PTX3/NF-κB/TLR4 Pathway Evaluation in the Follicular Fluid to Successfully Predict Blastocyst Implantation: A Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Participant Data
2.2. ELISA Kit
2.3. Statistical Analysis
3. Results
3.1. Preliminary Evaluations
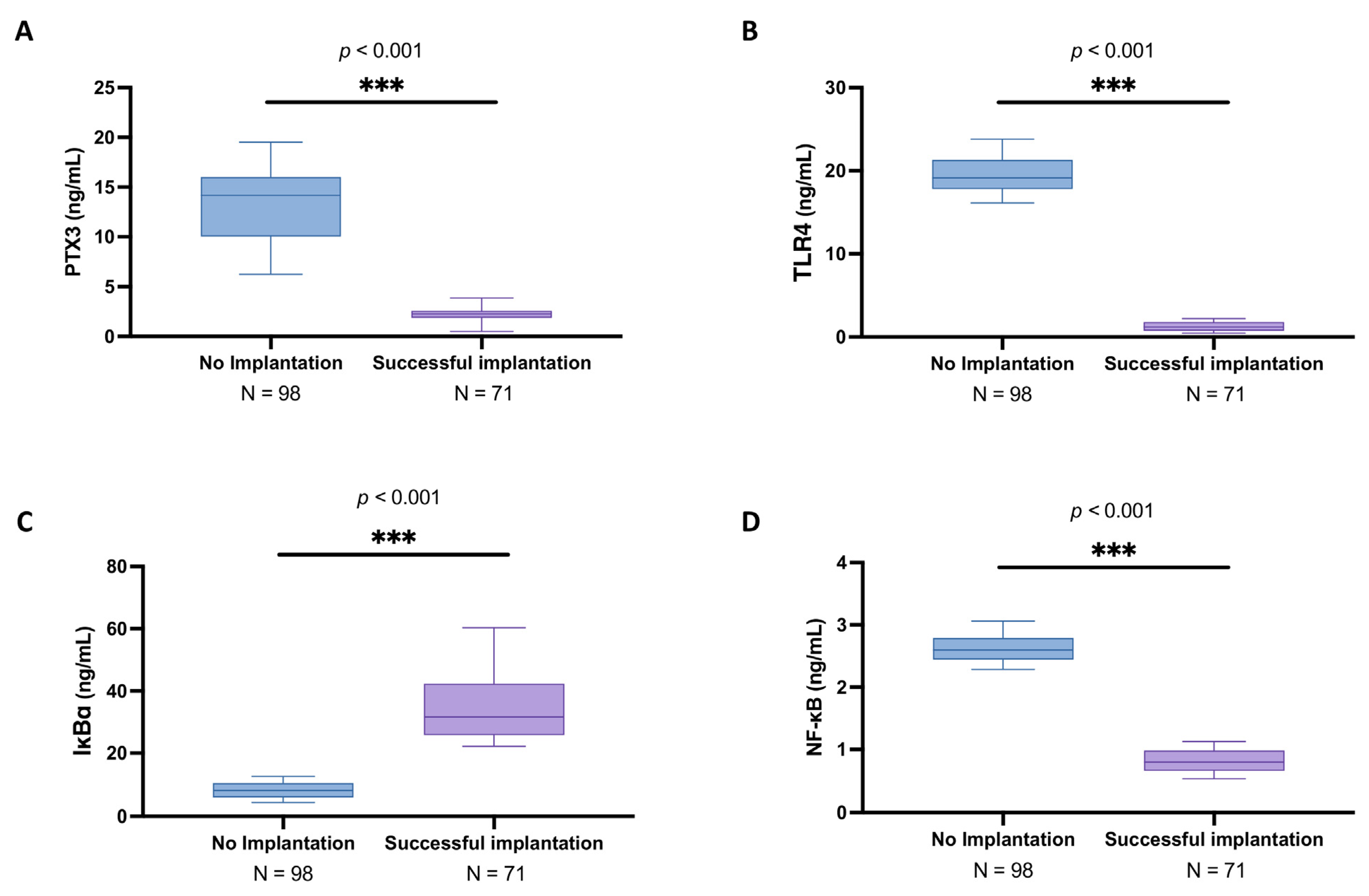
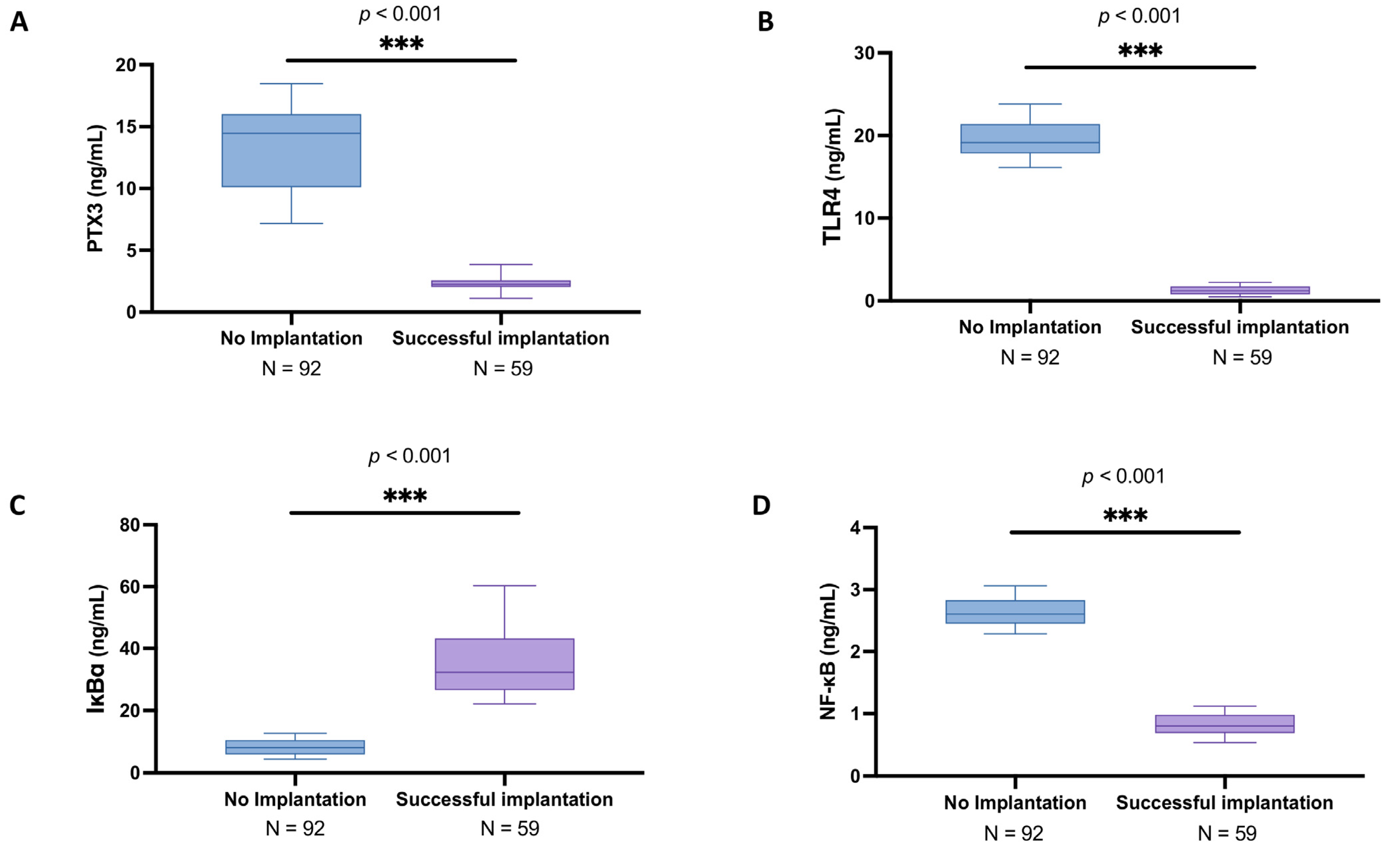
3.2. Analysis of PTX3 Levels and Associated Signaling Pathway in the Follicular Fluid Among Women with and Without Successful Implantation
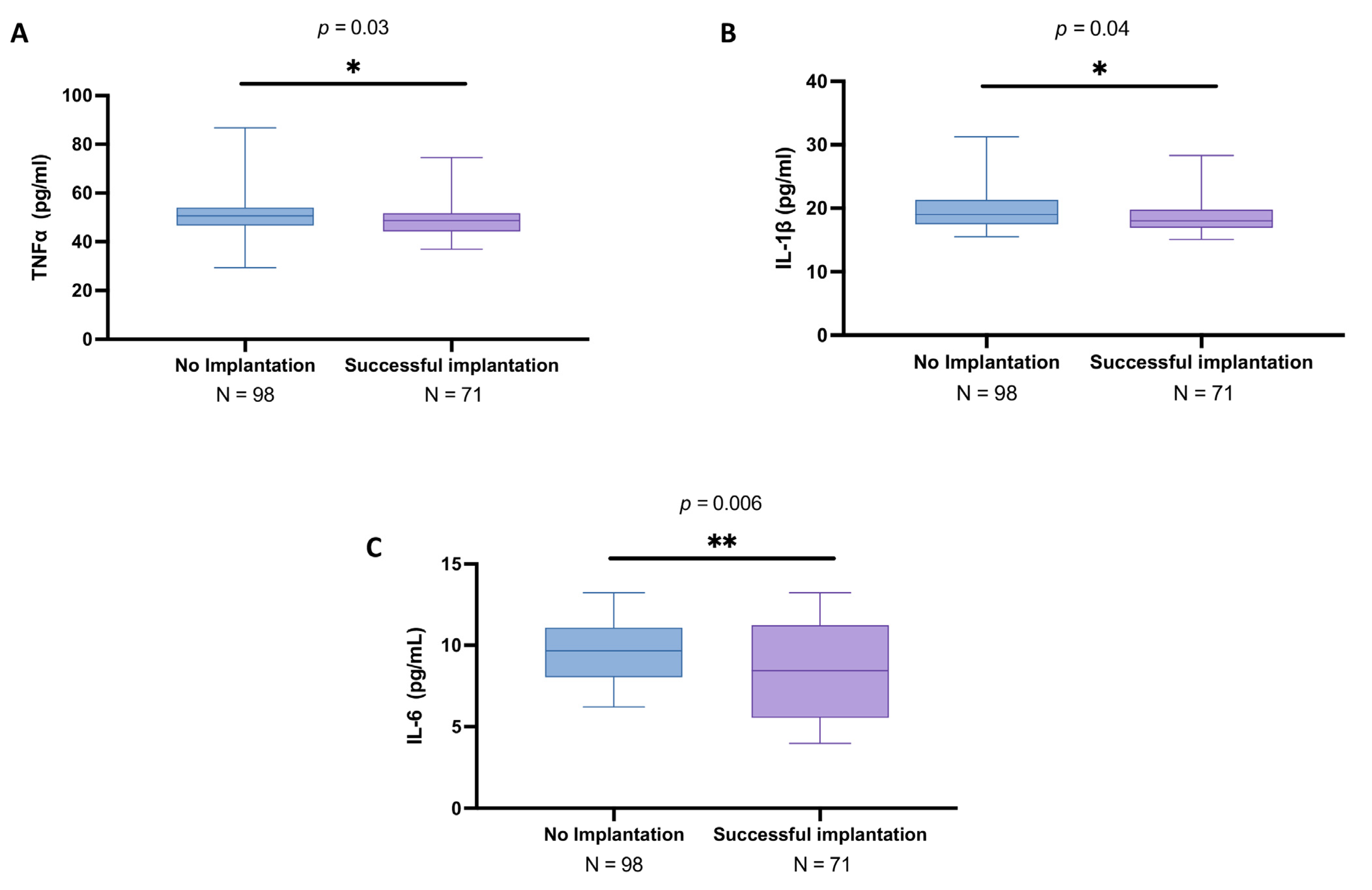
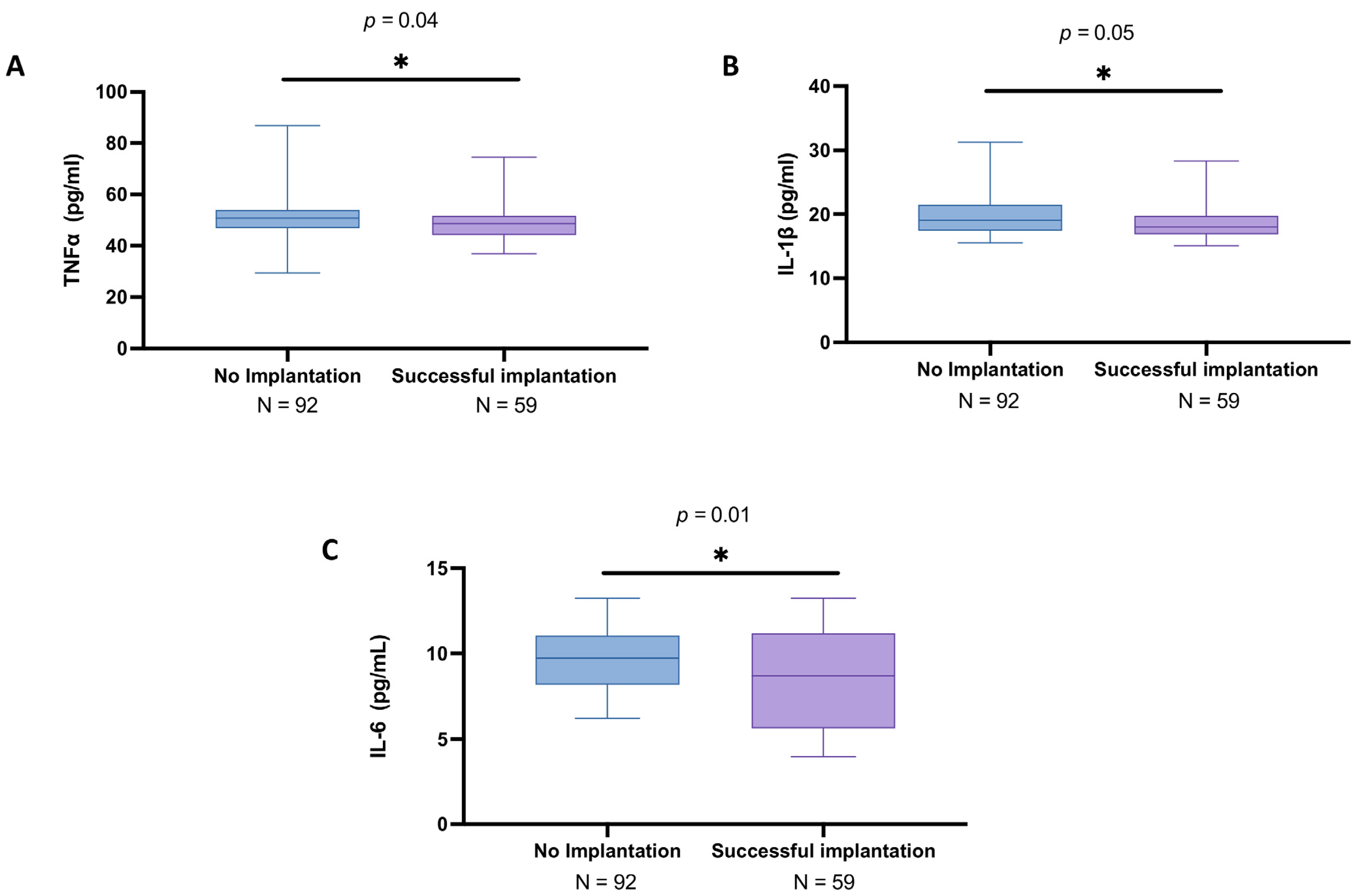
3.3. Analysis of Cytokines in the Follicular Fluid Among Women with and Without Successful Implantation
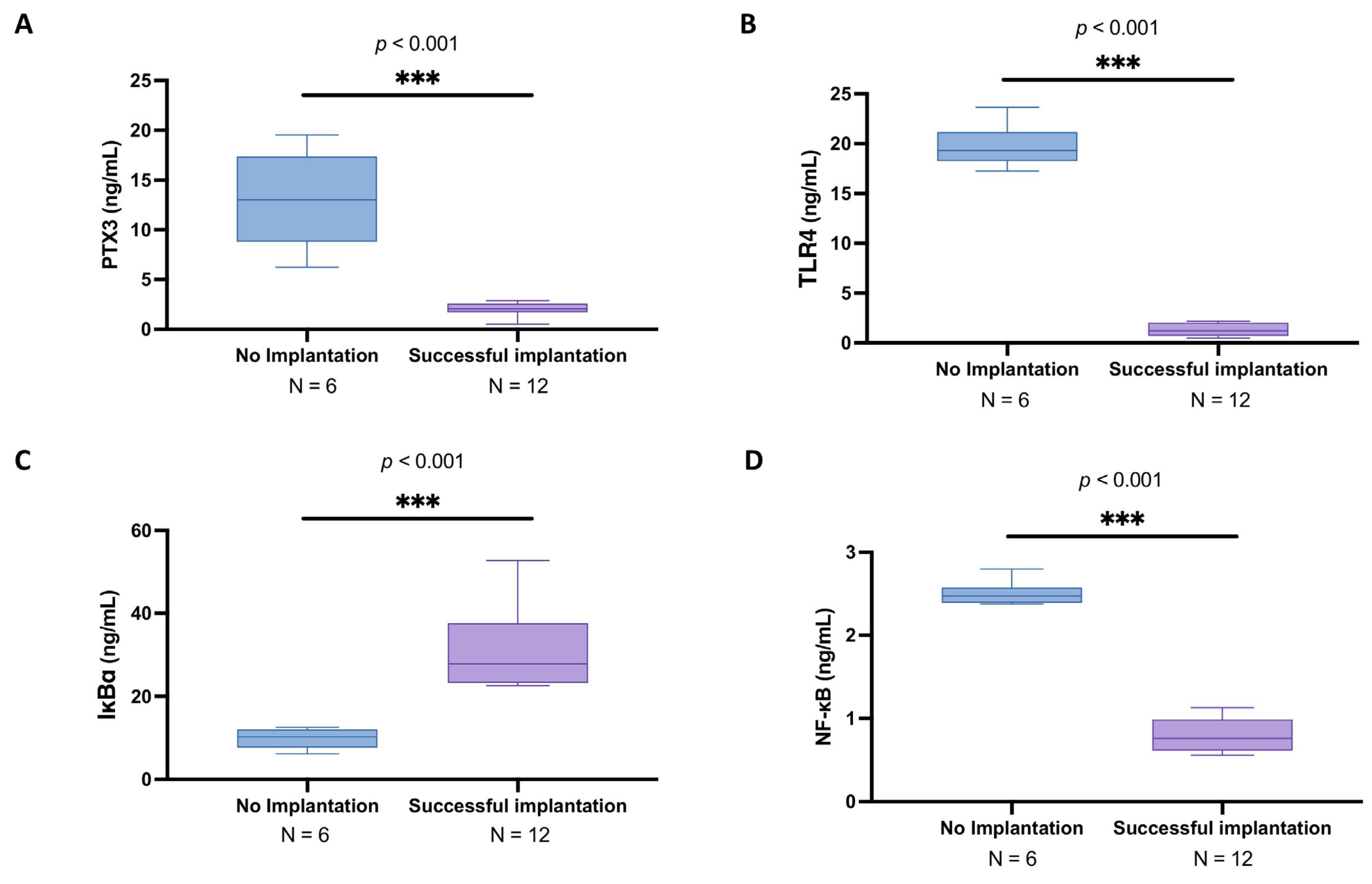
3.4. Subgroup Analysis of PTX3 Levels and Related Signaling Pathway in the Follicular Fluid of Women Under 30 Years Old with and Without Successful Implantation
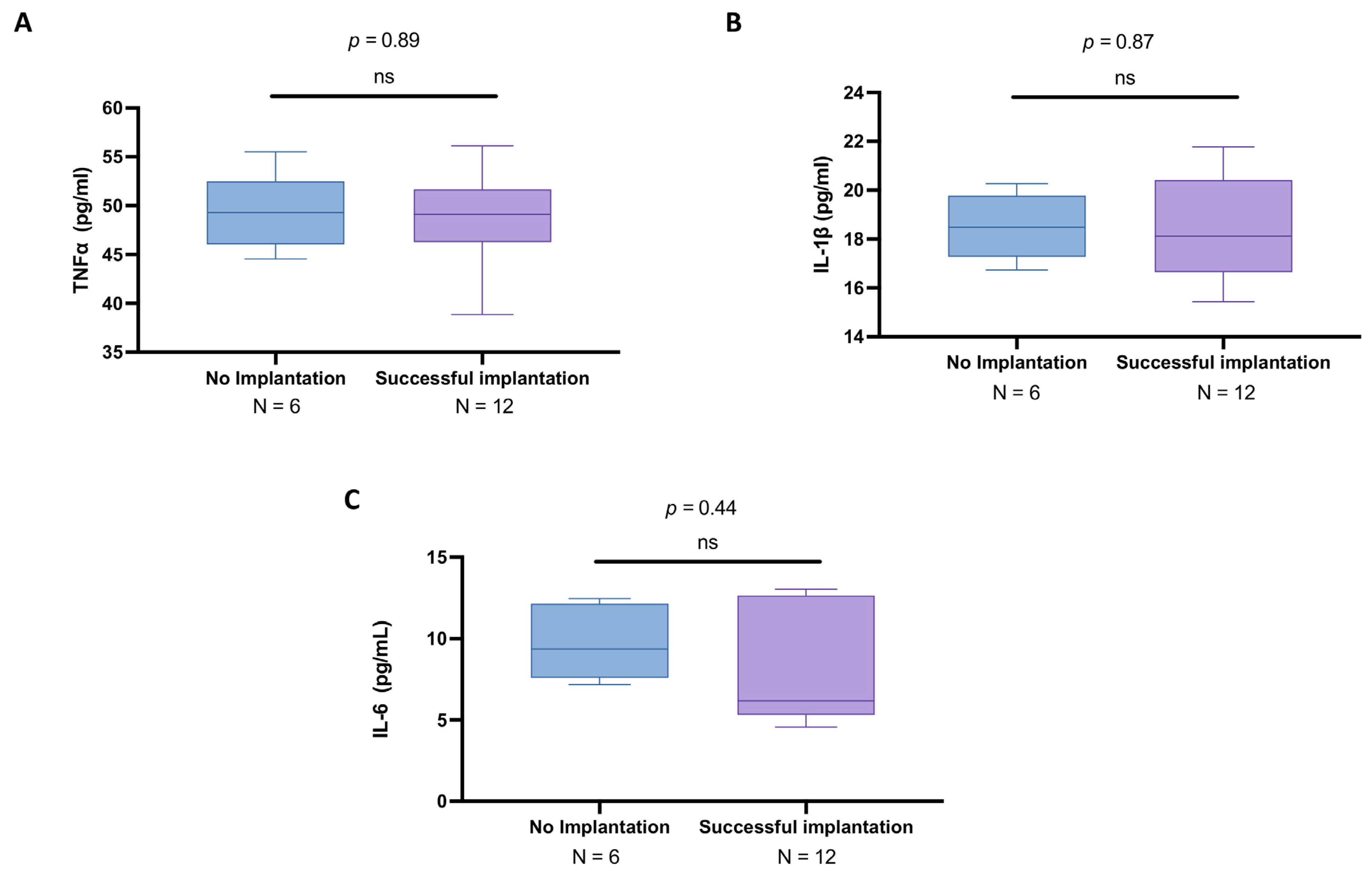
3.5. Subgroup Analysis of Cytokines in the Follicular Fluid of Women Under 30 Years Old with and Without Successful Implantation
3.6. Elevated PTX3 and NF-κB Pathway Alterations in Women over 30 with Unsuccessful Implantation
3.7. Cytokine Profile in Follicular Fluid of Women over 30 Years Old: A Comparison Between IVF Success and Failure
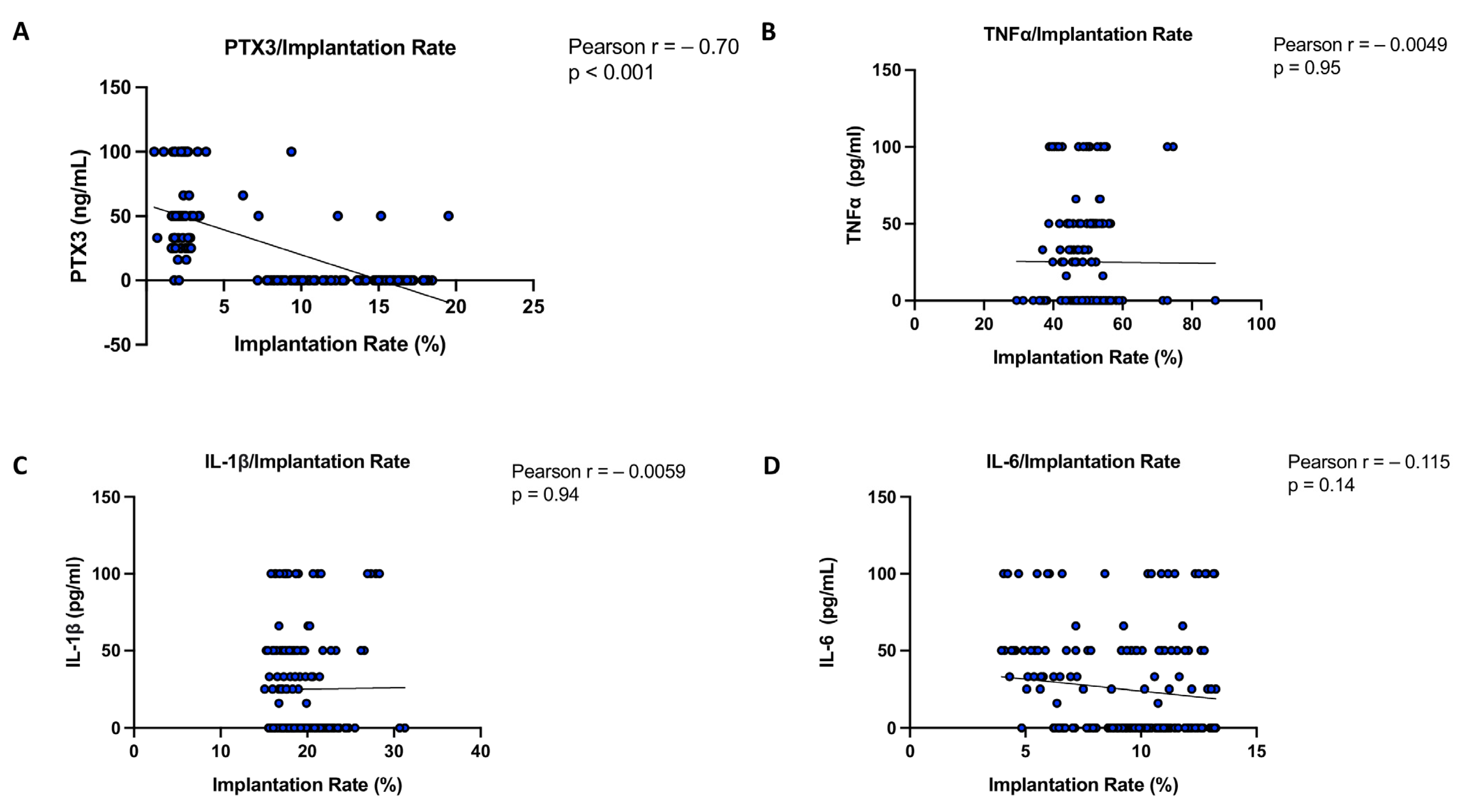
3.8. Correlation Analysis of PTX3 and Cytokines Relating to Implantation Rate in Women with and Without Successful Implantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Artificial reproductive treatment |
| PTX3 | Pentraxin 3 |
| IVF | In vitro fertilization |
| TNFα | Tumor necrosis factor alpha |
| IL | Interleukin |
| CRP | C-reactive protein |
| SAP | Serum amyloid P component |
| TNFαIP6 | Tumor necrosis factor a-induced protein 6 |
| ELISA | Enzyme-linked immunosorbent assays |
| AMH | Anti-Müllerian Hormone |
| AFC | Antral follicle count |
