Sulforaphane’s Role in Osteosarcoma Treatment: A Systematic Review and Meta-Analysis of Preclinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria and Study Selection
2.3. Outcomes Measures
2.4. Data Items and Data Extraction
2.5. Data Analysis
2.6. Quality Review
2.7. Certainty of Evidence
2.8. Publication Bias
3. Results
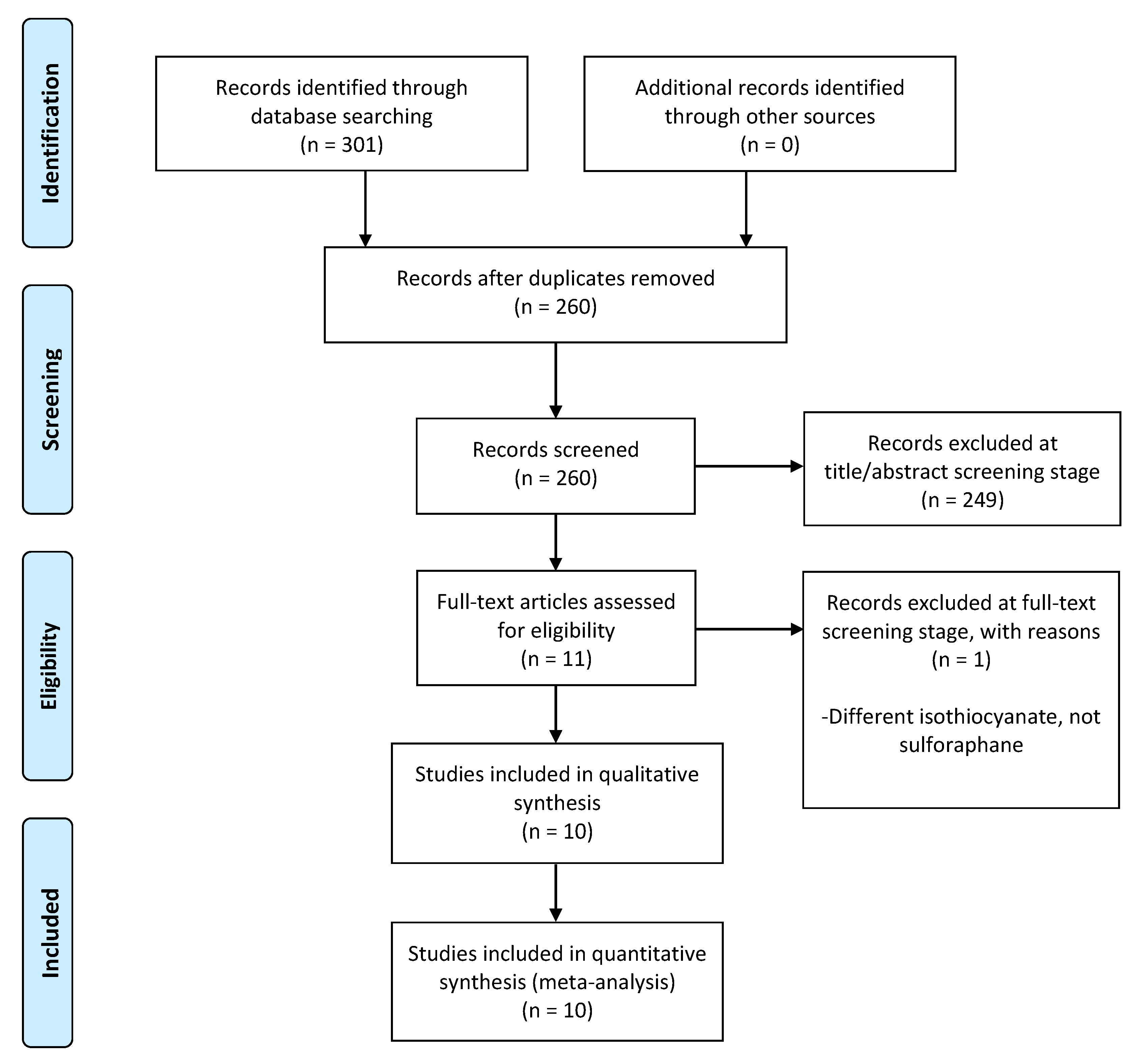
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Assessment of Certainty of Evidence
3.5. Meta-Analysis Interpretation and Narrative Synthesis
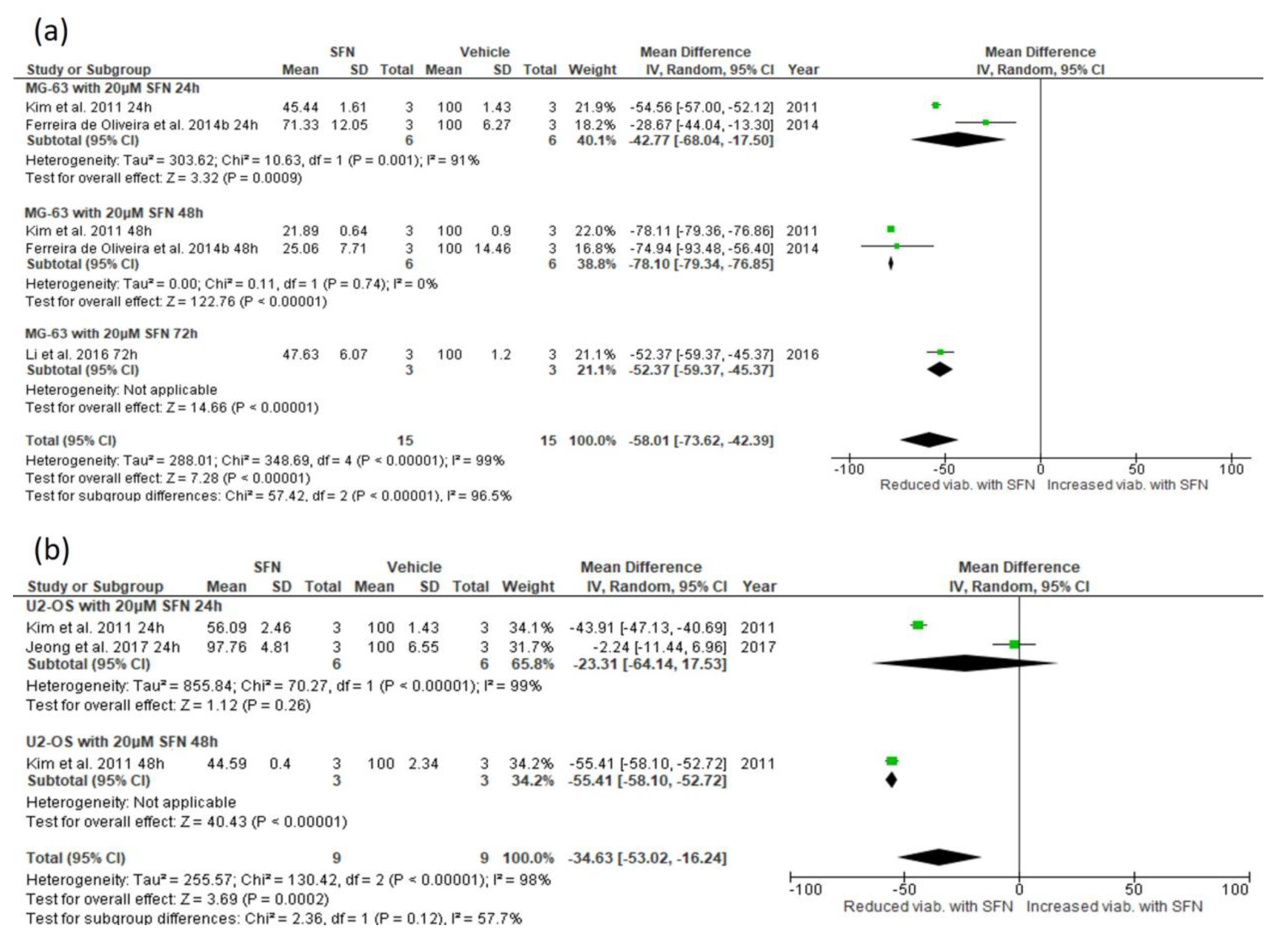
3.5.1. Effect of SFN on Cell Viability
3.5.2. Narrative Synthesis for the Effect of SFN on Cell Viability and Colony Number
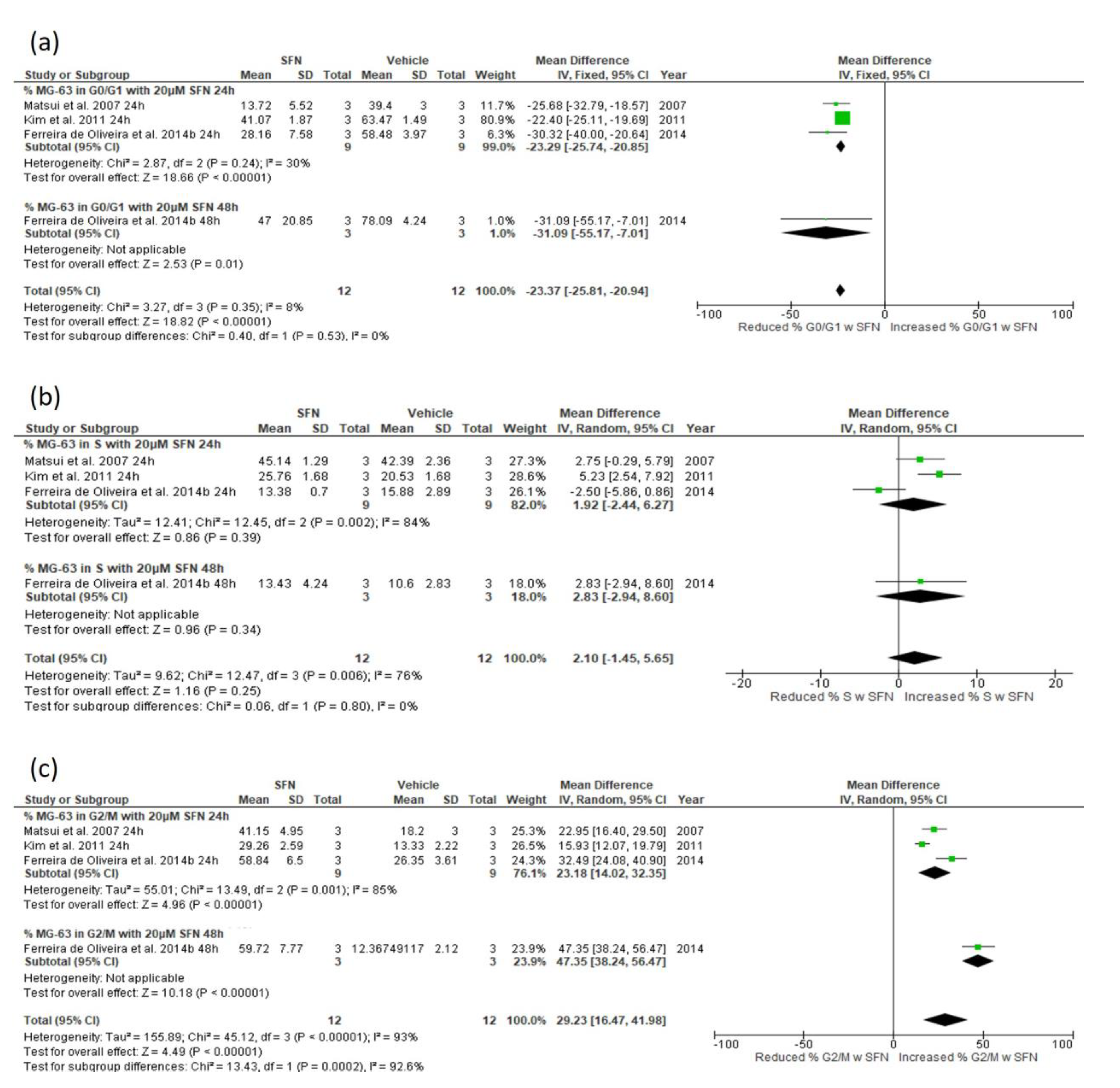
3.5.3. Effect of SFN on Cell Cycle Distribution
3.5.4. Narrative Synthesis for the Effect of SFN on Cell Cycle Distribution
3.5.5. Effect of SFN on Apoptosis Indices
3.5.6. Narrative Synthesis for the Effect of SFN on Apoptosis Indices
3.5.7. Effect of SFN on Percentage of Apoptotic Cells
3.5.8. Narrative Synthesis for the Effect of SFN on the Percentage of Apoptotic Cells
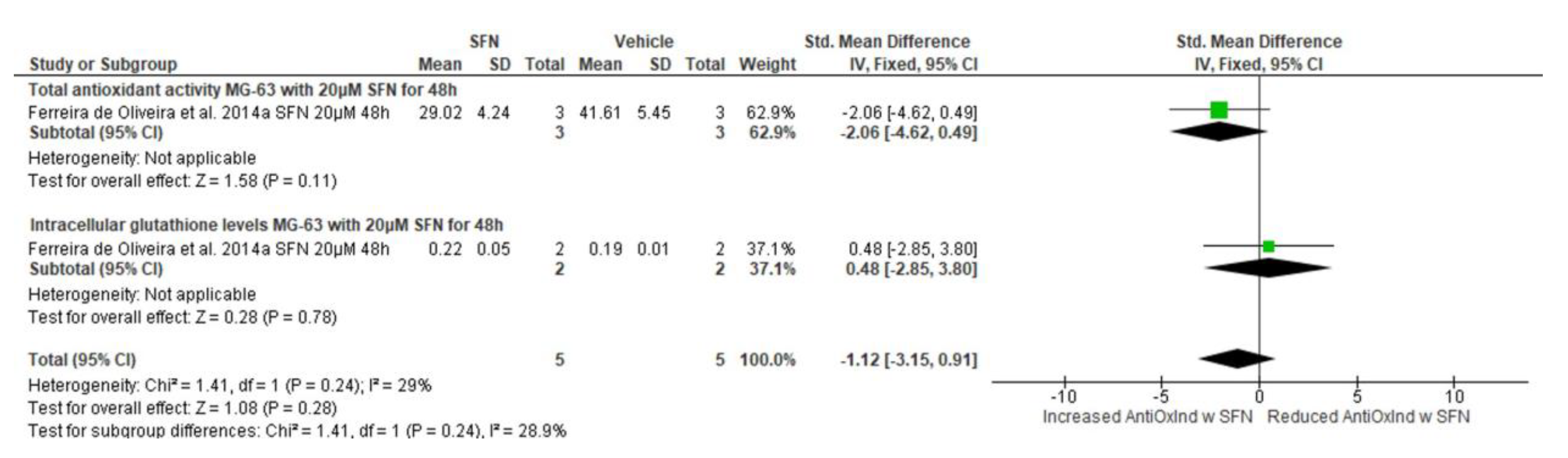
3.5.9. Effect of SFN on Antioxidant Indices
3.5.10. Narrative Synthesis for the Effect of SFN on Reactive Oxygen Species Formation
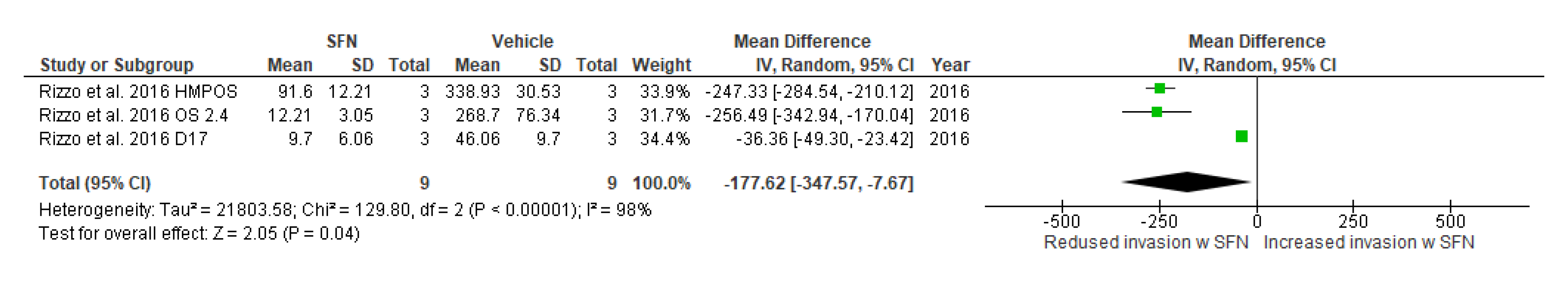
3.5.11. Effect of SFN on Cell Invasion and Cell Migration
3.5.12. Narrative Synthesis on the Effect of SFN on Cell Invasion and Cell Migration
3.5.13. Effect of SFN on Tumour Volume and Weight (Narrative Synthesis Only)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A


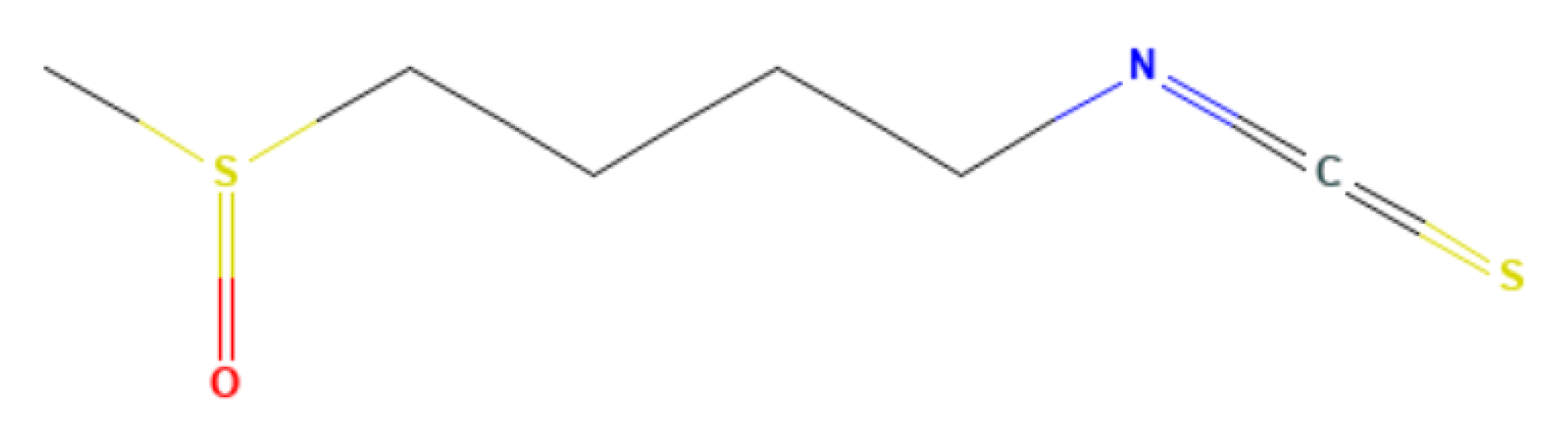
Appendix B
| Outcome | Studies Included | Cell line (Human/Mouse) | SFN Concentration | Treatment Duration (Hours) | Subgroup (std.) Mean Difference (95% CI) | Overall (std.) Mean Difference (95% CI) | Statistical Method | Test for Heterogeneity | Test for Overall Effect |
|---|---|---|---|---|---|---|---|---|---|
| Cell viability | Kim et al., 2011 [18]; Ferreira de Oliveira et al., 2014b [7]; Li et al., 2016 [29] | MG-63 (human) | 5 μΜ | 24 48 72 | −3.13 (−18.02, 11.75) −38.93 (−78.30, 0.45) −23.89 (−31.41, −16.37) | −26.05 (−53.30, 1.19) | Mean difference, IV, Random effects, 95% CI | Chi2 = 130.38, df = 3, p < 0.00001, I2 = 98% | Z = 1.87, p = 0.06 |
| MG-63 (human) | 10 μΜ | 24 48 72 | −1.69 (−10.97, 7.6) −69.14 (−70.73, −67.56) −36.50 (−42.98, −30.01) | −41.40 (−73.50, −9.31) | Mean difference, IV, Random effects, 95% CI | Chi2 = 279.26, df = 3, p < 0.00001, I2 = 99% | Z = 2.53, p = 0.01 | ||
| Kim et al., 2011 [18]; Jeong et al., 2017 [28] | U-2 OS (human) | 5 μΜ | 24 48 | −4.09 (−20.41, 12.22) −8.38 (−13.83, −2.92) | −7.95 (−13.12, −2.78) | Mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.24, df = 1, p = 0.63, I2 = 0% | Z = 3.01, p = 0.003 | |
