Advancing Head and Neck Cancer Therapies: From Conventional Treatments to Emerging Strategies
Abstract
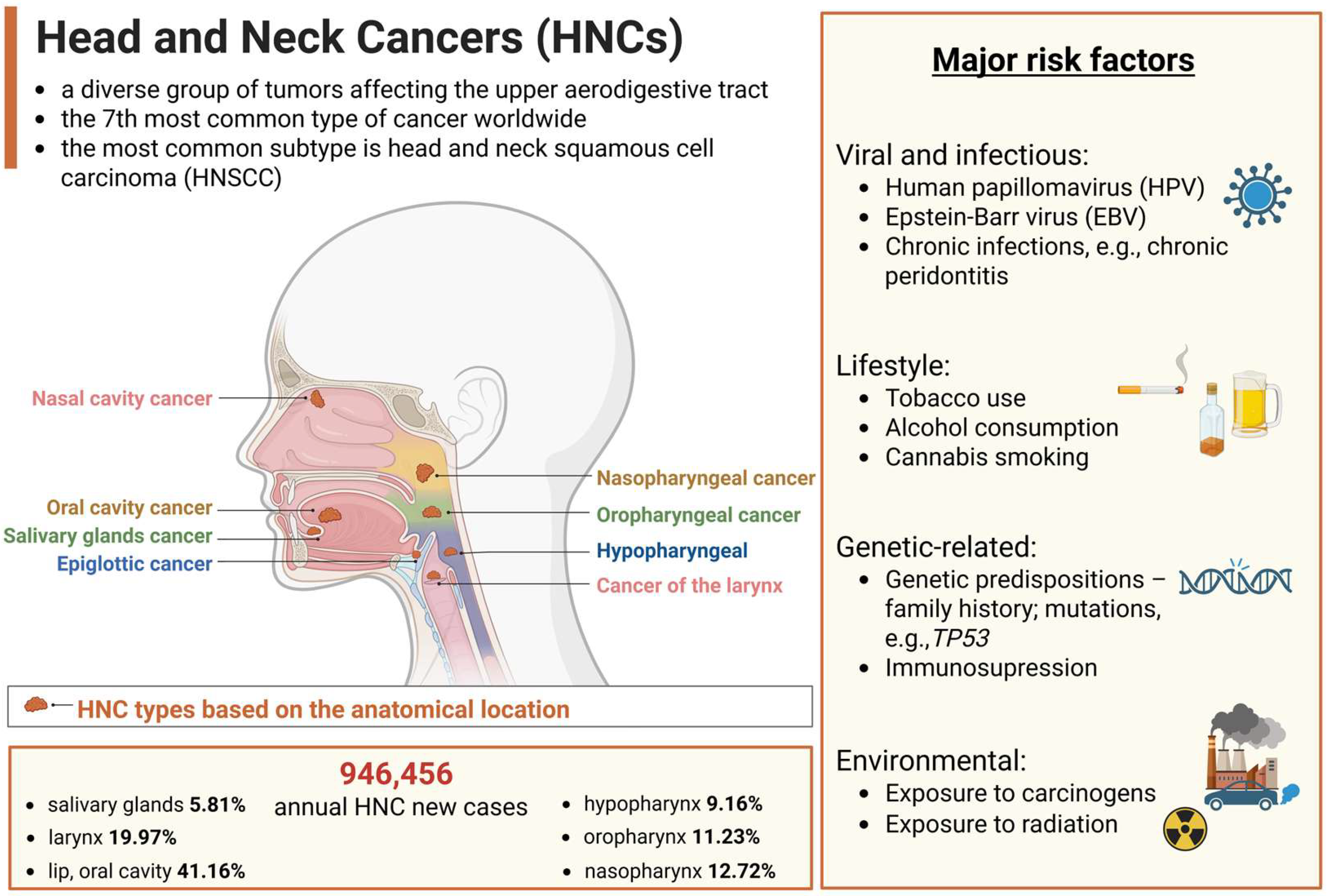
1. Introduction
2. Conventional Treatment Options for HNCs
2.1. Surgery
2.2. Radiotherapy
2.3. Chemotherapy
2.4. Limitations of Conventional HNC Treatments
3. Emerging Therapies in HNC
3.1. Targeted Therapy
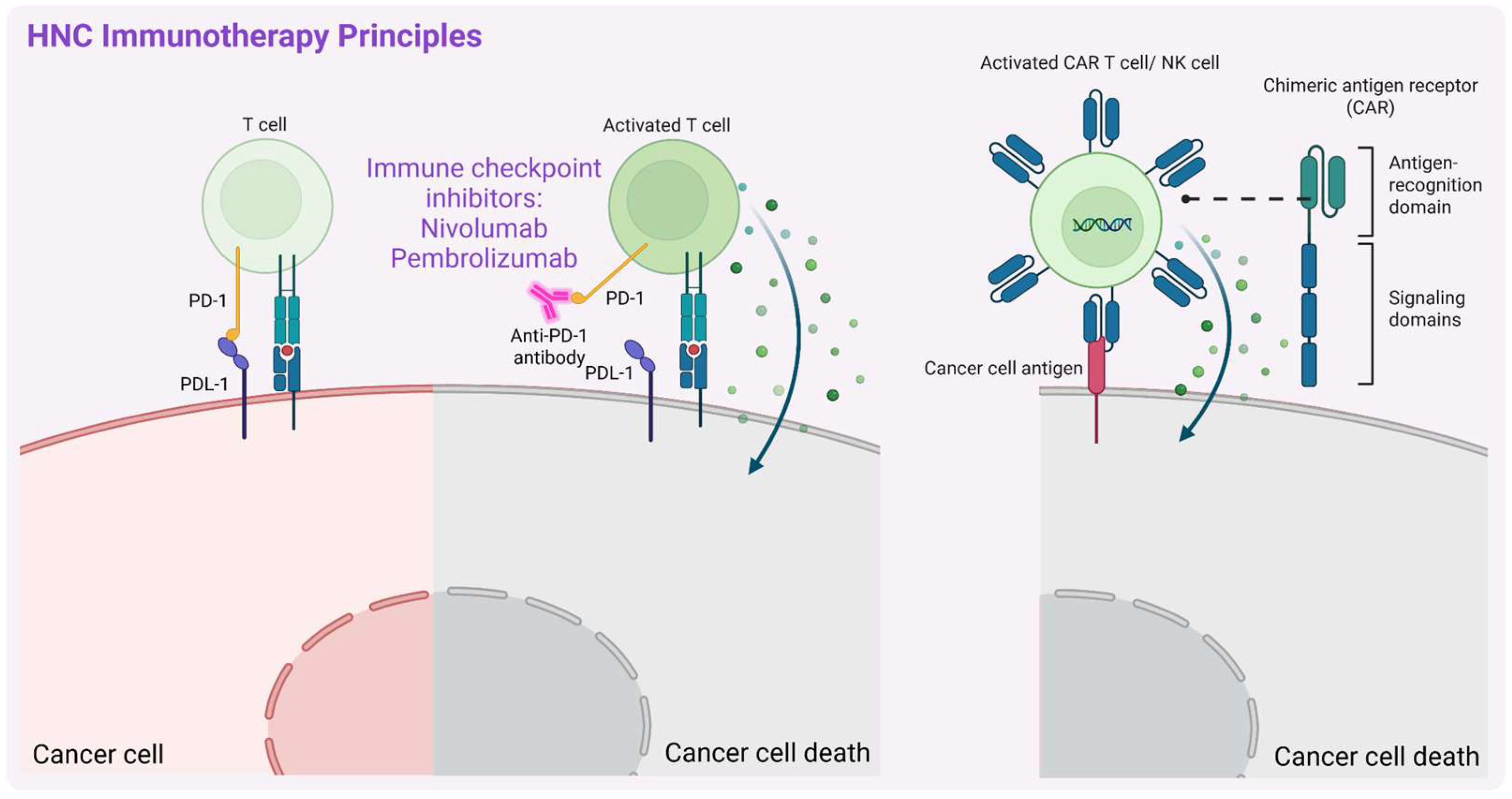
3.2. Immunotherapy
3.3. Nanomedicine in HNC Treatment
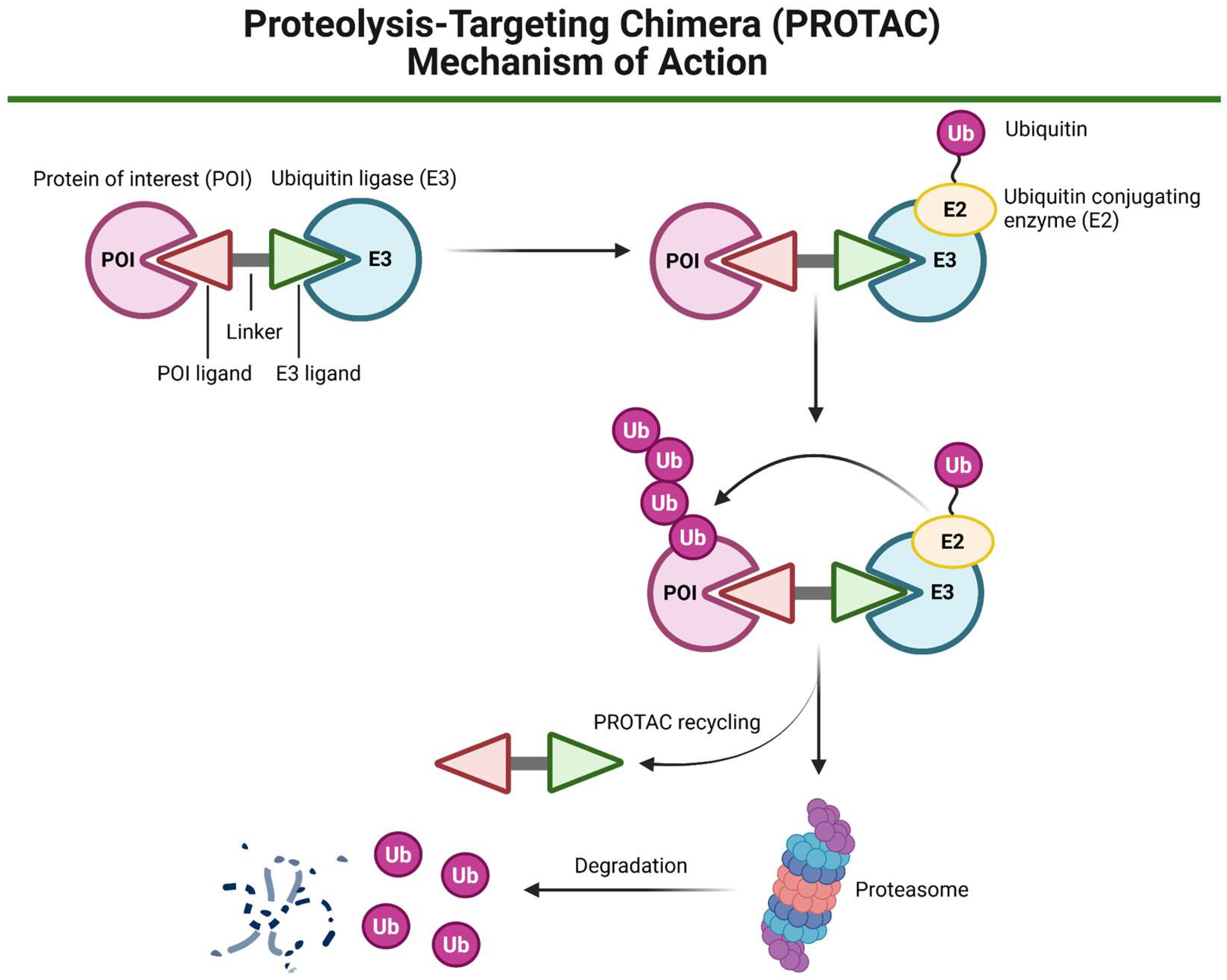
3.4. PROTAC Technology
| Feature | Traditional Inhibitors | Example References | PROTACs | Example References |
|---|---|---|---|---|
| Mechanism of action | Inhibition of the activity of oncogenic proteins | [32] | Induction of oncogenic proteins’ degradation | [131,144] |
| Examples of HNC targets | EGFR, PD-L1, MET, PI3K | [32] | STAT3, LZK (leucine zipper-bearing kinase) | [139,145] |
| Therapy effect duration | Dependent on pharmacokinetic exposure; continuous presence of the drug required | [146] | Longer effect (prolonged suppression) | [147] |
| Selectivity | Common off-target effects | [148] | Higher specificity due to the E3 ligase recruitment | [149] |
| HNC resistance | Frequent due to, e.g., EGFR mutations, MET pathway activation | [150,151] | Potentially lower due to protein degradation | [137] |
| Clinical stage in HNC | Some FDA-approved, e.g., cetuximab | [47] | Still limited for this cancer type | [142] |
4. Brief Summary and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugate |
| AI | Artificial intelligence |
| AKT | Serine/threonine kinase |
| AuNP | Gold nanoparticle |
| BiTE | Bispecific T-cell engagers |
| BsAbs | Bispecific antibodies |
| BRD4 | Bromodomain-containing protein 4 |
| CAR-NK | Chimeric antigen receptor natural killer |
| CAR-T | Chimeric antigen receptor T |
| CD117 | Cluster of differentiation 117 |
| CDDP | Cisplatin |
| CRT | Chemoradiotherapy |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DNMT | DNA methyltransferase |
| EBV | Epstein–Barr virus |
| EGFR | Epidermal growth factor receptor |
| EPR | Enhanced permeability and retention |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| FAS | Fatty acid synthase |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast growth factor receptor |
| 5-FU | 5-Fluorouracil |
| Gel-N | Gelatin nanoparticles |
| HDAC | Histone deacetylase |
| HER2 | Human epidermal growth factor receptor 2 |
| HNC | Head and neck cancer |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papillomavirus |
| ICB | Immune checkpoint blockage |
| ICG | Indocyanine green |
| ICI | Immune checkpoint inhibitor |
| IMRT | Intensity-modulated radiotherapy |
| ITGB6 | Integrin subunit beta 6 |
| JAK | Janus kinase |
| LA | Locoregionally advanced |
| LRT | Local radiotherapy |
| MEK | Mitogen-activated protein kinase |
| MET | Mesenchymal–epithelial transition factor |
| Met-AP-2 | Methionine aminopeptidase-2 |
| MMP | Matrix metalloproteinase |
| mRNA | Messenger RNA |
| mTOR | Mechanistic target of rapamycin |
| NIR | Near-infrared |
| Nkcc1 | Sodium–potassium–chloride cotransporter 1 |
| NP | Nanoparticle |
| NPC | Nasopharyngeal cancer |
| OS | Overall survival |
| OSCC | Oral squamous cell carcinoma |
| PCC | Paclitaxel, carboplatin, and cetuximab |
| PD-1 | Programmed cell death 1 |
| PDGFR | Platelet-derived growth factor receptor |
| PD-L1 | Programmed death-ligand 1 |
| PDO | Patient-derived organoids |
| PDX | Patient-derived xenografts |
| PFS | Progression-free survival |
| PI3K | Phosphatidylinositol 3-kinase |
| Pkcδ | Protein kinase C delta |
| POI | Protein of interest |
| PROTAB | Proteolysis-targeting antibody |
| PROTAC | Proteolysis-targeting chimera |
| PTT | Photothermal therapy |
| R/M | Recurrent or metastatic |
| Raf | Rapidly accelerated fibrosarcoma |
| RET | Rearranged during transfection |
| RNA-LPX | Ribonucleic acid lipoplex |
| RR | Response rates |
| RRM2 | Ribonucleotide reductase subunit M2 |
| RT | Radiotherapy |
| SCF | Skp1-Cullin-F box |
| SG | Salivary glands |
| siRNA | Small interfering RNA |
| SMG | Submandibular glands |
| SQLE | Squalene epoxidase |
| STAT3 | Signal transducer and activator of transcription 3 |
| tLNPs | Targeted lipid-based nanoparticles |
| TAA | Tumor-associated antigens |
| TME | Tumor microenvironment |
| TPD | Targeted protein degradation |
| Trop-2 | Trophoblast cell surface antigen 2 |
| TSA | Tumor-specific antigens |
| TSN | Toosendanin |
