Obesity and Systemic Inflammation Disrupt the Compensatory Role of Physical Activity in Chronic Pain Conditions
Abstract
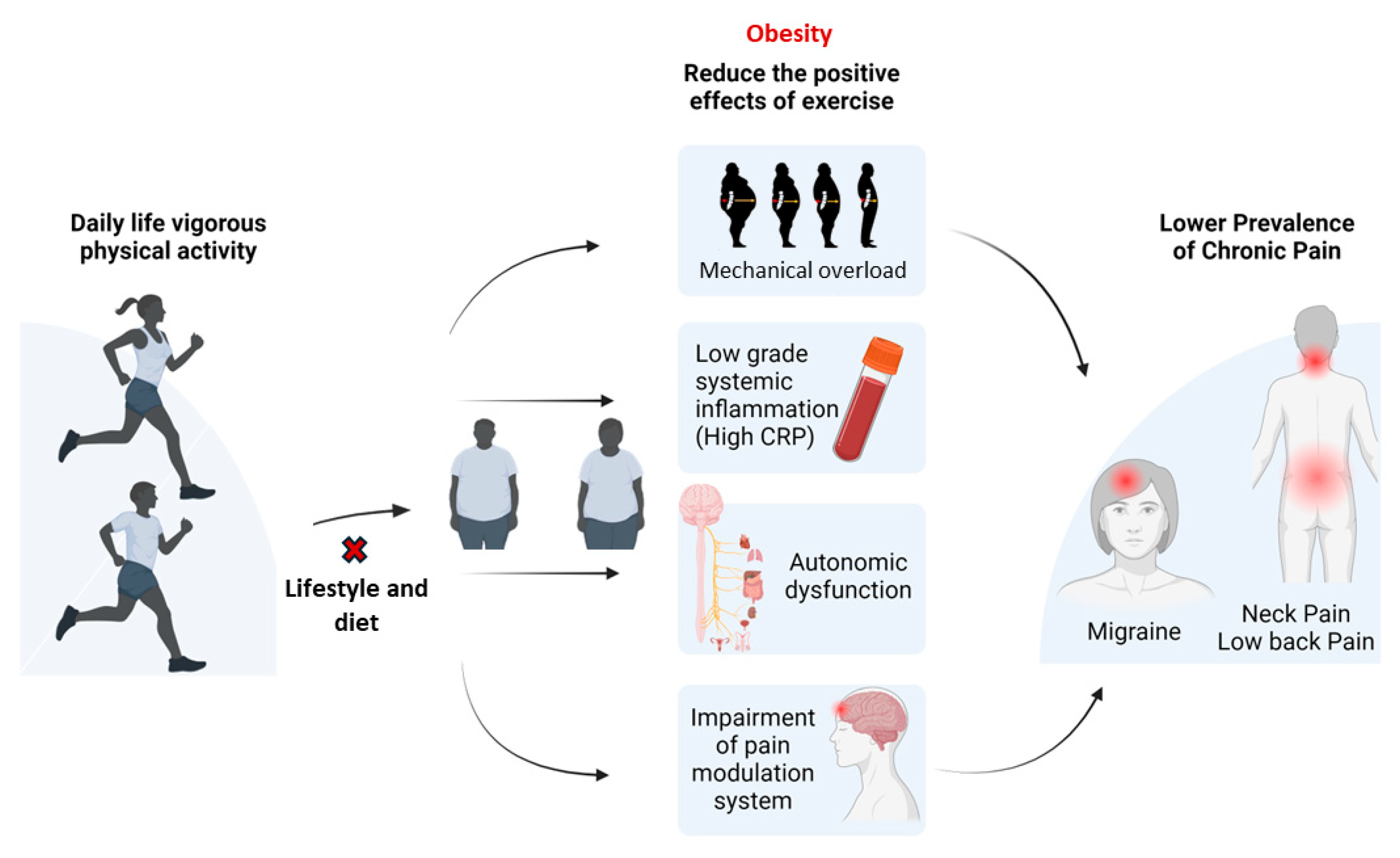
1. Introduction
2. Materials and Methods
2.1. Data Source and Sample
2.2. Demographic Variables
2.3. Health Status
2.4. Chronic Pain Outcomes
2.5. Physical Activity Exposure
2.6. Obesity and Systemic Inflammation
2.7. Dietary Inflammatory Index (DII)
2.8. Data Analysis
3. Results
3.1. Physical Activity Association with Neck, Low Back, and Hip Pain
3.2. BMI as an Effect Modifier
3.3. CRP Serum Levels as an Effect Modifier
3.4. DII Association with Pain
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, L.J.; Schur, E.; Noonan, C.; Ahumada, S.; Buchwald, D.; Afari, N. Chronic pain, overweight, and obesity: Findings from a community-based twin registry. J. Pain 2010, 11, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.C.; Xie, W.; Lundberg, D.J.; Hempstead, K.; Zajacova, A.; Zimmer, Z.; Glei, D.A.; Meara, E.; Preston, S.H. Increases in BMI and chronic pain for US adults in midlife, 1992 to 2016. SSM—Popul. Health 2020, 12, 100644. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23 (Suppl. 1), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Marques Miranda, C.; de Lima Campos, M.; Leite-Almeida, H. Diet, body weight and pain susceptibility—A systematic review of preclinical studies. Neurobiol. Pain 2021, 10, 100066. [Google Scholar] [CrossRef]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef]
- Lee, J.; Dunlop, D.; Ehrlich-Jones, L.; Semanik, P.; Song, J.; Manheim, L.; Chang, R.W. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res. 2012, 64, 488–493. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Hernandez-Molina, G.; Reichenbach, S. Exercise for osteoarthritis of the hip. Cochrane Database Syst. Rev. 2014, 2014, Cd007912. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.C.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; Schafer, L.A.; Danyliw, A.; Sawant, A.; Bello-Haas, V.D.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, Cd010884. [Google Scholar] [CrossRef]
- Finan, P.H.; Smith, M.T. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med. Rev. 2013, 17, 173–183. [Google Scholar] [CrossRef]
- Cramp, F.; Hewlett, S.; Almeida, C.; Kirwan, J.R.; Choy, E.H.; Chalder, T.; Pollock, J.; Christensen, R. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst. Rev. 2013, 2013, Cd008322. [Google Scholar] [CrossRef]
- Hurkmans, E.; van der Giesen, F.J.; Vliet Vlieland, T.P.; Schoones, J.; Van den Ende, E.C. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, 2009, Cd006853. [Google Scholar] [CrossRef] [PubMed]
- Koopman, F.S.; Beelen, A.; Gilhus, N.E.; de Visser, M.; Nollet, F. Treatment for postpolio syndrome. Cochrane Database Syst. Rev. 2015, 2015, Cd007818. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, Cd011279. [Google Scholar] [PubMed]