| U-2 OS (human) | 10 μΜ | 24 48 | −2.24 (−12.62, 8.14) −26.22 (−29.30, −23.13) | −14.76 (−38.23, 8.71) | Mean difference, IV, Random effects, 95% CI | Chi2 = 18.82, df = 1, p < 0.0001, I2 = 95% | Z = 1.23, p = 0.22 | ||
| Cell cycle distribution: G0/G1 phase | Kim et al., 2011 [18]; Ferreira de Oliveira et al., 2014b [7] | MG-63 (human) | 5 μΜ | 24 48 | −8.98 (−16.38, −1.58) −15.55 (−26.64, −4.46) | −10.39 (−16.96, −3.83) | Mean difference, IV, Random effects, 95% CI | Chi2 = 5.12, df = 2, p = 0.08, I2 = 61% | Z = 3.10, p = 0.002 |
| MG-63 (human) | 10 μΜ | 24 48 | −19.93 (−23.00, −16.86) −31.10 (−55.17, −7.02) | −20.11 (−23.15, −17.06) | Mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.89, df = 2, p = 0.64, I2 = 0% | Z = 12.94, p < 0.00001 | ||
| Cell cycle distribution: S phase | Kim et al., 2011 [18]; Ferreira de Oliveira et al., 2014b [7] | MG-63 (human) | 5 μΜ | 24 48 | 0.78 (−9.38, 10.94) 6.01 (2.01, 10.01) | 2.54 (−5.19, 10.27) | Mean difference, IV, Random effects, 95% CI | Chi2 = 20.18, df = 2, p < 0.0001, I2 = 90% | Z = 0.64, p = 0.52 |
| MG-63 (human) | 10 μΜ | 24 48 | −1.19 (−8.32, 5.95) −6.71 (−0.44, 13.87) | 0.93 (−5.03, 6.89) | Mean difference, IV, Random effects, 95% CI | Chi2 = 9.67, df = 2, p = 0.008, I2 = 79% | Z = 0.31, p = 0.76 | ||
| Cell cycle distribution: G2/M phase | Kim et al., 2011 [18]; Ferreira de Oliveira et al., 2014b [7] | MG-63 (human) | 5 μΜ | 24 48 | 10.39 (6.53, 14.24) 10.25 (1.51, 18.98) | 10.36 (6.84, 13.89) | Mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.36, df = 2, p = 0.84, I2 = 0% | Z = 5.76, p < 0.00001 |
| MG-63 (human) | 10 μΜ | 24 48 | 16.61 (12.91, 20.31) 24.03 (7.86, 40.20) | 16.98 (13.37, 20.59) | Mean difference, IV, Fixed effects, 95% CI | Chi2 = 3.15, df = 2, p = 0.21, I2 = 36% | Z = 9.22, p < 0.00001 | ||
| Apoptosis indices (Sub-G1 phase & Active caspase-3) | Matsui et al., 2006 [19]; Ferreira de Oliveira et al., 2014a [27] | MG-63 (human) | 20 μΜ | 24 (Sub-G1) 48 (Active caspase-3) | 3.34 (−0.26, 6.94) 1.26 (−6.13, 8.66) | 2.94 (−0.30, 6.18) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.25, df = 1, p = 0.62, I2 = 0% | Z = 1.78, p = 0.07 |
| Trypan blue dye exclusion test | Matsui et al., 2007 [17]; Sawai et al., 2013 [20] | LM8 (murine) | 5 μΜ 10 μΜ 20 μΜ | 24 | −3.16 (−5.88, −0.45) −5.21 (−9.42, −0.99) −9.49 (−16.61, −2.37) | −4.30 (−6.47, −2.12) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 7.49, df = 5, p = 0.19, I2 = 33% | Z = 3.87, p = 0.0001 |