| UPS | Ubiquitin-proteasome system |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the Epidemiology of Head and Neck Cancer: Definitions, Trends and Risk Factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Zhu, Y.; Huang, N.; Qu, N. New Advances in the Therapeutic Strategy of Head and Neck Squamous Cell Carcinoma: A Review of Latest Therapies and Cutting-Edge Research. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189230. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Bravi, F.; Lee, Y.-C.A.; Hashibe, M.; Boffetta, P.; Conway, D.I.; Ferraroni, M.; La Vecchia, C.; Edefonti, V.; INHANCE Consortium investigators. Lessons Learned from the INHANCE Consortium: An Overview of Recent Results on Head and Neck Cancer. Oral Dis. 2021, 27, 73–93. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Yan, F.; Knochelmann, H.M.; Morgan, P.F.; Kaczmar, J.M.; Neskey, D.M.; Graboyes, E.M.; Nguyen, S.A.; Ogretmen, B.; Sharma, A.K.; Day, T.A. The Evolution of Care of Cancers of the Head and Neck Region: State of the Science in 2020. Cancers 2020, 12, 1543. [Google Scholar] [CrossRef]
- Menezes, F.D.S.; Fernandes, G.A.; Antunes, J.L.F.; Villa, L.L.; Toporcov, T.N. Global Incidence Trends in Head and Neck Cancer for HPV-Related and -Unrelated Subsites: A Systematic Review of Population-Based Studies. Oral Oncol. 2021, 115, 105177. [Google Scholar] [CrossRef]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck: The Active8 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in Oropharyngeal Cancer: The Basics to Know in Clinical Practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar] [PubMed]
- Hu, H.; Li, B.; Wang, J.; Tan, Y.; Xu, M.; Xu, W.; Lu, H. New Advances into Cisplatin Resistance in Head and Neck Squamous Carcinoma: Mechanisms and Therapeutic Aspects. Biomed. Pharmacother. 2023, 163, 114778. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Long-Term Follow-up of the RTOG 9501/intergroup Phase III Trial: Postoperative Concurrent Radiation Therapy and Chemotherapy in High-Risk Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Late Side Effects of Radiation Treatment for Head and Neck Cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-Checkpoint Inhibitors: Long-Term Implications of Toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Kanno, Y.; Chen, C.-Y.; Lee, H.-L.; Chiou, J.-F.; Chen, Y.-J. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front. Oncol. 2021, 11, 640392. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral Squamous Cell Carcinomas: State of the Field and Emerging Directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Runnels, J.; Bloom, J.R.; Hsieh, K.; Dickstein, D.R.; Shi, Y.; Jones, B.M.; Lehrer, E.J.; Bakst, R.L. Combining Radiotherapy and Immunotherapy in Head and Neck Cancer. Biomedicines 2023, 11, 2097. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.; Handley, T.P.B.; Bobinskas, A.; Elsapagh, M.; Anwar, H.S.; Ricciardo, P.V.; McLaren, A.; Davis, R.; Syyed, N.; MacIver, C.; et al. Postoperative Complications after Head and Neck Operations That Require Free Tissue Transfer—Prevalent, Morbid, and Costly. Br. J. Oral Maxillofac. Surg. 2017, 55, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.; Tapiovaara, L.; Bäck, L.; Lindford, A.; Lassus, P.; Lehtonen, L.; Mäkitie, A.; Keski-Säntti, H. Enhanced Recovery after Surgery (ERAS) Protocol Improves Patient Outcomes in Free Flap Surgery for Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 2024, 281, 907–914. [Google Scholar] [CrossRef]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern Radiotherapy for Head and Neck Cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef]
- Budach, V.; Thieme, A. Proton Therapy for Head and Neck Cancer. In Critical Issues in Head and Neck Oncology; Springer International Publishing: Cham, Switzerland, 2023; pp. 95–121. ISBN 9783031231742. [Google Scholar]
- Kiafi, P.; Chalkia, M.; Kouri, M.A.; Patatoukas, G.; Kollaros, N.; Kougioumtzopoulou, A.; Nikolatou-Galitis, O.; Kyrodimos, E.; Perisanidis, C.; Kouloulias, V.; et al. Photon vs. Proton Radiation Therapy in Head and Neck Cancer: A Review of Dosimetric Advantages and Patient Quality of Life. J. Cancer Metastasis Treat. 2024, 10, 31. [Google Scholar] [CrossRef]
- Iorio, G.C.; Denaro, N.; Livi, L.; Desideri, I.; Nardone, V.; Ricardi, U. Editorial: Advances in Radiotherapy for Head and Neck Cancer. Front. Oncol. 2024, 14, 1437237. [Google Scholar] [CrossRef]
- Jumaniyazova, E.; Smyk, D.; Vishnyakova, P.; Fatkhudinov, T.; Gordon, K. Photon- and Proton-Mediated Biological Effects: What Has Been Learned? Life 2022, 13, 30. [Google Scholar] [CrossRef]
- Meyer, F.; Fortin, A.; Wang, C.S.; Liu, G.; Bairati, I. Predictors of Severe Acute and Late Toxicities in Patients with Localized Head-and-Neck Cancer Treated with Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1454–1462. [Google Scholar] [CrossRef]
- Minicucci, E.M.; da Silva, G.N.; Salvadori, D.M.F. Relationship between Head and Neck Cancer Therapy and Some Genetic Endpoints. World J. Clin. Oncol. 2014, 5, 93–102. [Google Scholar] [CrossRef]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted Therapy for Head and Neck Cancer: Signaling Pathways and Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Brockstein, B.E.; Vokes, E.E. Oral Chemotherapy in Head and Neck Cancer. Drugs 1999, 58, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Guidi, A.; Codecà, C.; Ferrari, D. Chemotherapy and Immunotherapy for Recurrent and Metastatic Head and Neck Cancer: A Systematic Review. Med. Oncol. 2018, 35, 37. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Metch, B.; Schuller, D.E.; Ensley, J.F.; Hutchins, L.F.; Triozzi, P.; Kish, J.A.; McClure, S.; VonFeldt, E.; Williamson, S.K. Randomized Comparison of Cisplatin plus Fluorouracil and Carboplatin plus Fluorouracil versus Methotrexate in Advanced Squamous-Cell Carcinoma of the Head and Neck: A Southwest Oncology Group Study. J. Clin. Oncol. 1992, 10, 1245–1251. [Google Scholar] [CrossRef]
