Route of Application and Dose Evaluation of Dental Pulp Stem Cells for the Treatment of Sialadenitis Caused by Sjögren’s Syndrome: A Preclinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Experiments
2.3. DPSC Treatment
2.4. DPSC Tracking
2.5. Stimulated SFR Measurement
2.6. Histological Evaluation of SMG
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results
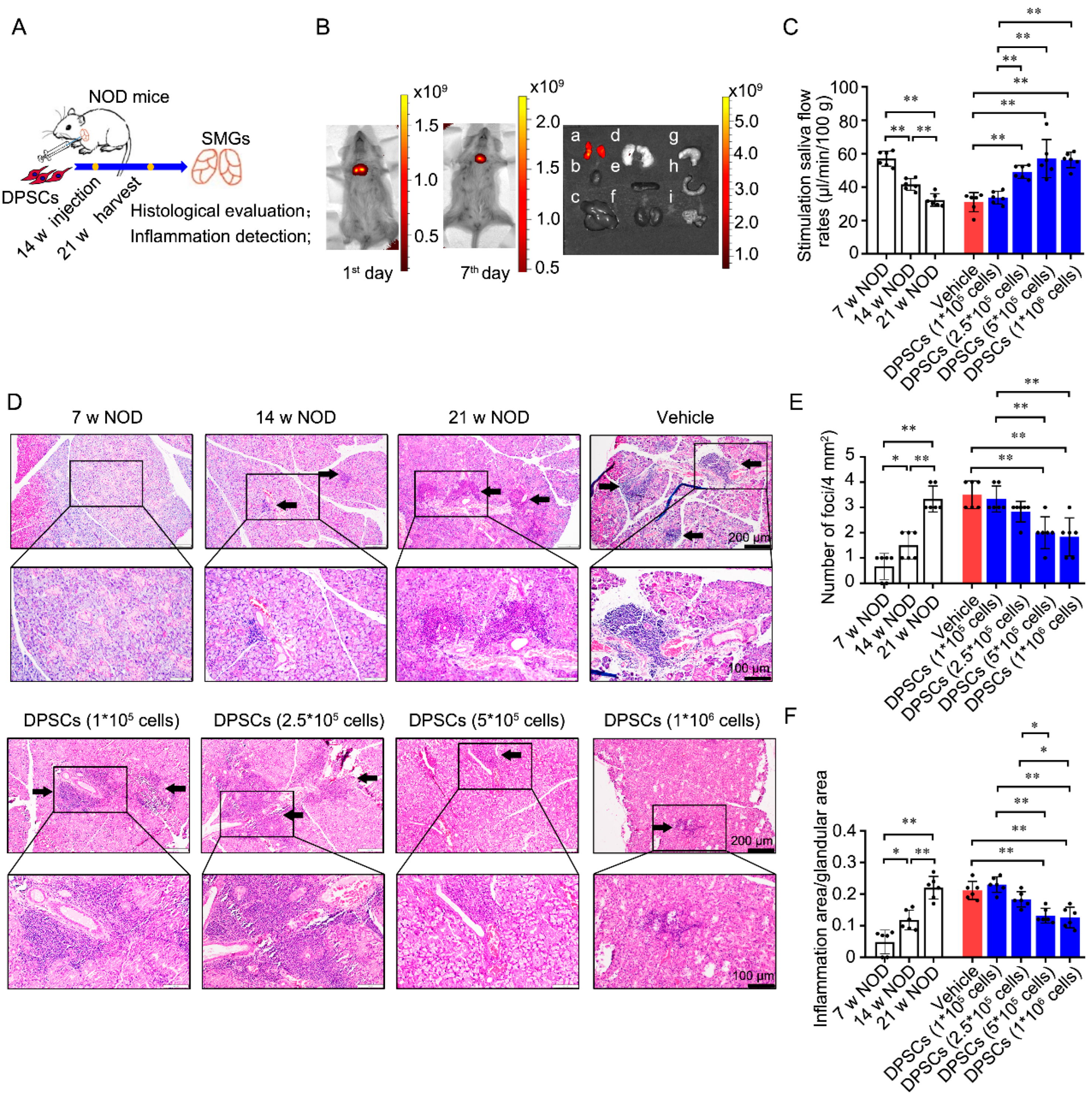
3.1. Injection of DPSCs into the SMG Promotes Saliva Secretion and Ameliorates Ectopic Lymphocyte Infiltration into the Glandular Tissues of NOD Mice
3.2. Injection of DPSCs into the SMG Decreases CD4+ Cell Infiltration and the Levels of SS-Specific Inflammatory Cytokines in the Glandular Tissues of NOD Mice
3.3. Intraductal Perfusion of DPSCs into the SMG Restores Saliva Secretion and Ameliorates Ectopic Lymphocyte Infiltration into the Glandular Tissues of NOD Mice
3.4. Comparison of the Two Application Routes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Margaretten, M. Neurologic Manifestations of Primary Sjögren Syndrome. Rheum. Dis. Clin. N. Am. 2017, 43, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, S.; Li, Q.; Wang, Y.H.; Zhao, J.L.; Li, M.T.; Zhao, Y.; Zeng, X.F. Characteristics of Chinese patients with primary Sjögren’s syndrome: Preliminary report of a multi-centre registration study. Lupus 2020, 29, 45–51. [Google Scholar] [CrossRef]
- Vivino, F.B.; Bunya, V.Y.; Massaro-Giordano, G.; Johr, C.R.; Giattino, S.L.; Schorpion, A.; Shafer, B.; Peck, A.; Sivils, K.; Rasmussen, A.; et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clin. Immunol. 2019, 203, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef]
- Srivastava, A.; Makarenkova, H.P. Innate Immunity and Biological Therapies for the Treatment of Sjögren’s Syndrome. Int. J. Mol. Sci. 2020, 21, 9172. [Google Scholar] [CrossRef]
- Carsons, S.E.; Vivino, F.B.; Parke, A.; Carteron, N.; Sankar, V.; Brasington, R.; Brennan, M.T.; Ehlers, W.; Fox, R.; Scofield, H.; et al. Treatment Guidelines for Rheumatologic Manifestations of Sjögren’s Syndrome: Use of Biologic Agents, Management of Fatigue, and Inflammatory Musculoskeletal Pain. Arthritis Care Res. 2017, 69, 517–527. [Google Scholar] [CrossRef]
- Price, E.J.; Rauz, S.; Tappuni, A.R.; Sutcliffe, N.; Hackett, K.L.; Barone, F.; Granata, G.; Ng, W.F.; Fisher, B.A.; Bombardieri, M.; et al. The British Society for Rheumatology guideline for the management of adults with primary Sjögren’s Syndrome. Rheumatology 2017, 56, 1643–1647. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Guillevin, L.; Lambotte, O.; Combe, B.; Allanore, Y.; Cantagrel, A.; Larroche, C.; Soubrier, M.; Bouillet, L.; Dougados, M.; et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann. Rheum. Dis. 2005, 64, 913–920. [Google Scholar] [CrossRef]