- Rossi, H.L.; Luu, A.K.; Kothari, S.D.; Kuburas, A.; Neubert, J.K.; Caudle, R.M.; Recober, A. Effects of diet-induced obesity on motivation and pain behavior in an operant assay. Neuroscience 2013, 235, 87–95. [Google Scholar] [CrossRef]
- Glei, D.A.; Stokes, A.C.; Weinstein, M. Widening Socioeconomic Disparities in Pain and Physical Function Among Americans Are Linked with Growing Obesity. J. Aging Health 2022, 34, 78–87. [Google Scholar] [CrossRef]
- Case, A.; Deaton, A.; Stone, A.A. Decoding the mystery of American pain reveals a warning for the future. Proc. Natl. Acad. Sci. USA 2020, 117, 24785–24789. [Google Scholar] [CrossRef]
- Glei, D.A.; Weinstein, M. Economic distress, obesity, and the rise in pain. Soc. Sci. Med. 2023, 339, 116399. [Google Scholar] [CrossRef]
- Fang, X.X.; Zhai, M.N.; Zhu, M.; He, C.; Wang, H.; Wang, J.; Zhang, Z.J. Inflammation in pathogenesis of chronic pain: Foe and friend. Mol. Pain 2023, 19, 17448069231178176. [Google Scholar] [CrossRef]
- Narouze, S.; Souzdalnitski, D. Obesity and chronic pain: Opportunities for better patient care. Pain Manag. 2015, 5, 217–219. [Google Scholar] [CrossRef]
- Narouze, S.; Souzdalnitski, D. Obesity and chronic pain: Systematic review of prevalence and implications for pain practice. Reg. Anesth. Pain Med. 2015, 40, 91–111. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Overwyk, K.J.; Zhao, L.; Zhang, Z.; Wiltz, J.L.; Dunford, E.K.; Cogswell, M.E. Trends in Blood Pressure and Usual Dietary Sodium Intake Among Children and Adolescents, National Health and Nutrition Examination Survey 2003 to 2016. Hypertension 2019, 74, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lean, M.E.; Han, T.S.; Morrison, C.E. Waist circumference as a measure for indicating need for weight management. BMJ 1995, 311, 158–161. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Paley, C.A.; Johnson, M.I. Physical Activity to Reduce Systemic Inflammation Associated with Chronic Pain and Obesity: A Narrative Review. Clin. J. Pain 2016, 32, 365–370. [Google Scholar] [CrossRef]
- Messier, S.P.; Mihalko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA 2013, 310, 1263–1273. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Carolyna Gianlorenço, A.; Machado, R.; Queiroga, M.; Zeng, H.; Shaikh, E.; Yang, Y.; Nogueira, B.; Castelo-Branco, L.; Fregni, F. Exercise-induced pain threshold modulation in healthy subjects: A systematic review and meta-analysis. Princ. Pract. Clin. Res. 2020, 6, 11–28. [Google Scholar] [CrossRef]
- Naugle, K.M.; Naugle, K.E.; Teegardin, M.; Kaleth, A.S. Physical Activity to Prevent the Age-Related Decline of Endogenous Pain Modulation. Exerc. Sport Sci. Rev. 2023, 51, 169–175. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Fehrmann, E.; Gajsar, H.; Kreddig, N. Endogenous Modulation of Pain: The Role of Exercise, Stress, and Cognitions in Humans. Clin. J. Pain 2020, 36, 150–161. [Google Scholar] [CrossRef]