- Jacobs, C.; Lyman, G.; Velez-García, E.; Sridhar, K.S.; Knight, W.; Hochster, H.; Goodnough, L.T.; Mortimer, J.E.; Einhorn, L.H.; Schacter, L. A Phase III Randomized Study Comparing Cisplatin and Fluorouracil as Single Agents and in Combination for Advanced Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 1992, 10, 257–263. [Google Scholar] [CrossRef]
- Ma, S.J.; Zhu, S.; Virk, J.; Koempel, A.; Bhateja, P.; Gogineni, E.; Baliga, S.; Konieczkowski, D.; Mitchell, D.; Jhawar, S.; et al. Weekly Cisplatin Cycles and Outcomes for Chemoradiation in Head and Neck Cancer. JAMA Netw. Open 2024, 7, e2450272. [Google Scholar] [CrossRef]
- Catimel, G.; Verweij, J.; Mattijssen, V.; Hanauske, A.; Piccart, M.; Wanders, J.; Franklin, H.; Le Bail, N.; Clavel, M.; Kaye, S.B. Docetaxel (Taxotere): An Active Drug for the Treatment of Patients with Advanced Squamous Cell Carcinoma of the Head and Neck. EORTC Early Clinical Trials Group. Ann. Oncol. 1994, 5, 533–537. [Google Scholar] [CrossRef]
- Dreyfuss, A.I.; Clark, J.R.; Norris, C.M.; Rossi, R.M.; Lucarini, J.W.; Busse, P.M.; Poulin, M.D.; Thornhill, L.; Costello, R.; Posner, M.R. Docetaxel: An Active Drug for Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 1996, 14, 1672–1678. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Kitamura, N.; Sento, S.; Yoshizawa, Y.; Sasabe, E.; Kudo, Y.; Yamamoto, T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 22, 240. [Google Scholar] [CrossRef]
- Forman, R.; Deshpande, H.; Burtness, B.; Bhatia, A.K. Efficacy and Toxicity of Weekly Paclitaxel, Carboplatin, and Cetuximab as Induction Chemotherapy or in Cases of Metastases or Relapse for Head and Neck Cancer with a Focus on Elderly or Frail Patients. Head Neck 2022, 44, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Samman, N. Speech and Swallowing Following Tongue Cancer Surgery and Free Flap Reconstruction—A Systematic Review. Oral Oncol. 2013, 49, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar Upadhyay, A.; Prakash, A.; Singodia, P.; Ravi Kiran, S.; Shankar, R. Treatment Complications of Head and Neck Cancers and Rehabilitation Measures: A Narrative Review. Cureus 2024, 16, e61173. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Early Side Effects of Radiation Treatment for Head and Neck Cancer. Cancer Radiother. 2021, 25, 507–513. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Sallah, S.; Karlsson, U.; Antoine, J.E. Combined Chemotherapy and Radiation Therapy for Head and Neck Malignancies: Quality of Life Issues. Cancer 2002, 94, 1131–1141. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Gulati, S.; Crist, M.; Riaz, M.K.; Takiar, V.; Lehn, M.; Monroe, I.; Palackdharry, S.; Kurtzweil, N.; Jandarov, R.; Harun, N.; et al. Durvalumab plus Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Nonrandomized, Phase II Clinical Trial. Clin. Cancer Res. 2023, 29, 1906–1915. [Google Scholar] [CrossRef]
- Gong, Y.; Bao, L.; Xu, T.; Yi, X.; Chen, J.; Wang, S.; Pan, Z.; Huang, P.; Ge, M. The Tumor Ecosystem in Head and Neck Squamous Cell Carcinoma and Advances in Ecotherapy. Mol. Cancer 2023, 22, 68. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, J.; Wang, H.; Zhao, Y.; Qu, S.; Chen, N.; Chen, X.; Sun, Y.; He, X.; Hu, C.; et al. Tislelizumab plus Chemotherapy as First-Line Treatment for Recurrent or Metastatic Nasopharyngeal Cancer: A Multicenter Phase 3 Trial (RATIONALE-309). Cancer Cell 2023, 41, 1061–1072.e4. [Google Scholar] [CrossRef]
- Wong, W.; Cracchiolo, J.R.; Riaz, N.; Ganly, I.; Sherman, E.J.; Ho, A.L.; Morris, L.; Ghossein, R.A.; Haque, S.; Hung, K.W.; et al. Neoadjuvant Cemiplimab with Platinum-Doublet Chemotherapy and Cetuximab to de-Escalate Surgery and Omit Adjuvant Radiation in Locoregionally Advanced Head & Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2023, 41, 6019. [Google Scholar]
- Dunn, L.; Ho, A.L.; Eng, J.; Michel, L.S.; Fetten, J.V.; Warner, E.; Kriplani, A.; Zhi, W.I.; Ng, K.K.; Haque, S.; et al. A Phase I/Ib Study of Lenvatinib and Cetuximab in Patients with Recurrent/metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2020, 38, 6541. [Google Scholar] [CrossRef]
- Hendler, F.J.; Ozanne, B.W. Human Squamous Cell Lung Cancers Express Increased Epidermal Growth Factor Receptors. J. Clin. Investig. 1984, 74, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zouhair, A.; Azria, D.; Ozsahin, M. The Epidermal Growth Factor Receptor (EGFR) in Head and Neck Cancer: Its Role and Treatment Implications. Radiat. Oncol. 2006, 1, 11. [Google Scholar] [CrossRef]