- Meijer, J.M.; Meiners, P.M.; Vissink, A.; Spijkervet, F.K.; Abdulahad, W.; Kamminga, N.; Brouwer, E.; Kallenberg, C.G.; Bootsma, H. Effectiveness of rituximab treatment in primary Sjögren’s syndrome: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 960–968. [Google Scholar] [CrossRef]
- Bowman, S.J.; Everett, C.C.; O’Dwyer, J.L.; Emery, P.; Pitzalis, C.; Ng, W.F.; Pease, C.T.; Price, E.J.; Sutcliffe, N.; Gendi, N.S.T.; et al. Randomized Controlled Trial of Rituximab and Cost-Effectiveness Analysis in Treating Fatigue and Oral Dryness in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2017, 69, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Devauchelle-Pensec, V.; Mariette, X.; Jousse-Joulin, S.; Berthelot, J.M.; Perdriger, A.; Puéchal, X.; Le Guern, V.; Sibilia, J.; Gottenberg, J.E.; Chiche, L.; et al. Treatment of primary Sjögren syndrome with rituximab: A randomized trial. Ann. Intern. Med. 2014, 160, 233–242. [Google Scholar] [CrossRef] [PubMed]
- de Wolff, L.; van Nimwegen, J.F.; Mossel, E.; van Zuiden, G.S.; Stel, A.J.; Majoor, K.I.; Olie, L.; Los, L.I.; Vissink, A.; Spijkervet, F.K.L.; et al. Long-term abatacept treatment for 48 weeks in patients with primary Sjögren’s syndrome: The open-label extension phase of the ASAP-III trial. Semin. Arthritis Rheum. 2022, 53, 151955. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, L.; Li, X. Composition and regulation of the immune microenvironment of salivary gland in Sjögren’s syndrome. Front. Immunol. 2022, 13, 967304. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, J.; Miao, M.; Zhang, R.; Cheng, G.; Wang, Y.; Feng, R.; Huang, B.; Luan, H.; Jia, Y.; et al. Efficacy and Safety of Low-Dose Interleukin 2 for Primary Sjögren Syndrome: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2241451. [Google Scholar] [CrossRef]
- Bentley, D.; Fisher, B.A.; Barone, F.; Kolb, F.A.; Attley, G. A randomized, double-blind, placebo-controlled, parallel group study on the effects of a cathepsin S inhibitor in primary Sjögren’s syndrome. Rheumatology 2023, 62, 3644–3653. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Liu, D.; Fan, Z.; Zhang, H.; Liu, O.; Ding, G.; Gao, R.; Zhang, C.; Ding, Y.; et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood 2012, 120, 3142–3151. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Wang, S.; Guo, J.; Guo, J.; Fu, J.; Ren, L.; An, Y.; He, J.; Li, Z. Human umbilical cord mesenchymal stem cells confer potent immunosuppressive effects in Sjögren’s syndrome by inducing regulatory T cells. Mod. Rheumatol. 2021, 31, 186–196. [Google Scholar] [CrossRef]