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J. Pain 2012, 13, 936–944. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Shields, M.R.; Stegner, A.J.; Cook, D.B. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J. Pain 2012, 13, 195–206. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J.H. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Taylor, J.L.; Booth, J.; Barry, B.K. Exploring the Mechanisms of Exercise-Induced Hypoalgesia Using Somatosensory and Laser Evoked Potentials. Front. Physiol. 2016, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.F. Analgesia following exercise: A review. Sports Med. 2000, 29, 85–98. [Google Scholar] [CrossRef]
- Slemenda, C.; Brandt, K.D.; Heilman, D.K.; Mazzuca, S.; Braunstein, E.M.; Katz, B.P.; Wolinsky, F.D. Quadriceps weakness and osteoarthritis of the knee. Ann. Intern. Med. 1997, 127, 97–104. [Google Scholar] [CrossRef]
- Mork, P.J.; Vasseljen, O.; Nilsen, T.I. Association between physical exercise, body mass index, and risk of fibromyalgia: Longitudinal data from the Norwegian Nord-Trøndelag Health Study. Arthritis Care Res. 2010, 62, 611–617. [Google Scholar] [CrossRef]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef]
- Sakamoto, K.; Butera, M.A.; Zhou, C.; Maurizi, G.; Chen, B.; Ling, L.; Shawkat, A.; Patlolla, L.; Thakker, K.; Calle, V.; et al. Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity. Cell Metab. 2025, 37, 121–137.E6. [Google Scholar] [CrossRef]
- Garfield, A.S.; Heisler, L.K. Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. 2009, 587, 49–60. [Google Scholar] [CrossRef]
- Mazier, W.; Saucisse, N.; Gatta-Cherifi, B.; Cota, D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. TEM. 2015, 26, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Woliński, K.; Płazińska, M.T.; Mikołajczak, P.; Ruchała, M. The effects of cannabinoids on the endocrine system. Endokrynol. Polska 2018, 69, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Pang, Z. Endocannabinoids and obesity. In Vitamins and Hormones; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 91, pp. 325–368. [Google Scholar]
- Rohbeck, E.; Eckel, J.; Romacho, T. Cannabinoid Receptors in Metabolic Regulation and Diabetes. Physiology 2021, 36, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Watkins, B.A. Dietary PUFAs and Exercise Dynamic Actions on Endocannabinoids in Brain: Consequences for Neural Plasticity and Neuroinflammation. Adv. Nutr. 2022, 13, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.M.; Toledo, E.; Rodriguez-Diez, M.C.; Gea, A.; Lopez-Iracheta, R.; Shivappa, N.; Hébert, J.R.; Martinez-Gonzalez, M.A. Dietary indexes, food patterns and incidence of metabolic syndrome in a Mediterranean cohort: The SUN project. Clin. Nutr. 2015, 34, 508–514. [Google Scholar] [CrossRef]
- Naja, F.; Shivappa, N.; Nasreddine, L.; Kharroubi, S.; Itani, L.; Hwalla, N.; Sibai, M.; Hebert, J.R. Role of inflammation in the association between the western dietary pattern and metabolic syndrome among Lebanese adults. Int. J. Food Sci. Nutr. 2017, 68, 997–1004. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Feng, Y. Association between the dietary inflammatory index and pain in US adults from NHANES. Nutr. Neurosci. 2024, 27, 460–469. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, A.; Wang, Y.; Meng, L.; Szeto, I.M.; Li, T.; Gong, H.; Tian, Z.; Zhang, Y.; Wang, P. Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2018, 10, 831. [Google Scholar] [CrossRef]