| FSH | Follicle stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| hCG | Human chorionic gonadotropin |
| ICSI | Intracytoplasmic sperm injection |
| BMI | Body mass index |
| SD | Standard deviation |
References
- Freis, A.; Von Horn, K.; Goggl, T.; Hecht, S.; Roesner, S.; Strowitzki, T.; Germeyer, A. Serum levels of Pentraxin 3 differ significantly at the time of blastocyst transfer depending on implantation success: A pilot study. Arch. Gynecol. Obstet. 2018, 297, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Ardizzone, A.; Panto, G.; Paterniti, I.; Campolo, M.; Crupi, L.; Squeri, R.; Esposito, E. The Prognostic Value of Pentraxin-3 in COVID-19 Patients: A Systematic Review and Meta-Analysis of Mortality Incidence. Int. J. Mol. Sci. 2023, 24, 3537. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Klinger, F.G.; Campagnolo, L.; Salustri, A. The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility. Front. Immunol. 2018, 9, 2808. [Google Scholar] [CrossRef] [PubMed]
- Salustri, A.; Garlanda, C.; Hirsch, E.; De Acetis, M.; Maccagno, A.; Bottazzi, B.; Doni, A.; Bastone, A.; Mantovani, G.; Beck Peccoz, P.; et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 2004, 131, 1577–1586. [Google Scholar] [CrossRef]
- Sayutti, N.; Abu, M.A.; Ahmad, M.F. PCOS and Role of Cumulus Gene Expression in Assessing Oocytes Quality. Front. Endocrinol. 2022, 13, 843867. [Google Scholar] [CrossRef]
- Zhang, X.; Jafari, N.; Barnes, R.B.; Confino, E.; Milad, M.; Kazer, R.R. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil. Steril. 2005, 83, 1169–1179. [Google Scholar] [CrossRef]
- McKenzie, L.; Pangas, S.; Carson, S.; Kovanci, E.; Cisneros, P.; Buster, J.; Amato, P.; Matzuk, M. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum. Reprod. 2004, 19, 2869–2874. [Google Scholar] [CrossRef]
- Cillo, F.; Brevini, T.A.; Antonini, S.; Paffoni, A.; Ragni, G.; Gandolfi, F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction 2007, 134, 645–650. [Google Scholar] [CrossRef]