| Antioxidant activity (total antioxidant activity & intracellular glutathione levels) | Ferreira de Oliveira et al., 2014a [27] | MG-63 (human) | 5 μΜ | 24 (total antioxidant activity) 24 (intracellular glutathione levels) | −0.84 (−2.63, 0.96) 0.81 (−4.17, 5.78) | −0.65 (−2.33, 1.04) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.37, df = 1, p = 0.54, I2 = 0% | Z = 0.75, p = 0.45 |
| Ferreira de Oliveira et al., 2014a [27] | MG-63 (human) | 10 μΜ | 24 (total antioxidant activity) 24 (intracellular glutathione levels) | −1.52 (−3.70, 0.65) 0.10 (−1.94, 2.13) | −0.66 (−2.15, 0.83) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 1.14, df = 1, p = 0.29, I2 = 12% | Z = 0.87, p = 0.38 | |
| MG-63 (human) | 20 μΜ | 24 (total antioxidant activity) 24 (intracellular glutathione levels) | −3.36 (−6.99, 0.26) −1.65 (−11.18, 7.88) | −3.15 (−6.53, 0.24) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.11, df = 1, p = 0.74, I2 = 0% | Z = 1.82, p = 0.07 | ||
| Antioxidant activity (total antioxidant activity & intracellular glutathione levels) | Ferreira de Oliveira et al., 2014b [7] | MG-63 (human) | 5 μΜ | 48 (total antioxidant activity) 48 (intracellular glutathione levels) | −0.18 (−1.79, 1.43) 2.17 (−10.26, 14.59) | −0.14 (−1.74, 1.45) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.14, df = 1, p = 0.71, I2 = 0% | Z = 0.18, p = 0.86 |
| MG-63 (human) | 10 μΜ | 48 (total antioxidant activity) 48 (intracellular glutathione levels) | −0.21 (−1.82, 1.41) 2.29 (−10.79, 15.37) | −0.17 (−1.77, 1.43) | Std. mean difference, IV, Fixed effects, 95% CI | Chi2 = 0.14, df = 1, p = 0.71, I2 = 0% | Z = 0.21, p = 0.84 | ||
| Cellinvasion capabilities | Rizzo et al., 2017 [30] | D17, OS 2.4, HMPOS (canine) | 10 μΜ | 24 | -------------------------- | −25.21 (−42.01, −8.42) | Mean difference, IV, effects, 95% CI | Chi2 = 2.92, df = 2, p = 0.23, I2 = 31% | Z = 2.94, p = 0.003, |
Appendix C
| Viability of Human Osteosarcoma Cell Lines (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|
| Study | Cell Line | Duration (Hours) | Vehicle | SFN 5 μM | SFN 10 μM | SFN 20 μM |
| Li et al., 2016 [29] | OS-732 | 72 | 100 ± 0.84 | 82.25 ± 6.29 ** | 69.21 ± 4.94 ** | 48.99 ± 4.94 *** |
| Zou et al., 2025 [31] | 143B | 24 | 100 ± 4.46 | 85.89 ± 1.78 | 57.05 ± 2.33 | 33.18 ± 2.26 |
| SJSA-1 | 24 | 100 ± 1.97 | 81.24 ± 4.25 | 66.98 ± 3.90 | 41.24 ± 3.22 | |
| 143B | 48 | 100 ± 1.09 | 56.78 ± 3.69 | 18.72 ± 1.44 | 11.96 ± 1.68 | |
| SJSA-1 | 48 | 100 ± 0.78 | 51.56 ± 2.26 | 42.05 ± 4.22 | 23.02 ± 1.31 | |
| Viability of D17, OS 2.4 and HMPOS for 48 h (Mean ± SD) | ||||||
| Study | Cell Line | Duration (Hours) | Vehicle | SFN 6.25μM | SFN 12.5μM | SFN 25μM |
| Rizzo et al., 2017 [30] | D17 | 48 | 100 ± 6.42 | 125.99 ± 11.01 * (↑) | 88.69 ± 13.45 | 58.10 ± 8.57 * (↓) |
