Relationship Between Depression and Neurodegeneration: Risk Factor, Prodrome, Consequence, or Something Else? A Scoping Review
Abstract
1. Introduction
- Investigating the first hypothesis is challenging, as another line of research suggests that depression may be more of a prodrome—a typical symptom that precedes the actual disease, serving as a “premonitory symptom” [32,33,34]. This suggests that early-diagnosed depression may predispose to pathological aging, while “late-life depression (LLD)” could serve as a prodrome for more severe latent conditions, supporting the thesis of a dichotomous relationship between early-life depression as a risk factor and LLD as a prodrome of dementia [34,35,36,37]. While it is methodologically possible to differentiate between early and later-life depression, studies have established arbitrary cut-off points since the timing of depressive symptoms relative to neurodegenerative disease onset cannot be objectively determined [32]. Two arguments appear to overlap and could both be valid [38].
- In parallel, other studies suggest neurodegeneration may be a key cause of depression, impacting emotional management circuits. While the psychological effects of a grim diagnosis are acknowledged, the role of neurodegeneration in depression should be emphasized [39].
- Finally, it cannot be ruled out that the association may depend on latent variables that covary with both; hence, it is well-known that these syndromes tend to manifest comorbidly due to medical factors and therefore, with each other. As reported above, the coexistence of neurodegeneration and depression is a phenomenon that has sparked various hypotheses. To provide a comprehensive understanding of this issue, this scoping review meticulously explores the four key theories that link these two pathological conditions, aiming to shed some light on this complex relationship.
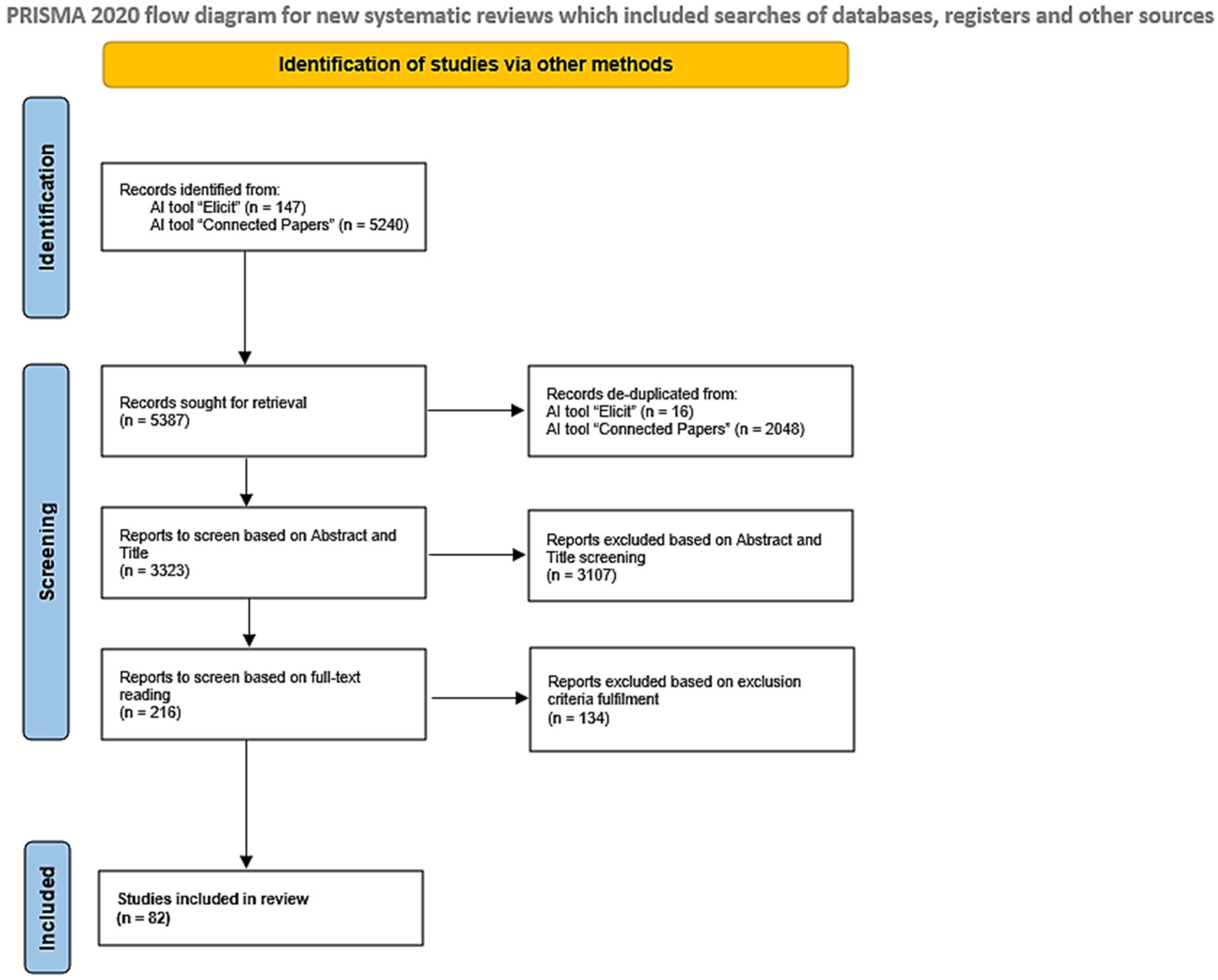
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Preselection Phase
2.3. Research Questions and Strategy
- “Can depression be a cause of neurodegeneration?”;
- “Can depression be a prodrome or early indicator of neurodegeneration?”;
- “Can depression be a consequence of neurodegeneration?”;
- “Is there a spurious or non-existent correlation between depression and neurodegeneration?”.