- Kollmann, Z.; Schneider, S.; Fux, M.; Bersinger, N.A.; von Wolff, M. Gonadotrophin stimulation in IVF alters the immune cell profile in follicular fluid and the cytokine concentrations in follicular fluid and serum. Hum. Reprod. 2017, 32, 820–831. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Y.; Zhang, M.; Xia, Y.; Chen, X.; Xian, Y.; Lin, D.; Xie, S.; Guo, X. Oxidative stress and inflammatory markers in ovarian follicular fluid of women with diminished ovarian reserve during in vitro fertilization. J. Ovarian Res. 2023, 16, 206. [Google Scholar] [CrossRef]
- Tarlatzis, B.C.; Zepiridis, L. Perimenopausal conception. Ann. N. Y. Acad. Sci. 2003, 997, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Buckshee, K.; Chatterjee, P.; Dhall, G.I.; Hazra, M.N.; Kodkany, B.S.; Lalitha, K.; Logambal, A.; Manchanda, P.; Nanda, U.K.; RaiChoudhury, G.; et al. Return of fertility following discontinuation of Norplant-II subdermal implants. ICMR Task Force on Hormonal Contraception. Contraception 1995, 51, 237–242. [Google Scholar] [CrossRef]
- Ardizzone, A.; Mannino, D.; Capra, A.P.; Repici, A.; Filippone, A.; Esposito, E.; Campolo, M. New Insights into the Mechanism of Ulva Pertusa on Colitis in Mice: Modulation of the Pain and Immune System. Mar. Drugs 2023, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, K.; Unkila-Kallio, L.; Alfthan, H.; Hamalainen, E.; Tiitinen, A.; Mikkola, T.; Tapanainen, J.; Savolainen-Peltonen, H. Plasma pentraxin 3 is higher in early ovarian hyperstimulation syndrome than in uncomplicated in vitro fertilization cycle of high-risk women. Arch. Gynecol. Obstet. 2020, 301, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, C.; Zhou, Z.; Yang, Z.; Dai, T.; Huang, H.; Jin, L. Elevated ovarian pentraxin 3 in polycystic ovary syndrome. J. Assist. Reprod. Genet. 2021, 38, 1231–1237. [Google Scholar] [CrossRef]