- Li, B.; Xing, Y.; Gan, Y.; He, J.; Hua, H. Labial gland-derived mesenchymal stem cells and their exosomes ameliorate murine Sjögren’s syndrome by modulating the balance of Treg and Th17 cells. Stem Cell Res. Ther. 2021, 12, 478. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Liu, H.; Gronthos, S.; Shi, S. Dental pulp stem cells. Methods Enzymol. 2006, 419, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, I.; Cerqueni, G.; Licini, C.; Lucarini, G.; Mattioli Belmonte, M. Dental pulp stem cells senescence and regenerative potential relationship. J. Cell. Physiol. 2019, 234, 7186–7197. [Google Scholar] [CrossRef]
- Osorio, R.; Rodríguez-Lozano, F.J.; Toledano, M.; Toledano-Osorio, M.; García-Bernal, D.; Murcia, L.; López-García, S. Mitigating lipopolysaccharide-induced impairment in human dental pulp stem cells with tideglusib-doped nanoparticles: Enhancing osteogenic differentiation and mineralization. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2024, 40, 1591–1601. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, Q.Z.; Karabucak, B.; Le, A.D. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-α/IDO Axis. J. Dent. Res. 2016, 95, 1274–1281. [Google Scholar] [CrossRef]
- Zayed, M.; Iohara, K. Immunomodulation and Regeneration Properties of Dental Pulp Stem Cells: A Potential Therapy to Treat Coronavirus Disease 2019. Cell Transplant. 2020, 29, 963689720952089. [Google Scholar] [CrossRef]
- Du, Z.H.; Ding, C.; Zhang, Q.; Zhang, Y.; Ge, X.Y.; Li, S.L.; Yu, G.Y. Stem cells from exfoliated deciduous teeth alleviate hyposalivation caused by Sjögren syndrome. Oral Dis. 2019, 25, 1530–1544. [Google Scholar] [CrossRef]
- Földes, A.; Kádár, K.; Kerémi, B.; Zsembery, Á.; Gyires, K.; Zádori, Z.S.; Varga, G. Mesenchymal Stem Cells of Dental Origin-Their Potential for Antiinflammatory and Regenerative Actions in Brain and Gut Damage. Curr. Neuropharmacol. 2016, 14, 914–934. [Google Scholar] [CrossRef] [PubMed]
- Lo Monaco, M.; Gervois, P.; Beaumont, J.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.M.; Lambrichts, I. Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells 2020, 9, 980. [Google Scholar] [CrossRef]
- Ishikawa, J.; Takahashi, N.; Matsumoto, T.; Yoshioka, Y.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Ishigro, N.; Ueda, M.; Furukawa, K.; et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone 2016, 83, 210–219. [Google Scholar] [CrossRef]
- Min, Q.; Yang, L.; Tian, H.; Tang, L.; Xiao, Z.; Shen, J. Immunomodulatory Mechanism and Potential Application of Dental Pulp-Derived Stem Cells in Immune-Mediated Diseases. Int. J. Mol. Sci. 2023, 24, 8068. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 130. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, S.J.; Meng, H.F.; Xu, H.Q.; Guo, Z.X.; Yan, J.F.; Gao, J.L.; Niu, L.N.; Wang, S.L.; Jiao, K. DPSCs regulate epithelial-T cell interactions in oral submucous fibrosis. Stem Cell Res. Ther. 2024, 15, 113. [Google Scholar] [CrossRef]