| OS 2.4 | 48 | 100 ± 6.13 | 130.06 ± 14.11 * (↑) | 69.94 ± 3.68 | 36.81 ± 3.06 * (↓) | |
| HMPOS | 48 | 100 ± 3.05 | 65.24 ± 12.19 * (↓) | 56.10 ± 4.88 * (↓) | 64.02 ± 10.36 * (↓) | |
| Colony Formation in 143B and SJSA-1 Cell Cultures | ||||||
| Study | Cell Line | Vehicle | SFN 1.25μM | SFN 2.5μM | SFN 5μM | |
| Zou et al., 2025 [31] | 143B | 98.36 ± 8.61 | 68.85 ± 7.99 * | 51.64 ± 4.92 ** | 2.46 ± 3.69 ** | |
| SJSA-1 | 97.13 ± 14.14 | 76.84 ± 15.37 | 55.33 ± 15.98 * | 12.91 ± 7.99 ** | ||
| Cell Cycle Distribution of OS-732 After 72 h Treatment with Vehicle DMSO | ||||
|---|---|---|---|---|
| Study | Cell Line | G0/G1 | S | G2/M |
| Li et al., 2016 [29] | OS-732 | 38.3 ± 2.13 | 14.47 ± 1.27 | 46.8 ± 2.56 |
| Cell cycle distribution of OS-732 after 72 h treatment with 15μM SFN | ||||
| Cell line | G0/G1 | S | G2/M | |
| OS-732 | 54.89 ± 2.13 | 11.07 ± 1.27 | 33.61 ± 2.56 | |
| Cell cycle distribution of MG-63 after 72 h treatment with vehicle DMSO | ||||
| Study | Cell line | G0/G1 | S | G2/M |
| Li et al., 2016 [29] | MG-63 | 43.27 ± 1.85 | 15.4 ± 1.23 | 42.04 ± 2.15 |
| Cell cycle distribution of MG-63 after 72 h treatment with 15μM SFN | ||||
| Cell line | G0/G1 | S | G2/M | |
| MG-63 | 59.23 ± 0.62 | 8.29 ± 0.92 | 31.92 ± 2.45 | |
| Cell cycle distribution of D17 after 48 h treatment with vehicle DMSO | ||||
| Study | Cell line | G0/G1 | S | G2/M |
| Rizzo et al., 2017 [30] | D17 | 56.6 ± 1.6 | 16.3 ± 0.7 | 26.4 ± 1.4 |
| Cell cycle distribution of D17 after 48 h treatment with 20μM SFN (* p < 0.05 from vehicle) | ||||
| Cell line | G0/G1 | S | G2/M | |
| D17 | 55.0 ± 1.7 | 12.2 ± 0.3 * | 32.5 ± 0.9 * | |
| Cell cycle distribution of OS 2.4 after 48 h treatment with vehicle DMSO | ||||
| Study | Cell line | G0/G1 | S | G2/M |
| Rizzo et al., 2017 [30] | OS 2.4 | 73.0 ± 1.4 | 7.2 ± 0.2 | 19.5 ± 1.4 |
| Cell cycle distribution of OS 2.4 after 48 h treatment with 20μM SFN (* p < 0.05 from vehicle) | ||||
| Cell line | G0/G1 | S | G2/M | |
| OS 2.4 | 54.1 ± 1.3 * | 13.0 ± 0.2 * | 31.8 ± 1.6 * | |
| Cell cycle distribution of HMPOS after 48 h treatment with vehicle DMSO | ||||
| Study | Cell line | G0/G1 | S | G2/M |
| Rizzo et al., 2017 [30] | HMPOS | 75.5 ± 1.1 | 8.0 ± 0.3 | 16.4 ± 1.0 |
| Cell cycle distribution of HMPOS after 48 h treatment with 20μM SFN (* p < 0.05 from vehicle) | ||||
| Cell line | G0/G1 | S | G2/M | |
| HMPOS | 70.1 ± 1.8 | 6.8 ± 0.8 | 23.3 ± 1.4* | |
| Cell cycle distribution of LM8 after 24 h treatment with vehicle DMSO | ||||
| Study | Cell line | G1 | S | G2/M |
| Matsui et al., 2007 + [17] | LM8 | 31.10 | 55.98 | 12.92 |
| Cell cycle distribution of LM8 after 24 h treatment with 20μM SFN | ||||
| Cell line | G1 | S | G2/M | |