- Robert, F.; Ezekiel, M.P.; Spencer, S.A.; Meredith, R.F.; Bonner, J.A.; Khazaeli, M.B.; Saleh, M.N.; Carey, D.; LoBuglio, A.F.; Wheeler, R.H.; et al. Phase I Study of Anti--Epidermal Growth Factor Receptor Antibody Cetuximab in Combination with Radiation Therapy in Patients with Advanced Head and Neck Cancer. J. Clin. Oncol. 2001, 19, 3234–3243. [Google Scholar] [CrossRef]
- Baselga, J.; Pfister, D.; Cooper, M.R.; Cohen, R.; Burtness, B.; Bos, M.; D’Andrea, G.; Seidman, A.; Norton, L.; Gunnett, K.; et al. Phase I Studies of Anti-Epidermal Growth Factor Receptor Chimeric Antibody C225 Alone and in Combination with Cisplatin. J. Clin. Oncol. 2000, 18, 904–914. [Google Scholar] [CrossRef]
- Lefebvre, J.L.; Pointreau, Y.; Rolland, F.; Alfonsi, M.; Baudoux, A.; Sire, C.; de Raucourt, D.; Malard, O.; Degardin, M.; Tuchais, C.; et al. Induction Chemotherapy Followed by Either Chemoradiotherapy or Bioradiotherapy for Larynx Preservation: The TREMPLIN Randomized Phase II Study. J. Clin. Oncol. 2013, 31, 853–859. [Google Scholar] [CrossRef]
- Ghi, M.G.; Paccagnella, A.; Ferrari, D.; Foa, P.; Alterio, D.; Codecà, C.; Nolè, F.; Verri, E.; Orecchia, R.; Morelli, F.; et al. Induction TPF Followed by Concomitant Treatment versus Concomitant Treatment Alone in Locally Advanced Head and Neck Cancer. A Phase II-III Trial. Ann. Oncol. 2017, 28, 2206–2212. [Google Scholar] [CrossRef]
- Nakano, K. Progress of Molecular Targeted Therapy for Head and Neck Cancer in Clinical Aspects. Mol. Biomed. 2021, 2, 15. [Google Scholar] [CrossRef]
- Grade, H., III; Grade, H., IV. StatBite: Radiation Therapy plus Cetuximab: Skin Reactions from 71 Head and Neck Cancer Patients from 11 Institutions in Europe. J. Natl. Cancer Inst. 2010, 102, 75. [Google Scholar]
- Specenier, P.; Vermorken, J.B. Cetuximab: Its Unique Place in Head and Neck Cancer Treatment. Biologics 2013, 7, 77–90. [Google Scholar] [PubMed]
- Waris, W.; Naik, S.; Idrees, I.; Taha, H.; Camosino, L.; Mehrishi, A.; Saif, M.W. Severe Cutaneous Reaction to Cetuximab with Possible Association with the Use of over-the-Counter Skin Care Products in a Patient with Oropharyngeal Cancer. Cutan. Ocul. Toxicol. 2009, 28, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Kalanjeri, S.; Stover, D.; Lee, R. Acute Eosinophilic Pneumonia Associated with Cetuximab. Chest 2012, 142, 959A. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.-Z.; Shi, D.; Shi, X.-Y.; Zou, Z.; Zhao, J.-H. Incidence and Risk of Severe Neutropenia in Advanced Cancer Patients Treated with Cetuximab: A Meta-Analysis. Drugs R D 2011, 11, 317–326. [Google Scholar] [CrossRef]
- Cui, R.; Chu, L.; Liu, Z.-Q.; Xiao, Y.-Y.; Zhu, X.-L.; Chen, Y.-J.; Xu, Q. Hematologic Toxicity Assessment in Solid Tumor Patients Treated with Cetuximab: A Pooled Analysis of 18 Randomized Controlled Trials. Int. J. Cancer 2016, 138, 2771–2773. [Google Scholar] [CrossRef]
- Stanbouly, D.; Philipone, E.; Morlandt, A.B.; Kaleem, A.; Chuang, S.-K.; Patel, N. Adverse Events Secondary to Cetuximab Therapy in Head & Neck Cancer Therapy and Risk Factors for Serious Outcomes. Oral Oncol. 2022, 131, 105952. [Google Scholar]
- Singh, P.; Contente, M.; Bennett, B.; Hall, J.; Bailey, H.; Bailey, A.; Zarrelli, L.; Polanco Sanchez, C. Real-World Treatment Patterns and Outcomes in Patients with Head and Neck Cancer: Point-in-Time Survey of Oncologists in Italy and Spain. Adv. Ther. 2021, 38, 4722–4735. [Google Scholar] [CrossRef]
- Ng, K.; Metcalf, R.; Sacco, J.; Kong, A.; Wheeler, G.; Forsyth, S.; Bhat, R.; Ward, J.; Ensell, L.; Lowe, H.; et al. Protocol for the EACH Trial: A Multicentre Phase II Study Evaluating the Safety and Antitumour Activity of the Combination of Avelumab, an Anti-PD-L1 Agent, and Cetuximab, as Any Line Treatment for Patients with Recurrent/metastatic Head and Neck Squamous Cell Cancer (HNSCC) in the UK. BMJ Open 2023, 13, e070391. [Google Scholar]
- Chung, C.H.; Li, J.; Steuer, C.E.; Bhateja, P.; Johnson, M.; Masannat, J.; Poole, M.I.; Song, F.; Hernandez-Prera, J.C.; Molina, H.; et al. Phase II Multi-Institutional Clinical Trial Result of Concurrent Cetuximab and Nivolumab in Recurrent And/or Metastatic Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 2329–2338. [Google Scholar] [CrossRef]
- Dhillon, S. Capmatinib: First Approval. Drugs 2020, 80, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.P.; Gilbert, J.; Levine, M.A.; Chakravarti, A.; Haigentz, M., Jr.; Saba, N.F.; Ikpeazu, C.V.; et al. Phase III Randomized Trial of Chemotherapy with or without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, J.; Li, Y.; Yang, X.; Wang, Q.; Wen, Y.; Yan, M.; Zhang, J.; Xu, Q.; Wei, Y.; et al. Targeting Epigenetic Modulation of Cholesterol Synthesis as a Therapeutic Strategy for Head and Neck Squamous Cell Carcinoma. Cell Death Dis. 2021, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Yousefnia, S.; Seyed Forootan, F.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Diverse Roles of Fatty Acid Binding Proteins (FABPs) in Development and Pathogenesis of Cancers. Gene 2018, 676, 171–183. [Google Scholar] [CrossRef]