- Amato, G.; Conte, M.; Mazziotti, G.; Lalli, E.; Vitolo, G.; Tucker, A.T.; Bellastella, A.; Carella, C.; Izzo, A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet. Gynecol. 2003, 101, 1177–1182. [Google Scholar] [CrossRef]
- Ben-Shlomo, I.; Wiener-Megnagi, Z.; Golan, J.; Eyali, V.; Geslevich, J.; Shalev, E. Individual implantation rate: Proposal for a new index for evaluation of assisted reproduction results. Fertil. Steril. 1997, 68, 816–819. [Google Scholar] [CrossRef]
- Boots, C.E.; Jungheim, E.S. Inflammation and Human Ovarian Follicular Dynamics. Semin. Reprod. Med. 2015, 33, 270–275. [Google Scholar] [CrossRef]
- Freitas, C.; Neto, A.C.; Matos, L.; Silva, E.; Ribeiro, A.; Silva-Carvalho, J.L.; Almeida, H. Follicular Fluid redox involvement for ovarian follicle growth. J. Ovarian Res. 2017, 10, 44. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, Z.; Fan, H.; Yan, X.; Liu, X.; Xuan, J.; Feng, D.; Wei, X. The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment. Front. Immunol. 2021, 12, 685724. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Wang, Y.; Cai, Q.; Liu, H.; Xu, C.; Zhang, F. IL-15 Participates in the Pathogenesis of Polycystic Ovary Syndrome by Affecting the Activity of Granulosa Cells. Front. Endocrinol. 2022, 13, 787876. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef]
- Marianna, S.; Alessia, P.; Susan, C.; Francesca, C.; Angela, S.; Francesca, C.; Antonella, N.; Patrizia, I.; Nicola, C.; Emilio, C. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst. 2017, 13, 1213–1222. [Google Scholar] [CrossRef]
- Varani, S.; Elvin, J.A.; Yan, C.; DeMayo, J.; DeMayo, F.J.; Horton, H.F.; Byrne, M.C.; Matzuk, M.M. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol. Endocrinol. 2002, 16, 1154–1167. [Google Scholar] [CrossRef]