| LM8 | 15.55 | 51.91 | 32.54 | |
| Sub-G1% of Cells After 24 h Treatment with Vehicle or SFN (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|
| Study | Cell Line | Vehicle | 2.5 μM | 5 μM | 10 μM | 15 μM |
| Sawai et al., 2013 [20] | LM8 | 0.67 ± 0.005 | 0.95 ± 0.003 | 1.52 ± 0.004 | 1.90 ± 0.10 | 7.03 ± 1.81 |
| Trypan Blue Dye Exclusion Test for MG-63 Cells with SFN for 24 h (×104; Mean ± SD) (* p < 0.05) | |||||
|---|---|---|---|---|---|
| Study | Cell Line | Vehicle | SFN 5 μM | SFN 10 μM | SFN 20 μM |
| Matsui et al., 2007 [17] | MG-63 | 3.55 ± 0.47 | 3.08 ± 0.27 | 2.53 ± 0.16 * | 1.33 ± 0.27 * |
| Trypan blue dye exclusion test for MG-63 cells with SFN for 48 h (×104; mean ± SD) (* p < 0.05) | |||||
| Study | Cell line | Vehicle | SFN 5μM | SFN 10μM | SFN 20μM |
| Matsui et al., 2007 [17] | MG-63 | 10.97 ± 1.01 | 8.13 ± 0.62 * | 4.36 ± 0.62 * | 1.21 ± 0.31 * |
| Trypan blue dye exclusion test for D17, OS 2.4 and HMPOS cells with 20μM SFN for 48 h (% trypan blue positive cells, i.e., non-viable cells; mean ± SD) (* p < 0.05) | |||||
| Study | Cell line | Vehicle | SFN 20μM | ||
| Rizzo et al., 2017 [30] | D17 | 5 ± 3 | 11 ± 3 | ||
| OS 2.4 | 28 ± 3 | 5 ± 5 * (↓) | |||
| HMPOS | 7 ± 3 | 7 ± 5 | |||
| % Apoptotic OS-732 Cells with 15 μΜ SFN for 72 h (Mean ± SD) (* p < 0.001) | ||||
|---|---|---|---|---|
| Study | Cell Line | Vehicle | SFN 15 μM | |
| Li et al., 2016 [29] 72 h | OS-732 | 6.14 ± 1.62 | 23.28 ± 2.75 * | |
| % apoptotic ΜG-63 cells with SFN (mean ± SD) (* p < 0.05) | ||||
| Study | Cell line | Vehicle | SFN 5μM | SFN 10μM |
| Ferreira de Oliveira et al., 2014b [7] 24 h | MG-63 | 9.12 ± 1.83 | 11.23 ± 3.05 | 14.04 ± 2.44 * |
| Ferreira de Oliveira et al., 2014b [7] 48 h | 8.77 ± 1.78 | 10.31 ± 1.78 | 26.77 ± 3.56 * | |
| ROS Accumulation (Median Fluorescent Intensity) MG-63 Cells with SFN (Mean ± SD) (* p < 0.05) | ||||||
|---|---|---|---|---|---|---|
| Study | Cell Line | Vehicle | SFN 5 μM | SFN 10 μM | SFN 20 μM | |
| Ferreira de Oliveira et al., 2014a [27] SFN (for 24 h) | MG-63 | 0.92 ± 0.24 | 1.27 ± 0.19 | 3.22 ± 0.61 * | 3.84 ± 0.21 * | |
| Ferreira de Oliveira et al., 2014a [27] SFN (for 48 h) | 0.63 ± 0.09 | 1.02 ± 0.16 * | 4.22 ± 0.23 * | 4.00 ± 0.26 * | ||
| ROS accumulation/Dihydrorhodamine 123 fluorescence assay (mean ± SD) (* p < 0.05) | ||||||
| Study | Cell line | Vehicle | SFN 20μM | |||
| Rizzo et al., 2017 [30] | D17 | 652.46 ± 72.13 | 773.77 ± 144.26 | |||
| OS 2.4 | 186.89 ± 22.95 | 337.71 ± 16.39 * | ||||
| HMPOS | 459.02 ± 39.34 | 62.30 ± 9.11 * | ||||
| ROS accumulation (relative ROS fluorescence) (mean ± SD) (* p < 0.05; ** p < 0.01) | ||||||
| Study | Cell line | Vehicle | SFN 5μM | SFN 10μM | ||