- Fang, L.-Y.; Wong, T.-Y.; Chiang, W.-F.; Chen, Y.-L. Fatty-Acid-Binding Protein 5 Promotes Cell Proliferation and Invasion in Oral Squamous Cell Carcinoma: FABP5 Promotes Oral Cancer Cell Proliferation and Invasion. J. Oral Pathol. Med. 2010, 39, 342–348. [Google Scholar] [CrossRef]
- Vidotto, A.; Polachini, G.M.; de Paula-Silva, M.; Oliani, S.M.; Henrique, T.; López, R.V.M.; Cury, P.M.; Nunes, F.D.; Góis-Filho, J.F.; de Carvalho, M.B.; et al. Differentially Expressed Proteins in Positive versus Negative HNSCC Lymph Nodes. BMC Med. Genom. 2018, 11, 73. [Google Scholar] [CrossRef]
- Agostini, M.; Silva, S.D.; Zecchin, K.G.; Coletta, R.D.; Jorge, J.; Loda, M.; Graner, E. Fatty Acid Synthase Is Required for the Proliferation of Human Oral Squamous Carcinoma Cells. Oral Oncol. 2004, 40, 728–735. [Google Scholar] [CrossRef]
- Agostini, M.; Almeida, L.Y.; Bastos, D.C.; Ortega, R.M.; Moreira, F.S.; Seguin, F.; Zecchin, K.G.; Raposo, H.F.; Oliveira, H.C.F.; Amoêdo, N.D.; et al. The Fatty Acid Synthase Inhibitor Orlistat Reduces the Growth and Metastasis of Orthotopic Tongue Oral Squamous Cell Carcinomas. Mol. Cancer Ther. 2014, 13, 585–595. [Google Scholar] [CrossRef]
- Perri, F.; Della Vittoria Scarpati, G.; Pontone, M.; Marciano, M.L.; Ottaiano, A.; Cascella, M.; Sabbatino, F.; Guida, A.; Santorsola, M.; Maiolino, P.; et al. Cancer Cell Metabolism Reprogramming and Its Potential Implications on Therapy in Squamous Cell Carcinoma of the Head and Neck: A Review. Cancers 2022, 14, 3560. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Wen, Y.; Wen, J. Head and Neck Cancer: Pathogenesis and Targeted Therapy. MedComm 2024, 5, e702. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Licitra, L.; Fayette, J.; Even, C.; Blumenschein, G., Jr.; Harrington, K.J.; Guigay, J.; Vokes, E.E.; Saba, N.F.; Haddad, R.; et al. Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin. Cancer Res. 2019, 25, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, W.; Wang, W.; Wu, Y.; Fang, M.; Huang, X.; Han, P.; Zhang, Q.; Dong, P.; Zhou, X.; et al. Finotonlimab with Chemotherapy in Recurrent or Metastatic Head and Neck Cancer: A Randomized Phase 3 Trial. Nat. Med. 2024, 30, 2568–2575. [Google Scholar] [CrossRef]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current Perspectives on Statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef]
- Seol, S.; Choi, J.R.; Choi, B.; Kim, S.; Jeon, J.Y.; Park, K.N.; Park, J.H.; Park, M.W.; Eun, Y.-G.; Park, J.J.; et al. Effect of Statin Use on Head and Neck Cancer Prognosis in a Multicenter Study Using a Common Data Model. Sci. Rep. 2023, 13, 19770. [Google Scholar] [CrossRef]
- Demierre, M.-F.; Higgins, P.D.R.; Gruber, S.B.; Hawk, E.; Lippman, S.M. Statins and Cancer Prevention. Nat. Rev. Cancer 2005, 5, 930–942. [Google Scholar] [CrossRef]
- Sheridan, A.; Wheeler-Jones, C.P.D.; Gage, M.C. The Immunomodulatory Effects of Statins on Macrophages. Immuno 2022, 2, 317–343. [Google Scholar] [CrossRef]
- Kansal, V.; Burnham, A.J.; Kinney, B.L.C.; Saba, N.F.; Paulos, C.; Lesinski, G.B.; Buchwald, Z.S.; Schmitt, N.C. Statin Drugs Enhance Responses to Immune Checkpoint Blockade in Head and Neck Cancer Models. J. Immunother. Cancer 2023, 11, e005940. [Google Scholar] [CrossRef]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e4. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Hu, Z.; Xiong, F.; Yang, Y.; Peng, C.; Wang, D.; Li, X. Lipid Metabolism Reprogramming in Tumor-Associated Macrophages and Implications for Therapy. Lipids Health Dis. 2023, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, K.; Zhu, H.; Cheng, M.; Chen, S.; Ling, R.; Wang, C.; Chen, D. ITGB6 Modulates Resistance to Anti-CD276 Therapy in Head and Neck Cancer by Promoting PF4+ Macrophage Infiltration. Nat. Commun. 2024, 15, 7077. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.; Li, Y.; Zhao, Y.; Sharma, A.; Liu, H.; Schmidt-Wolf, I.G.H. Recent Advances and Future Perspectives of CAR-T Cell Therapy in Head and Neck Cancer. Front. Immunol. 2023, 14, 1213716. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Fu, R.; Man, Q.-W.; Yang, G.; Liu, B.; Bu, L.-L. Advances in CAR-T Cell Therapy in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 2173. [Google Scholar] [CrossRef]
- Manzar, G.S.; Rafei, H.; Kumar, B.; Shanley, M.; Acharya, S.; Liu, B.; Xu, A.; Wang, X.A.; Islam, S.; Kaplan, M.; et al. Radiation Therapy Sensitizes Head-and-Neck Cancer Cells to Killing by Chimeric Antigen Receptor (CAR)-NK Cells Targeting CD70. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, S167–S168. [Google Scholar] [CrossRef]
- Nowak, J.; Bentele, M.; Kutle, I.; Zimmermann, K.; Lühmann, J.L.; Steinemann, D.; Kloess, S.; Koehl, U.; Roßberg, W.; Ahmed, A.; et al. CAR-NK Cells Targeting HER1 (EGFR) Show Efficient Anti-Tumor Activity against Head and Neck Squamous Cell Carcinoma (HNSCC). Cancers 2023, 15, 3169. [Google Scholar] [CrossRef]