- Gebhardt, K.M.; Feil, D.K.; Dunning, K.R.; Lane, M.; Russell, D.L. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil. Steril. 2011, 96, 47–52.e2. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Crupi, L.; Panto, G.; Repici, A.; Calapai, F.; Squeri, R.; Ardizzone, A.; Esposito, E. Serum Pentraxin 3 as Promising Biomarker for the Long-Lasting Inflammatory Response of COVID-19. Int. J. Mol. Sci. 2023, 24, 14195. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, R.; Ukleja-Sokołowska, N.; Lis, K.; Dubiel, M. Function of follicular cytokines: Roles played during maturation, development and implantation of embryo. Medicina 2021, 57, 1251. [Google Scholar] [CrossRef]
- Wang, L.J.; Brannstrom, M.; Robertson, S.A.; Norman, R.J. Tumor necrosis factor alpha in the human ovary: Presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil. Steril. 1992, 58, 934–940. [Google Scholar] [CrossRef]
- Terranova, P.F. Potential roles of tumor necrosis factor-alpha in follicular development, ovulation, and the life span of the corpus luteum. Domest. Anim. Endocrinol. 1997, 14, 1–15. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola, K.; Xystra, P.; Pantou, A.; Kokkali, G.; Pappas, A.; Lambropoulou, M.; Sfakianoudis, K.; et al. The Role of Interleukins in Recurrent Implantation Failure: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2022, 23, 2198. [Google Scholar] [CrossRef]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular Signaling Regulating Endometrium-Blastocyst Crosstalk. Int. J. Mol. Sci. 2019, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Dimitriadis, E.; Sharkey, A.M.; Stoikos, C.J.; Salamonsen, L.A. Interleukin 1 beta is induced by interleukin 11 during decidualization of human endometrial stromal cells, but is not released in a bioactive form. J. Reprod. Immunol. 2007, 73, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Equils, O.; Kellogg, C.; McGregor, J.; Gravett, M.; Neal-Perry, G.; Gabay, C. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complicationsdagger. Biol. Reprod. 2020, 103, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Kreines, F.M.; Nasioudis, D.; Minis, E.; Irani, M.; Witkin, S.S.; Spandorfer, S. IL-1beta predicts IVF outcome: A prospective study. J. Assist. Reprod. Genet. 2018, 35, 2031–2035. [Google Scholar] [CrossRef]
- Simon, C.; Mercader, A.; Gimeno, M.J.; Pellicer, A. The interleukin-1 system and human implantation. Am. J. Reprod. Immunol. 1997, 37, 64–72. [Google Scholar] [CrossRef]
- Simon, C.; Frances, A.; Piquette, G.N.; el Danasouri, I.; Zurawski, G.; Dang, W.; Polan, M.L. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology 1994, 134, 521–528. [Google Scholar] [CrossRef]
- Kruessel, J.S.; Huang, H.Y.; Wen, Y.; Kloodt, A.R.; Bielfeld, P.; Polan, M.L. Different pattern of interleukin-1 beta-(IL-1 beta), interleukin-1 receptor antagonist- (IL-1ra) and interleukin-1 receptor type I- (IL-1R tI) mRNA-expression in single preimplantation mouse embryos at various developmental stages. J. Reprod. Immunol. 1997, 34, 103–120. [Google Scholar] [CrossRef]
- Simon, C.; Frances, A.; Piquette, G.; Hendrickson, M.; Milki, A.; Polan, M.L. Interleukin-1 system in the materno-trophoblast unit in human implantation: Immunohistochemical evidence for autocrine/paracrine function. J. Clin. Endocrinol. Metab. 1994, 78, 847–854. [Google Scholar] [CrossRef]
- Gavrilovic, A.Z.S.; Cekovic, J.M.; Parandilovic, A.Z.; Nikolov, A.B.; Sazdanovic, P.S.; Velickovic, A.M.; Andjelkovic, M.V.; Sorak, M.P. IL-6 of follicular fluid and outcome of in vitro fertilization. Medicine 2022, 101, e29624. [Google Scholar] [CrossRef]
- Dai, M.; Hong, L.; Yin, T.; Liu, S. Disturbed Follicular Microenvironment in Polycystic Ovary Syndrome: Relationship to Oocyte Quality and Infertility. Endocrinology 2024, 165, bqae023. [Google Scholar] [CrossRef]