| Zou et al. [31] | 143B | 100 ± 0.34 | 116.16 ± 5.05 * | 137.37 ± 12.12 ** | ||
| SJSA-1 | 100 ± 0.42 | 145.46 ± 8.08 ** | 150.50 ± 7.08 ** | |||
| Migration Activity in U-2OS Cells Treated with SFN 10 μM and 12-O-Tetradecanoylphorbol-13-Acetate (TPA) (% of Control, Mean ± SD) (* p < 0.05) | |||
|---|---|---|---|
| Study | Cell Line | Vehicle + TPA | SFN 10 μM + TPA |
| Jeong et al., 2017 [28] | U-2 OS | 100 ± 9.62 | 49.26 ± 5.77 * |
| Tumour Volume from LM8 Cells Following Treatment with SFN for 4 Weeks (mean ± SD) (* p < 0.05) | ||||
|---|---|---|---|---|
| Study | Parameter | Vehicle | SFN 5 mg/week | SFN 10 mg/week |
| Matsui et al., 2007 [17] | Tumour volume (cm3) | 906.82 ± 245.45 | 225.00 ± 81.82 * | 204.55 ± 115.91 * |
| Tumour volume from 143B cells following treatment with SFN for 18 days (mean ± SD) (* p < 0.05; ** p < 0.01) | ||||
| Study | Parameter | Vehicle | SFN 25 mg/kg | SFN 50 mg/kg |
| Zou et al., 2025 [31] | Tumour volume (mm3) | 1364 ± 519.29 | 734 ± 240.05 * | 511 ± 159.22 ** |
| Zou et al., 2025 [31] | Weight (g) | 0.823 ± 0.326 | 0.483 ± 0.073 * | 0.323 ± 0.166 ** |
References
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y.; He, Z.; Yang, J.; Sun, B.; Shi, X.; et al. Progress in the Chemotherapeutic Treatment of Osteosarcoma (Review). Oncol. Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Li, X.; Liu, W.; Liufu, Q.; Zhong, C. Global, Regional, and National Burden and Trends Analysis of Malignant Neoplasm of Bone and Articular Cartilage from 1990 to 2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Bone 2024, 188, 117212. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. In Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O., Bielack, S., Eds.; Cancer Treatment and Research; Springer: Boston, MA, USA, 2009; Volume 152, pp. 3–13. [Google Scholar]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Bone and Joint Cancer. Available online: https://seer.cancer.gov/statfacts/html/bones.html (accessed on 23 February 2025).
- PosthumaDeBoer, J.; Witlox, M.A.; Kaspers, G.J.L.; van Royen, B.J. Molecular Alterations as Target for Therapy in Metastatic Osteosarcoma: A Review of Literature. Clin. Exp. Metastasis 2011, 28, 493–503. [Google Scholar] [CrossRef]
- de Oliveira, J.M.P.F.; Remédios, C.; Oliveira, H.; Pinto, P.; Pinho, F.; Pinho, S.; Costa, M.; Santos, C. Sulforaphane Induces DNA Damage and Mitotic Abnormalities in Human Osteosarcoma MG-63 Cells: Correlation with Cell Cycle Arrest and Apoptosis. Nutr. Cancer 2014, 66, 325–334. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5350, Sulforaphane. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sulforaphane (accessed on 25 March 2025).