- Ciulean, I.S.; Fischer, J.; Quaiser, A.; Bach, C.; Abken, H.; Tretbar, U.S.; Fricke, S.; Koehl, U.; Schmiedel, D.; Grunwald, T. CD44v6 Specific CAR-NK Cells for Targeted Immunotherapy of Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2023, 14, 1290488. [Google Scholar] [CrossRef]
- Jiménez-Labaig, P.; Rullan, A.; Hernando-Calvo, A.; Llop, S.; Bhide, S.; O’Leary, B.; Braña, I.; Harrington, K.J. A Systematic Review of Antibody-Drug Conjugates and Bispecific Antibodies in Head and Neck Squamous Cell Carcinoma and Nasopharyngeal Carcinoma: Charting the Course of Future Therapies. Cancer Treat. Rev. 2024, 128, 102772. [Google Scholar] [CrossRef]
- Dewaele, L.; Fernandes, R.A. Bispecific T-Cell Engagers for the Recruitment of T Cells in Solid Tumors: A Literature Review. Immunother. Adv. 2025, 5, ltae005. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, in Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Virone-Oddos, A.; Beninga, J.; Rossi, B.; Nicolazzi, C.; Amara, C.; Blanchard-Alvarez, A.; Gourdin, N.; Courta, J.; Basset, A.; et al. Control of Acute Myeloid Leukemia by a Trifunctional NKp46-CD16a-NK Cell Engager Targeting CD123. Nat. Biotechnol. 2023, 41, 1296–1306. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Vetizou, M.; Blanchard Alvarez, A.; Vagne, C.; Habif, G.; Batista, L.; Baron, W.; Belaïd, N.; Girard-Madoux, M.; et al. Antitumor Immunity Induced by Antibody-Based Natural Killer Cell Engager Therapeutics Armed with Not-Alpha IL-2 Variant. Cell Rep. Med. 2022, 3, 100783. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-Infiltrating Lymphocyte Therapy for Human Papillomavirus-Associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Jimeno, A.; Papa, S.; Haigentz, M.; Rodríguez-Moreno, J.; Schardt, J.; Fardis, M.; Finckenstein, F.G.; Fiaz, R.; Chen, G.; Cacovean, A.; et al. 353 Safety and Efficacy of Tumor Infiltrating Lymphocytes (TIL, LN-145) in Combination with Pembrolizumab for Advanced, Recurrent or Metastatic HNSCC. J. Immunother. Cancer 2020, 8, A378. [Google Scholar]
- O’Malley, D.; Lee, S.; Psyrri, A.; Sukari, A.; Thomas, S.; Wenham, R.; Gogas, H.; Jazaeri, A.; Monk, B.; Rose, P.; et al. 492 Phase 2 Efficacy and Safety of Autologous Tumor-Infiltrating Lymphocyte (TIL) Cell Therapy in Combination with Pembrolizumab in Immune Checkpoint Inhibitor-Naïve Patients with Advanced Cancers. J. Immunother. Cancer 2021, 9, A523–A524. [Google Scholar]
- Albarrán, V.; San Román, M.; Pozas, J.; Chamorro, J.; Rosero, D.I.; Guerrero, P.; Calvo, J.C.; González, C.; García de Quevedo, C.; Pérez de Aguado, P.; et al. Adoptive T Cell Therapy for Solid Tumors: Current Landscape and Future Challenges. Front. Immunol. 2024, 15, 1352805. [Google Scholar] [CrossRef]
- Wang, H.; Yu, J.; Lu, X.; He, X. Nanoparticle Systems Reduce Systemic Toxicity in Cancer Treatment. Nanomedicine 2016, 11, 103–106. [Google Scholar] [CrossRef]
- Ruiz-Pulido, G.; Medina, D.I.; Barani, M.; Rahdar, A.; Sargazi, G.; Baino, F.; Pandey, S. Nanomaterials for the Diagnosis and Treatment of Head and Neck Cancers: A Review. Materials 2021, 14, 3706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Puri, S.; Mazza, M.; Roy, G.; England, R.M.; Zhou, L.; Nourian, S.; Anand Subramony, J. Evolution of Nanomedicine Formulations for Targeted Delivery and Controlled Release. Adv. Drug Deliv. Rev. 2023, 200, 114962. [Google Scholar] [CrossRef]
- Li, H.-X.; Gong, Y.-W.; Yan, P.-J.; Xu, Y.; Qin, G.; Wen, W.-P.; Teng, F.-Y. Revolutionizing Head and Neck Squamous Cell Carcinoma Treatment with Nanomedicine in the Era of Immunotherapy. Front. Immunol. 2024, 15, 1453753. [Google Scholar] [CrossRef]
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines 2022, 10, 1187. [Google Scholar] [CrossRef]
- Werner, M.E.; Copp, J.A.; Karve, S.; Cummings, N.D.; Sukumar, R.; Li, C.; Napier, M.E.; Chen, R.C.; Cox, A.D.; Wang, A.Z. Folate-Targeted Polymeric Nanoparticle Formulation of Docetaxel Is an Effective Molecularly Targeted Radiosensitizer with Efficacy Dependent on the Timing of Radiotherapy. ACS Nano 2011, 5, 8990–8998. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amin, A.R.M.R.; Wang, X.; Zuckerman, J.E.; Choi, C.H.J.; Zhou, B.; Wang, D.; Nannapaneni, S.; Koenig, L.; Chen, Z.; et al. Systemic Delivery of siRNA Nanoparticles Targeting RRM2 Suppresses Head and Neck Tumor Growth. J. Control. Release 2012, 159, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Arany, S.; Benoit, D.S.W.; Dewhurst, S.; Ovitt, C.E. Nanoparticle-Mediated Gene Silencing Confers Radioprotection to Salivary Glands in Vivo. Mol. Ther. 2013, 21, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Kampel, L.; Goldsmith, M.; Ramishetti, S.; Veiga, N.; Rosenblum, D.; Gutkin, A.; Chatterjee, S.; Penn, M.; Lerman, G.; Peer, D.; et al. Therapeutic Inhibitory RNA in Head and Neck Cancer via Functional Targeted Lipid Nanoparticles. J. Control. Release 2021, 337, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.; Kempson, I.; Bezak, E.; Phillips, W. Predictive Modeling of Hypoxic Head and Neck Cancers during Fractionated Radiotherapy with Gold Nanoparticle Radiosensitization. Med. Phys. 2021, 48, 3120–3133. [Google Scholar] [CrossRef]