- Wang, C.; Ng, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beer, A.; Beaman, K. Increased tumor necrosis factor-alpha level in infertility patient. Clin. Appl. Immunol. Rev. 2002, 3, 6. [Google Scholar] [CrossRef]
- Zeybek, S.; Tepeli, E.; Cetin, G.O.; Caner, V.; Senol, H.; Yildirim, B.; Bagci, G. Increased Expression of Pentraxin 3 in Placental Tissues from Patients with Unexplained Recurrent Pregnancy Loss. Balkan J. Med Genet. 2019, 22, 21–28. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; Liu, Z. Molecular insight into pentraxin-3: Update advances in innate immunity, inflammation, tissue remodeling, diseases, and drug role. Biomed. Pharmacother. 2022, 156, 113783. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Pan, M.; Sheng, Y.; Jia, E.; Wang, Y.; Dong, J.; Tu, J.; Bai, Y.; Cai, L.; Ge, Q. Extracellular cell-free RNA profile in human large follicles and small follicles. Front. Cell. Dev. Biol. 2022, 10, 940336. [Google Scholar] [CrossRef]
- Casteleiro Alves, M.M.; Oliani, L.; Almeida, M.; Cardoso, H.J.; Oliani, A.H.; Breitenfeld, L.; Ramalhinho, A.C. Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis. Diagnostics 2023, 13, 208. [Google Scholar] [CrossRef]
- Ardizzone, A.; Capra, A.P.; Mondello, S.; Briuglia, S.; La Rosa, M.A.; Campolo, M.; Esposito, E. H1299R Variant in Factor V and Recurrent Pregnancy Loss: A Systematic Review and Meta-Analysis Protocol. Genes 2022, 13, 1019. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Ardizzone, A.; Briuglia, S.; La Rosa, M.A.; Mondello, S.; Campolo, M.; Esposito, E. A Systematic Review and Meta-Analysis of the Association Between the FV H1299R Variant and the Risk of Recurrent Pregnancy Loss. Biology 2022, 11, 1608. [Google Scholar] [CrossRef]
- Hamed, S.; Behnes, M.; Pauly, D.; Lepiorz, D.; Barre, M.; Becher, T.; Lang, S.; Akin, I.; Borggrefe, M.; Bertsch, T.; et al. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect. Dis. 2017, 17, 554. [Google Scholar] [CrossRef]