- Cieslik, E.; Leszczynska, T.; Filipiak-Florkiewicz, A.; Sikora, E.; Pisulewski, P. Effects of Some Technological Processes on Glucosinolate Contents in Cruciferous Vegetables. Food Chem. 2007, 105, 976–981. [Google Scholar] [CrossRef]
- Sailo, B.L.; Liu, L.; Chauhan, S.; Girisa, S.; Hegde, M.; Liang, L.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. Harnessing Sulforaphane Potential as a Chemosensitizing Agent: A Comprehensive Review. Cancers 2024, 16, 244. [Google Scholar] [CrossRef]
- Ali, M.A.; Khan, N.; Kaleem, N.; Ahmad, W.; Alharethi, S.H.; Alharbi, B.; Alhassan, H.H.; Al-Enazi, M.M.; Razis, A.F.A.; Modu, B.; et al. Anticancer Properties of Sulforaphane: Current Insights at the Molecular Level. Front. Oncol. 2023, 13, 1168321. [Google Scholar]
- Liu, P.; Zhang, B.; Li, Y.; Yuan, Q. Potential Mechanisms of Cancer Prevention and Treatment by Sulforaphane, a Natural Small Molecule Compound of Plant-Derived. Mol. Med. 2024, 30, 94. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer, version 4.8; Software for data extraction from charts; Ankit Rohatgi: Notre Dame, IN, USA, 2020; Available online: https://apps.automeris.io/wpd4/ (accessed on 1 July 2024).
- Ma, J.; Liu, W.; Hunter, A.; Zhang, W. Performing Meta-Analysis with Incomplete Statistical Information in Clinical Trials. BMC Med. Res. Methodol. 2008, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.J.; Butcher, I.; Assi, V.; Lewis, S.C.; Murray, G.D.; Langhorne, P.; Brady, M.C. Dealing with Missing Standard Deviation and Mean Values in Meta-Analysis of Continuous Outcomes: A Systematic Review. BMC Med. Res. Methodol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Murata, H.; Sakabe, T.; Sowa, Y.; Horie, N.; Nakanishi, R.; Sakai, T.; Kubo, T. Sulforaphane Induces Cell Cycle Arrest and Apoptosis in Murine Osteosarcoma Cells in Vitro and Inhibits Tumor Growth in Vivo. Oncol. Rep. 2007, 18, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R. Induction of G2/M Arrest and Apoptosis by Sulforaphane in Human Osteosarcoma U2-OS Cells. Mol. Med. Rep. 2011, 4, 929–934. [Google Scholar] [CrossRef]
- Matsui, T.-A.; Sowa, Y.; Yoshida, T.; Murata, H.; Horinaka, M.; Wakada, M.; Nakanishi, R.; Sakabe, T.; Kubo, T.; Sakai, T. Sulforaphane Enhances TRAIL-Induced Apoptosis through the Induction of DR5 Expression in Human Osteosarcoma Cells. Carcinogenesis 2006, 27, 1768–1777. [Google Scholar] [CrossRef]
- Sawai, Y.; Murata, H.; Horii, M.; Koto, K.; Matsui, T.; Horie, N.; Tsuji, Y.; Ashihara, E.; Maekawa, T.; Kubo, T.; et al. Effectiveness of Sulforaphane as a Radiosensitizer for Murine Osteosarcoma Cells. Oncol. Rep. 2013, 29, 941–945. [Google Scholar] [CrossRef]
- Review Manager (RevMan), version 5.3; Cochrane Collaboration: London, UK, 2014.
- Office of Health Assessment and Translation (OHAT), National Toxicology Program (NTP), the National Institute of Environmental Health Sciences (NIEHS). OHAT Risk of Bias Rating Tool for Human and Animal Studies. 2015. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 29 January 2025).
- Ryan, R.; Hill, S. How to GRADE the Quality of the Evidence; Cochrane Consumers and Communication Group: Melbourne, Australia, 2016; Version 3; pp. 1–24. [Google Scholar]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating Healthcare Decisions by Assessing the Certainty in the Evidence from Preclinical Animal Studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the Certainty in Evidence in the Absence of a Single Estimate of Effect. Evid. Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- de Oliveira, J.M.P.F.; Costa, M.; Pedrosa, T.; Pinto, P.; Remédios, C.; Oliveira, H.; Pimentel, F.; Almeida, L.; Santos, C. Sulforaphane Induces Oxidative Stress and Death by P53-Independent Mechanism: Implication of Impaired Glutathione Recycling. PLoS ONE 2014, 9, e92980. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Cho, H.-J.; Chung, F.-L.; Wang, X.; Hoe, H.-S.; Park, K.-K.; Kim, C.-H.; Chang, H.-W.; Lee, S.-R.; Chang, Y.-C. Isothiocyanates Suppress the Invasion and Metastasis of Tumors by Targeting FAK/MMP-9 Activity. Oncotarget 2017, 8, 63949–63962. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.Y.; Zhou, C.J.; Ma, H.Z.; Sun, C.L.; Feng, R.J. Sulforaphane Enhances Cisplatin Sensitivity in Human Osteosarcoma Cells through Upregulation of P53–P21 Pathway by Enhancing G1 Arrest. Int. J. Clin. Exp. Med. 2016, 9, 1621–1629. [Google Scholar]
- Rizzo, V.L.; Levine, C.B.; Wakshlag, J.J. The Effects of Sulforaphane on Canine Osteosarcoma Proliferation and Invasion. Vet. Comp. Oncol. 2017, 15, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhou, X.; Lai, J.; Zhou, H.; Su, J.; Zhang, Z.; Zhuang, X.; Liu, L.; Yuan, R.; Li, S.; et al. Targeting P62 by Sulforaphane Promotes Autolysosomal Degradation of SLC7A11, Inducing Ferroptosis for Osteosarcoma Treatment. Redox Biol. 2025, 79, 103460. [Google Scholar] [CrossRef]
- Jin, C.Y.; Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Kang, S.H.; Park, C.; Kim, G.Y.; Choi, Y.H. TRAIL Attenuates Sulforaphane-Mediated Nrf2 and Sustains ROS Generation, Leading to Apoptosis of TRAIL-Resistant Human Bladder Cancer Cells. Toxicol. Appl. Pharmacol. 2018, 352, 132–141. [Google Scholar] [CrossRef]
- Labsch, S.; Liu, L.; Bauer, N.; Zhang, Y.; Aleksandrowicz, E.; Gladkich, J.; Schönsiegel, F.; Herr, I. Sulforaphane and TRAIL Induce a Synergistic Elimination of Advanced Prostate Cancer Stem-like Cells. Int. J. Oncol. 2014, 44, 1470–1480. [Google Scholar] [CrossRef]
