & Remorgida, V. (2025). Perma Curette and Hysteroscopy: An Observational Study About Endometrial Sampling. Biomedicines, 13(5), 1113. https://doi.org/10.3390/biomedicines13051113