- Dubey, P.; Sertorio, M.; Takiar, V. Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy. Cancers 2022, 14, 514. [Google Scholar] [CrossRef]
- Bu, L.-L.; Wang, H.-Q.; Pan, Y.; Chen, L.; Wu, H.; Wu, X.; Zhao, C.; Rao, L.; Liu, B.; Sun, Z.-J. Gelatinase-Sensitive Nanoparticles Loaded with Photosensitizer and STAT3 Inhibitor for Cancer Photothermal Therapy and Immunotherapy. J. Nanobiotechnol. 2021, 19, 379. [Google Scholar] [CrossRef]
- Salomon, N.; Selmi, A.; Grunwitz, C.; Kong, A.; Stanganello, E.; Neumaier, J.; Petschenka, J.; Diken, M.; Kreiter, S.; Türeci, Ö.; et al. Local Radiotherapy and E7 RNA-LPX Vaccination Show Enhanced Therapeutic Efficacy in Preclinical Models of HPV16+ Cancer. Cancer Immunol. Immunother. 2022, 71, 1975–1988. [Google Scholar] [CrossRef]
- Grunwitz, C.; Salomon, N.; Vascotto, F.; Selmi, A.; Bukur, T.; Diken, M.; Kreiter, S.; Türeci, Ö.; Sahin, U. HPV16 RNA-LPX Vaccine Mediates Complete Regression of Aggressively Growing HPV-Positive Mouse Tumors and Establishes Protective T Cell Memory. Oncoimmunology 2019, 8, e1629259. [Google Scholar] [CrossRef]
- Tsai, J.M.; Nowak, R.P.; Ebert, B.L.; Fischer, E.S. Targeted Protein Degradation: From Mechanisms to Clinic. Nat. Rev. Mol. Cell Biol. 2024, 25, 740–757. [Google Scholar] [CrossRef]
- Zhong, G.; Chang, X.; Xie, W.; Zhou, X. Targeted Protein Degradation: Advances in Drug Discovery and Clinical Practice. Signal Transduct. Target. Ther. 2024, 9, 308. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Li, G.; Yang, Z.; Han, C.; Sun, X.; Sheng, C.; Ding, K.; Rao, Y. Targeting the Undruggables-the Power of Protein Degraders. Sci. Bull. 2024, 69, 1776–1797. [Google Scholar] [CrossRef] [PubMed]
- Kenten, J.H.; Roberts, S.F. Controlling Protein Levels in Eucaryotic Organisms. US Patent US6306663B1, 23 October 2001. [Google Scholar]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric Molecules That Target Proteins to the Skp1-Cullin-F Box Complex for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the “Undruggable” Cancer Targets. Nat. Rev. Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, J.; Zhou, P.; Zhou, J.; Xie, S. Targeting Undruggable Transcription Factors with PROTACs: Advances and Perspectives. J. Med. Chem. 2022, 65, 10183–10194. [Google Scholar] [CrossRef]
- Burke, M.R.; Smith, A.R.; Zheng, G. Overcoming Cancer Drug Resistance Utilizing PROTAC Technology. Front. Cell Dev. Biol. 2022, 10, 872729. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Kim, J.-M. Targeted Protein Degradation to Overcome Resistance in Cancer Therapies: PROTAC and N-Degron Pathway. Biomedicines 2022, 10, 2100. [Google Scholar] [CrossRef]
- Jin, J.; Wu, Y.; Zhao, Z.; Wu, Y.; Zhou, Y.-D.; Liu, S.; Sun, Q.; Yang, G.; Lin, J.; Nagle, D.G.; et al. Small-Molecule PROTAC Mediates Targeted Protein Degradation to Treat STAT3-Dependent Epithelial Cancer. JCI Insight 2022, 7, e160606. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Gorai, S.; Ghosh, A.; Alexiou, A.; Thorat, N.D. Revolutionizing Human Papillomavirus (HPV)-Related Cancer Therapies: Unveiling the Promise of Proteolysis Targeting Chimeras (PROTACs) and Proteolysis Targeting Antibodies (PROTABs) in Cancer Nano-Vaccines. J. Med. Virol. 2023, 95, e29135. [Google Scholar] [CrossRef]
- Zhang, S.; Lai, Y.; Pan, J.; Saeed, M.; Li, S.; Zhou, H.; Jiang, X.; Gao, J.; Zhu, Y.; Yu, H.; et al. PROTAC Prodrug-Integrated Nanosensitizer for Potentiating Radiation Therapy of Cancer. Adv. Mater. 2024, 36, e2314132. [Google Scholar] [CrossRef]
- Mukerjee, N.; Mukherjee, D. PROTAC-Based Therapeutics for Targeting HPV Oncoproteins in Head and Neck Cancers. Nano TransMed 2025, 4, 100071. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, C.; Wang, H.; Zhang, L.; Liu, Z.; Xu, P. The State of the Art of PROTAC Technologies for Drug Discovery. Eur. J. Med. Chem. 2022, 235, 114290. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pu, W.; Zheng, Q.; Ai, M.; Chen, S.; Peng, Y. Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy. Mol. Cancer 2022, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.L.; Katerji, M.; Afifi, M.; Nyswaner, K.; Woodroofe, C.C.; Edwards, Z.C.; Lindberg, E.; Bergman, K.L.; Gough, N.R.; Rubin, M.R.; et al. Targeting c-MYC and Gain-of-Function p53 through Inhibition or Degradation of the Kinase LZK Suppresses the Growth of HNSCC Tumors. Sci. Signal. 2025, 18, eado2857. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; Mathijssen, R.H.J.; Beijnen, J.H.; Huitema, A.D.R.; Steeghs, N. Individualized Dosing of Oral Targeted Therapies in Oncology Is Crucial in the Era of Precision Medicine. Eur. J. Clin. Pharmacol. 2019, 75, 1309–1318. [Google Scholar] [CrossRef]