- Kim, H.; Kim, E.H.; Eom, Y.W.; Kim, W.-H.; Kwon, T.K.; Lee, S.J.; Choi, K.S. Sulforaphane Sensitizes Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL)–Resistant Hepatoma Cells to TRAIL-Induced Apoptosis through Reactive Oxygen Species–Mediated Up-Regulation of DR5. Cancer Res. 2006, 66, 1740–1750. [Google Scholar] [CrossRef]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Enhancement of Cisplatin-Mediated Apoptosis in Ovarian Cancer Cells through Potentiating G2/M Arrest and P21 Upregulation by Combinatorial Epigallocatechin Gallate and Sulforaphane. J. Oncol. 2013, 2013, 872957. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Yang, X.; Gao, Z.; Song, M.; Han, Y.; Chen, X.; Xiao, H. Synergistic Chemopreventive Effect of Allyl Isothiocyanate and Sulforaphane on Non-Small Cell Lung Carcinoma Cells. Food Funct. 2019, 10, 893–902. [Google Scholar] [CrossRef]
- Pappa, G.; Strathmann, J.; Löwinger, M.; Bartsch, H.; Gerhäuser, C. Quantitative Combination Effects between Sulforaphane and 3,3′-Diindolylmethane on Proliferation of Human Colon Cancer Cells in Vitro. Carcinogenesis 2007, 28, 1471–1477. [Google Scholar] [CrossRef]
- Lubelska, K.; Milczarek, M.; Modzelewska, K.; Krzysztoń-Russjan, J.; Fronczyk, K.; Wiktorska, K. Interactions between Drugs and Sulforaphane Modulate the Drug Metabolism Enzymatic System. Pharmacol. Rep. 2012, 64, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Hänsch, G.M.; Hübner, K.; Samstag, Y. Sulforaphane as Anticancer Agent: A Double-Edged Sword? Tricky Balance between Effects on Tumor Cells and Immune Cells. Adv. Biol. Regul. 2019, 71, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liang, H.; Yuan, Q.; Wang, T.; Yan, X. Preparation and Stability Investigation of the Inclusion Complex of Sulforaphane with Hydroxypropyl-β-Cyclodextrin. Carbohydr. Polym. 2010, 82, 613–617. [Google Scholar] [CrossRef]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative Determination of Dithiocarbamates in Human Plasma, Serum, Erythrocytes and Urine: Pharmacokinetics of Broccoli Sprout Isothiocyanates in Humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.S.A.; Stierer, T.; Garrett-Mayer, E.; Argani, P.; et al. Preclinical and Clinical Evaluation of Sulforaphane for Chemoprevention in the Breast. Carcinogenesis 2007, 28, 1485–1490. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and Chemopreventive Targets of Sulforaphane in Humans Following Consumption of Broccoli Sprouts or a Myrosinase-Treated Broccoli Sprout Extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.E.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]

| Study, Year | Osteosarcoma Cell Line (s) | Intervention | Reported Cancer Relevant Outcomes | Study Results | Funding Source |
|---|---|---|---|---|---|
| Ferreira de Oliveira et al., 2014a [27] | Human: MG-63 | In vitro: 0, 5, 10, and 20 μΜ SFN |
|
| Portuguese Foundation for Science and Technology |
| Ferreira de Oliveira et al., 2014b [7] | Human: MG-63 | In vitro: 0, 5, 10, and 20 μM SFN |
|
| Portuguese Foundation for Science and Technology |
| Jeong et al., 2017 [28] | Human: U-2 OS | In vitro: 10 µM SFN |
|
| National Research Foundation of Korea, Ministry of Science, ICT & Future Planning, Korea Research Institute of Bioscience & Biotechnology |
| Kim et al., 2011 [18] | Human: U-2 OS, MG-63 | In vitro: 0, 1, 5, 10, and 20 µM SFN |
|
| Chonbuk National University Hospital Research Institute of Clinical Medicine and research funds |
| Li et al., 2016 [29] | Human; OS-732, MG-63 | In vitro: 0, 1, 5, 10, 15, and 20 µM SFN |
|
| No information |
| Matsui et al., 2006 [19] | Human: Saos2, MG-63 | In vitro: 0–40 µM SFN |
|
| Ministry of Education, Culture, Sports, Science and Technology of Japan. |
| Matsui et al., 2007 [17] | Human: MG-63 Murine: LM8 | In vitro: 0–20 μΜ SFN In vivo: BALB/C mice, i.p. injection, 5 mg/week and 10 mg/week (for 4 weeks) |
|
| Ministry of Education, Culture, Sports, Science and Technology of Japan & Takeda Science Foundation |
| Rizzo et al., 2017 [30] | Canine: OS 2.4, HMPOS, D17 | In vitro: 0.8 to 100 μΜ SFN |
|
| No information |
| Sawai et al., 2013 [20] | Murine: LM8 | In vitro: 0, 2.5, 5, 10, 15, and 20 µM SFN |
|
| KAKENHI (Grant-in-Aid for Scientific Research) |
| Zou et al., 2025 [31] | Human: 143B, SJSA-1 | In vitro: 0–20 μΜ SFN In vivo: BALB/C mice, i.p. injection, 25 mg/kg or 50 mg/kg every two days (for 18 days) |
|
| Guangdong Basic and Applied Basic Research Foundation, National Natural Science Foundation of China, Science and Technology Projects in Guangzhou, Qilu Sanitation and Health LeadingTalent Cultivation Project and the Medical Joint Fund of Jinan University |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louka, P.; Ferreira, N.; Sophocleous, A. Sulforaphane’s Role in Osteosarcoma Treatment: A Systematic Review and Meta-Analysis of Preclinical Studies. Biomedicines 2025, 13, 1048. https://doi.org/10.3390/biomedicines13051048
Louka P, Ferreira N, Sophocleous A. Sulforaphane’s Role in Osteosarcoma Treatment: A Systematic Review and Meta-Analysis of Preclinical Studies. Biomedicines. 2025; 13(5):1048. https://doi.org/10.3390/biomedicines13051048
Chicago/Turabian StyleLouka, Polymnia, Nuno Ferreira, and Antonia Sophocleous. 2025. "Sulforaphane’s Role in Osteosarcoma Treatment: A Systematic Review and Meta-Analysis of Preclinical Studies" Biomedicines 13, no. 5: 1048. https://doi.org/10.3390/biomedicines13051048
APA StyleLouka, P., Ferreira, N., & Sophocleous, A. (2025). Sulforaphane’s Role in Osteosarcoma Treatment: A Systematic Review and Meta-Analysis of Preclinical Studies. Biomedicines, 13(5), 1048. https://doi.org/10.3390/biomedicines13051048