- Humphreys-Beher, M.G.; Hu, Y.; Nakagawa, Y.; Wang, P.L.; Purushotham, K.R. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjögren’s syndrome. Adv. Exp. Med. Biol. 1994, 350, 631–636. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Zhang, Z.; Yu, X.; Zheng, J. Recent Advances in Mouse Models of Sjögren’s Syndrome. Front. Immunol. 2020, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, J.; Yang, J.; Ge, Y.; Tian, J. Effects of iguratimod on inflammatory factors and apoptosis of submandibular gland epithelial cells in NOD mice. Sci. Rep. 2023, 13, 18205. [Google Scholar] [CrossRef]
- Yamano, S.; Atkinson, J.C.; Baum, B.J.; Fox, P.C. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin. Immunol. 1999, 92, 265–275. [Google Scholar] [CrossRef]
- Su, Y.C.; Xiang, R.L.; Zhang, Y.; Ding, C.; Cong, X.; Guo, X.H.; Yang, N.Y.; Hua, H.; Wu, L.L.; Yu, G.Y. Decreased submandibular adiponectin is involved in the progression of autoimmune sialoadenitis in non-obese diabetic mice. Oral Dis. 2014, 20, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.X.; Ding, C.; Du, Z.H.; Wei, P.; Wang, Y.X.; Ge, X.J.; Yu, G.Y. SHED-exos promote saliva secretion by suppressing p-ERK1/2-mediated apoptosis in glandular cells. Oral Dis. 2024, 30, 3066–3080. [Google Scholar] [CrossRef]
- Du, Z.; Wei, P.; Jiang, N.; Wu, L.; Ding, C.; Yu, G. SHED-derived exosomes ameliorate hyposalivation caused by Sjögren’s syndrome via Akt/GSK-3β/Slug-mediated ZO-1 expression. Chin. Med. J. 2023, 136, 2596–2608. [Google Scholar] [CrossRef]
- Du, Z.H.; Chu, W.X.; Peng, X.; Wu, L.L.; Liu, Y.; Yu, G.Y.; Ding, C. SHED-Derived Exosomes Ameliorate Sjögren’s Syndrome-Induced Hyposalivation by Suppressing Th1 Cell Response via the miR-29a-3p/T-bet Axis. ACS Appl. Mater. Interfaces 2025, 17, 5752–5761. [Google Scholar] [CrossRef] [PubMed]
- Mitsias, D.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. The role of epithelial cells in the initiation and perpetuation of autoimmune lesions: Lessons from Sjogren’s syndrome (autoimmune epithelitis). Lupus 2006, 15, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I. The salivary gland epithelial cell in Sjogren’s Syndrome: What are the steps involved in wounding or killing their secretory function? J. Rheumatol. 2012, 39, 1117–1119. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, J.F.; Chang, C.; Xu, T.; Gao, C.Y.; Gershwin, M.E.; Lian, Z.X. Immunobiology of T Cells in Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2021, 60, 111–131. [Google Scholar] [CrossRef]