- Moreau, K.; Coen, M.; Zhang, A.X.; Pachl, F.; Castaldi, M.P.; Dahl, G.; Boyd, H.; Scott, C.; Newham, P. Proteolysis-Targeting Chimeras in Drug Development: A Safety Perspective. Br. J. Pharmacol. 2020, 177, 1709–1718. [Google Scholar] [CrossRef]
- Dietz, A.; Boehm, A.; Mozet, C.; Wichmann, G.; Giannis, A. Current Aspects of Targeted Therapy in Head and Neck Tumors. Eur. Arch. Otorhinolaryngol. 2008, 265 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- Smith, B.E.; Wang, S.L.; Jaime-Figueroa, S.; Harbin, A.; Wang, J.; Hamman, B.D.; Crews, C.M. Differential PROTAC Substrate Specificity Dictated by Orientation of Recruited E3 Ligase. Nat. Commun. 2019, 10, 131. [Google Scholar] [CrossRef]
- Sok, J.C.; Coppelli, F.M.; Thomas, S.M.; Lango, M.N.; Xi, S.; Hunt, J.L.; Freilino, M.L.; Graner, M.W.; Wikstrand, C.J.; Bigner, D.D.; et al. Mutant Epidermal Growth Factor Receptor (EGFRvIII) Contributes to Head and Neck Cancer Growth and Resistance to EGFR Targeting. Clin. Cancer Res. 2006, 12, 5064–5073. [Google Scholar] [CrossRef]
- Novoplansky, O.; Fury, M.; Prasad, M.; Yegodayev, K.; Zorea, J.; Cohen, L.; Pelossof, R.; Cohen, L.; Katabi, N.; Cecchi, F.; et al. MET Activation Confers Resistance to Cetuximab, and Prevents HER2 and HER3 Upregulation in Head and Neck Cancer: MET/MAPK Drives Resistance to Cetuximab in HNSCC. Int. J. Cancer 2019, 145, 748–762. [Google Scholar] [CrossRef]
- Constantin, M.; Chifiriuc, M.C.; Bleotu, C.; Vrancianu, C.O.; Cristian, R.-E.; Bertesteanu, S.V.; Grigore, R.; Bertesteanu, G. Molecular Pathways and Targeted Therapies in Head and Neck Cancers Pathogenesis. Front. Oncol. 2024, 14, 1373821. [Google Scholar] [CrossRef]
- Yan, Y.; Kumar, A.B.; Finnes, H.; Markovic, S.N.; Park, S.; Dronca, R.S.; Dong, H. Combining Immune Checkpoint Inhibitors with Conventional Cancer Therapy. Front. Immunol. 2018, 9, 1739. [Google Scholar] [CrossRef]
- Kearney, V.; Chan, J.W.; Valdes, G.; Solberg, T.D.; Yom, S.S. The Application of Artificial Intelligence in the IMRT Planning Process for Head and Neck Cancer. Oral Oncol. 2018, 87, 111–116. [Google Scholar] [CrossRef]



| Compound | Class | Mechanism of Action | Exemplified Clinical Trial(s) | Use in HNC Therapy | FDA Approval | References |
|---|---|---|---|---|---|---|
| Cetuximab | Monoclonal antibody | Targets EGFR | NCT00004227 | R/M-HNCs alone and in combination therapies | Approved | [47] |
| Cetuximab with cisplatin or carboplatin and 5-fluorouracil | NCT00122460 | [48] | ||||
| Pembrolizumab monotherapy or pembrolizumab, and cisplatin or carboplatin in combination with 5-fluorouracil (‘pembro combo’) | Monoclonal antibody | Inhibits PD-1, enhances immune response | NCT02358031 | R/M-HNSCC | Approved | [17] |
| Nivolumab | Monoclonal antibody | Inhibits PD-1, enhances immune response | NCT02105636 | R/M-HNCs | Approved | [15] |
| Durvalumab with cetuximab | Monoclonal antibody | Inhibits PD-L1 interaction with PD-1, enhances immune response | NCT03691714 | Previously treated R/M-HNSCC | Phase II clinical trial | [49] |
| Atezolizumab | Monoclonal antibody | Inhibits PD-L1, enhances immune response | NCT04939480 | Local HNSCC | Phase II clinical trial | [50] |
| Tislelizumab with gemcitabine and cisplatin | Monoclonal antibody | Inhibits PD-1, enhances immune response | NCT03924986 | R/M nasopharyngeal cancer (NPC) | Phase III clinical trial | [51] |
| Cemiplimab with platinum-doublet chemotherapy, and cetuximab | Monoclonal antibody | Inhibits PD-1, enhances immune response | NCT04722523 | Locoregionally advanced (LA) HNSCC | Phase I clinical trial | [52] |
| Lenvatinib with cetuximab | Multikinase inhibitor | Inhibits VEGFR, FGFR, PDGFR, RET, and CD117 * | NCT03524326 | R/M-HNSCC | Phase I clinical trial | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordzińska-Rak, A.; Telejko, I.; Adamczuk, G.; Trombik, T.; Stepulak, A.; Błaszczak, E. Advancing Head and Neck Cancer Therapies: From Conventional Treatments to Emerging Strategies. Biomedicines 2025, 13, 1046. https://doi.org/10.3390/biomedicines13051046
Mordzińska-Rak A, Telejko I, Adamczuk G, Trombik T, Stepulak A, Błaszczak E. Advancing Head and Neck Cancer Therapies: From Conventional Treatments to Emerging Strategies. Biomedicines. 2025; 13(5):1046. https://doi.org/10.3390/biomedicines13051046
Chicago/Turabian StyleMordzińska-Rak, Aleksandra, Ilona Telejko, Grzegorz Adamczuk, Tomasz Trombik, Andrzej Stepulak, and Ewa Błaszczak. 2025. "Advancing Head and Neck Cancer Therapies: From Conventional Treatments to Emerging Strategies" Biomedicines 13, no. 5: 1046. https://doi.org/10.3390/biomedicines13051046
APA StyleMordzińska-Rak, A., Telejko, I., Adamczuk, G., Trombik, T., Stepulak, A., & Błaszczak, E. (2025). Advancing Head and Neck Cancer Therapies: From Conventional Treatments to Emerging Strategies. Biomedicines, 13(5), 1046. https://doi.org/10.3390/biomedicines13051046