| Character | 2003–2004 n = 4703 | 2009–2010 n = 5106 |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 48.90 (18.5) | 44.27 (14.1) |
| Gender | ||
| Female | 2433 (51.5%) | 2632 (51.6%) |
| Male | 2288 (48.5%) | 2474 (48.5%) |
| Race/Ethnicity | ||
| Mexican American | 944 (20.0%) | 1026 (20.1%) |
| Non-Hispanic Black | 949 (20.1%) | 963 (18.9%) |
| Non-Hispanic White | 2468 (52.3%) | 2245 (43.9%) |
| Other Hispanic | 147 (3.1%) | 576 (11.3%) |
| Other Race—Including Multi-Racial | 213 (4.5%) | 296 (5.8%) |
| Country of birth | ||
| Born Elsewhere | 469 (9.9%) | 862 (16.9%) |
| Born in 50 US States or Washington, DC | 3720 (78.8%) | 3590 (70.3%) |
| Born in Mexico | 531 (11.3%) | 652 (12.8%) |
| Educational level | ||
| 9–11th Grade (Includes 12th with no diploma) | 696 (14.7%) | 802 (15.7%) |
| College Graduate or above | 879 (18.6%) | 1060 (20.8%) |
| High School Grad/GED or Equivalent | 1182 (25.0%) | 1174 (22.9%) |
| Less Than 9th Grade | 656 (13.9%) | 581 (11.4%) |
| Some College or AA degree | 1298 (27.5%) | 1477 (28.9%) |
| Marital status | ||
| Divorced | 435 (9.2%) | 595 (11.7%) |
| Living with partner | 294 (6.2%) | 474 (9.3%) |
| Married | 2561 (54.3%) | 2605 (51.0%) |
| Never married | 827 (17.5%) | 1063 (20.8%) |
| Separated | 127 (2.7%) | 193 (3.8%) |
| Widowed | 476 (10.1%) | 172 (3.4%) |
| Annual family income | ||
| $ 0 to $ 19,999 | 1.406 (30.2%) | 1.167 (23.0%) |
| $20,000 to $24,999 | 1.474 (31.7%) | 1.447 (28.5%) |
| $45,000 to $54,999 | 806 (17.3%) | 847 (16.7%) |
| $75,000 and over | 740 (15.9%) | 439 (8.7%) |
| Poverty income ratio | ||
| Mean (SD) | 2.59 (1.6) | 2.43 (1.7) |
| Alcohol consumption (number of drinks/last 12 months) | ||
| Mean (SD) | 5.24 (38.8) | 5.07 (24.8) |
| Smoking (number of cigarettes/day last 30 days | ||
| Mean (SD) | 16.02 (44.3) | 12.41 (9.9) |
| CRP serum levels (mg/dL) | ||
| Mean (SD) | 0.47 (0.9) | 0.41 (0.8) |
| Physical, mental and emotional limitations | ||
| No | 3415 (97.3%) | 3944 (98.4%) |
| Yes | 95 (2.7%) | 64 (1.6%) |
| Sleep disorders | ||
| No | N.A. | 4728 (92.6%) |
| Yes | N.A. | 371 (7.3%) |
| DII | ||
| Mean (SD) | −0.02 (1.5) | 0.02 (1.6) |
| DII (categorical) | ||
| 0 (No—anti-inflammatory) | 2028 (48.3%) | 2269 (47.6%) |
| 1 (Yes—pro-inflammatory) | 2175 (51.8%) | 2496 (52.4%) |
| Physical activity | ||
| Vigorous | 1243 (26.3%) | 1019 (19.9%) |
| Moderate | 2.464 (52.4%) | 1.886 (36.9%) |
| BMI (kg/m2) | ||
| Mean (SD) | 28.36 (6.2) | 29.29 (7.0) |
| Non-obese < 30 kg/m2 | 2.941 (67.2%) | 3.034 (61.1%) |
| Obese ≥ 30 kg/m2 | 1.434 (32.8%) | 1.928 (38.9%) |
| Pain | ||
| Low back/hip/neck | 1752 (37.3%) | 1.508 (29.5%) |
| Migraine | 939 (19.9%) | N.A. |