- Keller, C.W.; Adamopoulos, I.E.; Lünemann, J.D. Autophagy pathways in autoimmune diseases. J. Autoimmun. 2023, 136, 103030. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Yin, J.; Fu, J.; Yang, X.; Wang, B.; Yu, C.; Zheng, L.; Zhang, Z. Lactate-induced mtDNA Accumulation Activates cGAS-STING Signaling and the Inflammatory Response in Sjögren’s Syndrome. Int. J. Med. Sci. 2023, 20, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.V.; Hopkins, A.M.; Nusrat, A. Modulation of tight junction structure and function by cytokines. Adv. Drug Deliv. Rev. 2000, 41, 303–313. [Google Scholar] [CrossRef]
- Ewert, P.; Aguilera, S.; Alliende, C.; Kwon, Y.J.; Albornoz, A.; Molina, C.; Urzúa, U.; Quest, A.F.; Olea, N.; Pérez, P.; et al. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010, 62, 1280–1289. [Google Scholar] [CrossRef]
- Janebodin, K.; Horst, O.V.; Ieronimakis, N.; Balasundaram, G.; Reesukumal, K.; Pratumvinit, B.; Reyes, M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS ONE 2011, 6, e27526. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tsuneto, M.; Yoshino, M.; Yamamura, K.; Hayashi, S. Potential of dental mesenchymal cells in developing teeth. Stem Cells 2007, 25, 78–87. [Google Scholar] [CrossRef]
- Sui, B.; Wu, D.; Xiang, L.; Fu, Y.; Kou, X.; Shi, S. Dental Pulp Stem Cells: From Discovery to Clinical Application. J. Endod. 2020, 46, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Sang, Y.; Zhang, F.; Liu, Z.; Qi, N.; Chen, Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016, 2016, 3516574. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Racz, G.Z.; Kadar, K.; Foldes, A.; Kallo, K.; Perczel-Kovach, K.; Keremi, B.; Nagy, A.; Varga, G. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 327–339. [Google Scholar]
- Mora, A.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Ghilotti, J.; Lozano, A.; López-García, S. Biocompatibility and osteogenic potential of novel tricalcium silicate-based materials in human dental pulp stem cells: Advancing vital pulp therapies. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2025, in press. [CrossRef]
- Zhao, Y.; Wang, L.; Jin, Y.; Shi, S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J. Dent. Res. 2012, 91, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Prateeksha, P.; Das, H. Dental Pulp-Derived Stem Cells Reduce Inflammation, Accelerate Wound Healing and Mediate M2 Polarization of Myeloid Cells. Biomedicines 2022, 10, 1999. [Google Scholar] [CrossRef]
- Ogasawara, N.; Kano, F.; Hashimoto, N.; Mori, H.; Liu, Y.; Xia, L.; Sakamaki, T.; Hibi, H.; Iwamoto, T.; Tanaka, E.; et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthr. Cartil. 2020, 28, 831–841. [Google Scholar] [CrossRef]
- Chen, Y.R.; Lai, P.L.; Chien, Y.; Lee, P.H.; Lai, Y.H.; Ma, H.I.; Shiau, C.Y.; Wang, K.C. Improvement of Impaired Motor Functions by Human Dental Exfoliated Deciduous Teeth Stem Cell-Derived Factors in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3807. [Google Scholar] [CrossRef]