| No Implantation (n = 98) | Successful Implantation (n = 71) | p-Value (t-Test) | |
|---|---|---|---|
| Mean age | 34.7 ± 3.16 | 34.4 ± 3.56 | 0.56 |
| BMI (kg/m2) | 23.1 ± 2.9 | 23.0 ± 1.9 | 0.80 |
| Smokers | 30/98 (30.61%) | 20/71 (28.17%) | |
| Male factor | 32/98 (32.65%) | 44/71 (61.97%) | |
| GnRH long protocol | 14/98 (14.29%) | 3/71 (4.23%) | |
| Oocytes retrieved | 4.85 ± 3.24 | 5.9 ± 4.00 | 0.06 |
| Embryos formed | 2.40 ± 1.81 | 2.9 ± 1.63 | 0.07 |
| Embryos transferred into each patient | 2.05 ± 1.25 | 2.38 ± 1.17 | 0.08 |
| Number of attempts | 1.17 ± 0.65 | 1.35 ± 0.56 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardizzone, A.; Liuzzo, C.; Ferro, A.; Galletta, M.; Esposito, E.; Capra, A.P. PTX3/NF-κB/TLR4 Pathway Evaluation in the Follicular Fluid to Successfully Predict Blastocyst Implantation: A Pilot Study. Biomedicines 2025, 13, 1071. https://doi.org/10.3390/biomedicines13051071
Ardizzone A, Liuzzo C, Ferro A, Galletta M, Esposito E, Capra AP. PTX3/NF-κB/TLR4 Pathway Evaluation in the Follicular Fluid to Successfully Predict Blastocyst Implantation: A Pilot Study. Biomedicines. 2025; 13(5):1071. https://doi.org/10.3390/biomedicines13051071
Chicago/Turabian StyleArdizzone, Alessio, Carmelo Liuzzo, Arianna Ferro, Marco Galletta, Emanuela Esposito, and Anna Paola Capra. 2025. "PTX3/NF-κB/TLR4 Pathway Evaluation in the Follicular Fluid to Successfully Predict Blastocyst Implantation: A Pilot Study" Biomedicines 13, no. 5: 1071. https://doi.org/10.3390/biomedicines13051071
APA StyleArdizzone, A., Liuzzo, C., Ferro, A., Galletta, M., Esposito, E., & Capra, A. P. (2025). PTX3/NF-κB/TLR4 Pathway Evaluation in the Follicular Fluid to Successfully Predict Blastocyst Implantation: A Pilot Study. Biomedicines, 13(5), 1071. https://doi.org/10.3390/biomedicines13051071