| Joint pain | 2124 (45.2%) | N.A. |
| 2003–2004 | 2009–2010 | ||||||
|---|---|---|---|---|---|---|---|
| O.R. | C.I. (2.5–97.5%) | p Value | O.R. | C.I. (2.5–97.5%) | p Value | ||
| Unadjusted | Vigorous physical activity | 0.820 | 0.704–0.955 | 0.014 a | 0.748 | 0.599–0.934 | 0.013 b |
| Moderate physical activity | 0.755 | 0.603–0.945 | 0.012 a | 1.146 | 0.934–1.406 | 0.175 | |
| Adjusted | Vigorous physical activity | 0.798 | 0.647–0.984 | 0.037 a | 0.629 | 0.474–0.833 | 0.004 b |
| Moderate physical activity | 0.804 | 0.622–1.039 | 0.08 | 1.306 | 0.949–1.796 | 0.090 | |
| O.R. | C.I. (2.5–97.5%) | p Value | |||
|---|---|---|---|---|---|
| Unadjusted | Joint pain | Vigorous physical activity | 0.860 | 0.726–1.018 | 0.077 |
| Moderate physical activity | 0.925 | 0.783–1.092 | 0.331 | ||
| Migraine | Vigorous physical activity | 0.781 | 0.640–0.952 | 0.018 * | |
| Moderate physical activity | 0.904 | 0.731–1.116 | 0.324 | ||
| Adjusted | Joint pain | Vigorous physical activity | 1.366 | 1.031–1.810 | 0.033 * |
| Moderate physical activity | 1.144 | 0.866–1.509 | 0.296 | ||
| Migraine | Vigorous physical activity | 0.697 | 0.517–0.939 | 0.022 * | |
| Moderate physical activity | 0.848 | 0.665–1.081 | 0.162 |
| 2003–2004 | 2009–2010 | ||||||
|---|---|---|---|---|---|---|---|
| Interaction p Value = 0.015 * | Interaction p Value = 0.011 * | ||||||
| O.R. | C.I. (2.5–97.5%) | p Value | O.R. | C.I. (2.5–97.5%) | p Value | ||
| BMI | Obese ≥30 kg/m2 | 0.924 | 0.741–1.152 | 0.449 | 0.75 | 0.530–1.062 | 0.096 |
| Non-obese <30 kg/m2 | 0.637 | 0.460–0.883 | 0.011 a | 0.568 | 0.429–0.752 | 0.0011 b | |
| 2003–2004 | ||||
|---|---|---|---|---|
| Interaction p Value = 0.006 ** | ||||
| O.R. | C.I. (2.5–97.5%) | p Value | ||
| CRP | High CRP (≥0.22 mg/dL) | 1.198 | 0.783–1.831 | 0.364 |
| Low CRP (<0.22 mg/dL) | 0.560 | 0.339–0.924 | 0.027 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galassi, T.; Pacheco-Barrios, K.; Gianlorenco, A.C.; Fregni, F. Obesity and Systemic Inflammation Disrupt the Compensatory Role of Physical Activity in Chronic Pain Conditions. Biomedicines 2025, 13, 1111. https://doi.org/10.3390/biomedicines13051111
Galassi T, Pacheco-Barrios K, Gianlorenco AC, Fregni F. Obesity and Systemic Inflammation Disrupt the Compensatory Role of Physical Activity in Chronic Pain Conditions. Biomedicines. 2025; 13(5):1111. https://doi.org/10.3390/biomedicines13051111
Chicago/Turabian StyleGalassi, Taynah, Kevin Pacheco-Barrios, Anna C. Gianlorenco, and Felipe Fregni. 2025. "Obesity and Systemic Inflammation Disrupt the Compensatory Role of Physical Activity in Chronic Pain Conditions" Biomedicines 13, no. 5: 1111. https://doi.org/10.3390/biomedicines13051111
APA StyleGalassi, T., Pacheco-Barrios, K., Gianlorenco, A. C., & Fregni, F. (2025). Obesity and Systemic Inflammation Disrupt the Compensatory Role of Physical Activity in Chronic Pain Conditions. Biomedicines, 13(5), 1111. https://doi.org/10.3390/biomedicines13051111