- Chen, T.F.; Chen, K.W.; Chien, Y.; Lai, Y.H.; Hsieh, S.T.; Ma, H.Y.; Wang, K.C.; Shiau, C.Y. Dental Pulp Stem Cell-Derived Factors Alleviate Subarachnoid Hemorrhage-Induced Neuroinflammation and Ischemic Neurological Deficits. Int. J. Mol. Sci. 2019, 20, 3747. [Google Scholar] [CrossRef]
- Li, X.X.; Yuan, X.J.; Zhai, Y.; Yu, S.; Jia, R.X.; Yang, L.P.; Ma, Z.Z.; Zhao, Y.M.; Wang, Y.X.; Ge, L.H. Treatment with Stem Cells from Human Exfoliated Deciduous Teeth and Their Derived Conditioned Medium Improves Retinal Visual Function and Delays the Degeneration of Photoreceptors. Stem Cells Dev. 2019, 28, 1514–1526. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, T.; Sun, H.; Lin, R.; Zheng, X.; Bian, Q.; Zhang, J.; Chen, S.; Wu, H.; Xu, D.; et al. High Targeting Specificity toward Pulmonary Inflammation Using Mesenchymal Stem Cell-Hybrid Nanovehicle for an Efficient Inflammation Intervention. Adv. Healthc. Mater. 2023, 12, e2300376. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef]
- Chen, X.; Aqrawi, L.A.; Utheim, T.P.; Tashbayev, B.; Utheim, Ø.A.; Reppe, S.; Hove, L.H.; Herlofson, B.B.; Singh, P.B.; Palm, Ø.; et al. Elevated cytokine levels in tears and saliva of patients with primary Sjögren’s syndrome correlate with clinical ocular and oral manifestations. Sci. Rep. 2019, 9, 7319. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Meng, S.; Tang, D.; Wang, T.; Ding, L.; Yu, H.; Li, H.; Liu, D.; Dai, Y.; Yang, M. Single-Cell RNA Sequencing Reveals the Expansion of Cytotoxic CD4+ T Lymphocytes and a Landscape of Immune Cells in Primary Sjögren’s Syndrome. Front. Immunol. 2020, 11, 594658. [Google Scholar] [CrossRef] [PubMed]
- Dörner, T.; Hucko, M.; Mayet, W.J.; Trefzer, U.; Burmester, G.R.; Hiepe, F. Enhanced membrane expression of the 52 kDa Ro(SS-A) and La(SS-B) antigens by human keratinocytes induced by TNF alpha. Ann. Rheum. Dis. 1995, 54, 904–909. [Google Scholar] [CrossRef]
- Baker, O.J.; Camden, J.M.; Redman, R.S.; Jones, J.E.; Seye, C.I.; Erb, L.; Weisman, G.A. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am. J. physiology. Cell Physiol. 2008, 295, C1191–C1201. [Google Scholar] [CrossRef] [PubMed]
- Karabiyik, A.; Peck, A.B.; Nguyen, C.Q. The important role of T cells and receptor expression in Sjögren’s syndrome. Scand. J. Immunol. 2013, 78, 157–166. [Google Scholar] [CrossRef]
- James, J.A.; Guthridge, J.M.; Chen, H.; Lu, R.; Bourn, R.L.; Bean, K.; Munroe, M.E.; Smith, M.; Chakravarty, E.; Baer, A.N.; et al. Unique Sjögren’s syndrome patient subsets defined by molecular features. Rheumatology 2020, 59, 860–868. [Google Scholar] [CrossRef]
- Rizzo, C.; Grasso, G.; Destro Castaniti, G.M.; Ciccia, F.; Guggino, G. Primary Sjogren Syndrome: Focus on Innate Immune Cells and Inflammation. Vaccines 2020, 8, 272. [Google Scholar] [CrossRef] [PubMed]




| DPSC Injection | 1 × 105 Cells | 2.5 × 105 Cells | 5 × 105 Cells | 1 × 106 Cells |
|---|---|---|---|---|
| Increase the stimulated SFR | No | Yes | Yes | Yes |
| Decrease the lesion score | No | No | Yes | Yes |
| Decrease the lesion ratio | No | No | Yes | Yes |
| Alleviate the infiltration of CD4+ cells | No | Yes | Yes | Yes |
| Reduce the level of TNF-α | No | Yes | Yes | Yes |
| Reduce the level of IFN-γ | No | No | Yes | Yes |
| DPSC Perfusion | 7.5 × 104 Cells | 1 × 105 Cells | 2 × 105 Cells | 3 × 105 Cells | 4 × 105 Cells |
|---|---|---|---|---|---|
| Increase the stimulated SFR | No | Yes | Yes | Yes | Yes |
| Decrease the lesion score | No | Yes | Yes | Yes | Yes |
| Decrease the lesion ratio | No | Yes | Yes | Yes | Yes |
| Alleviate the infiltration of CD4+ cells | No | No | Yes | Yes | Yes |
| Reduce the level of TNF-α | No | Yes | Yes | No | No |
| Reduce the level of IFN-γ | No | No | Yes | Yes | No |
| 5 × 105 injection vs. 2 × 105 perfusion | |
| Application routes’ advantage | 2 × 105 perfusion is non-invasive |
| Increase the stimulated SFR | No statistical difference |
| Decrease the lesion score | No statistical difference |
| Decrease the lesion ratio | 2 × 105 perfusion is better |
| Alleviate the infiltration of CD4+ cells | 5 × 105 injection is better |
| Reduce the level of TNF-α | No statistical difference |
| Reduce the level of IFN-γ | 5 × 105 injection is better |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Feng, L.; Zhang, Y.; Peng, X.; Zhang, S.; Zhao, R.; Lei, J.; Li, X.; Yu, G.; Ding, C. Route of Application and Dose Evaluation of Dental Pulp Stem Cells for the Treatment of Sialadenitis Caused by Sjögren’s Syndrome: A Preclinical Study. Biomedicines 2025, 13, 1068. https://doi.org/10.3390/biomedicines13051068
Du Z, Feng L, Zhang Y, Peng X, Zhang S, Zhao R, Lei J, Li X, Yu G, Ding C. Route of Application and Dose Evaluation of Dental Pulp Stem Cells for the Treatment of Sialadenitis Caused by Sjögren’s Syndrome: A Preclinical Study. Biomedicines. 2025; 13(5):1068. https://doi.org/10.3390/biomedicines13051068
Chicago/Turabian StyleDu, Zhihao, Lifang Feng, Yu Zhang, Xin Peng, Shan Zhang, Rui Zhao, Jia Lei, Xiaotong Li, Guangyan Yu, and Chong Ding. 2025. "Route of Application and Dose Evaluation of Dental Pulp Stem Cells for the Treatment of Sialadenitis Caused by Sjögren’s Syndrome: A Preclinical Study" Biomedicines 13, no. 5: 1068. https://doi.org/10.3390/biomedicines13051068
APA StyleDu, Z., Feng, L., Zhang, Y., Peng, X., Zhang, S., Zhao, R., Lei, J., Li, X., Yu, G., & Ding, C. (2025). Route of Application and Dose Evaluation of Dental Pulp Stem Cells for the Treatment of Sialadenitis Caused by Sjögren’s Syndrome: A Preclinical Study. Biomedicines, 13(5), 1068. https://doi.org/10.3390/biomedicines13051068