2.4. Data Processing and Expansion
3. Results
3.1. Depression as the Cause of Neurodegeneration
3.2. Depression as a Prodrome of Neurodegenerative Pathologies
3.3. Depression as a Consequence of Neurodegeneration
3.4. Depression and Neurodegeneration as a Spurious or Dissociated Relationship
3.5. Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| VaD | Vascular dementia |
| PD | Parkinson’s disease |
| DLB | Dementia with Lewy bodies |
| CBD | Corticobasal degeneration |
| PSP | Progressive supranuclear palsy |
| FTD | Frontotemporal dementia |
| ALS | Amyotrophic lateral sclerosis |
| MCI | Mild cognitive impairment |
| LLD | Late-life depression |
| PRISMA-ScR | PRISMA guidelines for scoping reviews |
| HPA | Hypothalamic–pituitary–adrenal |
| BDNF | Brain-derived neurotrophic factor |
| ROS | Reactive oxygen species |
References
- Knapskog, A.-B.; Barca, M.L.; Engedal, K. Prevalence of depression among memory clinic patients as measured by the Cornell Scale of Depression in Dementia. Aging Ment. Health 2014, 18, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Heterogeneity and comorbidity in dementia-depression syndromes. Int. J. Geriatr. Psychiatry 1991, 6, 125–127. [Google Scholar] [CrossRef]
- Tetsuka, S. Depression and Dementia in Older Adults: A Neuropsychological Review. Aging Dis. 2021, 12, 1920–1934. [Google Scholar] [CrossRef] [PubMed]
- Baquero, M.; Martín, N. Depressive symptoms in neurodegenerative diseases. World J. Clin. Cases 2015, 3, 682–693. [Google Scholar] [CrossRef]
- Winter, Y.; Korchounov, A.; Zhukova, T.V.; Bertschi, N.E. Depression in elderly patients with Alzheimer dementia or vascular dementia and its influence on their quality of life. J. Neurosci. Rural Pract. 2011, 2, 27–32. [Google Scholar] [CrossRef]
- Rotaru, L.; Gavriliuc, O.; Grosu, O. Depression in patients with Parkinson’s disease. Preliminary results of the cohort study. Bull. Acad. Sci. Moldova. Med. Sci. 2023, 74, 91–94. [Google Scholar] [CrossRef]
- Yapici Eser, H.; Bora, H.A.; Kuruoğlu, A. Depression and Parkinson disease: Prevalence, temporal relationship, and determinants. Turk. J. Med. Sci. 2017, 47, 499–503. [Google Scholar] [CrossRef]
- Chiu, P.-Y.; Wang, C.-W.; Tsai, C.-T.; Li, S.-H.; Lin, C.-L.; Lai, T.-J. Depression in dementia with Lewy bodies: A comparison with Alzheimer’s disease. PLoS ONE 2017, 12, e0179399. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Damangir, S.; Cermakova, P.; Aarsland, D.; Eriksdotter, M.; Religa, D. Comorbidity profile in dementia with Lewy bodies versus Alzheimer’s disease: A linkage study between the Swedish Dementia Registry and the Swedish National Patient Registry. Alzheimer’s Res. Ther. 2014, 6, 65. [Google Scholar] [CrossRef]
- Sakai, K.; Yamane, Y.; Yamamoto, Y.; Maeda, K. Depression in dementia with Lewy bodies. Seishin Shinkeigaku Zasshi = Psychiatr. Et Neurol. Jpn. 2013, 115, 1127–1134. [Google Scholar]
- Yamane, Y.; Sakai, K.; Maeda, K. Dementia with Lewy Bodies is associated with higher scores on the Geriatric Depression Scale than is Alzheimer’s disease. Psychogeriatrics 2011, 11, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bruns, M.B.; Josephs, K.A. Neuropsychiatry of corticobasal degeneration and progressive supranuclear palsy. Int. Rev. Psychiatry 2013, 25, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Sepehry, A.; Chakrabarty, T.; Jacova, C.; Robin Hsiung, G. P3–147: Prevalence of depressive mood in frontotemporal dementia (FTD): A meta-analysis of studies from 2000 to present. Alzheimer’s Dement. 2013, 9 Pt 15, 607. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Sepehry, A.A.; Jacova, C.; Hsiung, G.-Y.R. The Prevalence of Depressive Symptoms in Frontotemporal Dementia: A Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2015, 39, 257–271. [Google Scholar] [CrossRef]
- Young, C.A.; Ealing, J.; McDermott, C.J.; Williams, T.L.; Al-Chalabi, A.; Majeed, T.; Talbot, K.; Harrower, T.; Faull, C.; Malaspina, A.; et al. Prevalence of depression in amyotrophic lateral sclerosis/motor neuron disease: Multi-attribute ascertainment and trajectories over 30 months. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 82–90. [Google Scholar] [CrossRef]
- Heidari, M.E.; Nadali, J.; Parouhan, A.; Azarafraz, M.; Tabatabai, S.M.; Irvani, S.S.N.; Eskandari, F.; Gharebaghi, A. Prevalence of depression among amyotrophic lateral sclerosis (ALS) patients: A systematic review and meta-analysis. J. Affect. Disord. 2021, 287, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.; Mariosa, D.; Ingre, C.; Lundholm, C.; Wirdefeldt, K.; Roos, P.M.; Fang, F. Depression in amyotrophic lateral sclerosis. Neurology 2016, 86, 2271–2277. [Google Scholar] [CrossRef]
- Pisa, F.E.; Logroscino, G.; Casetta, A.; Cecotti, L.; Verriello, L.; Bratina, A.; Sartori, A.; Lazzarino De Lorenzo, L.; Eleopra, R.; Barbone, F. The Use of Antidepressant Medication before and after the Diagnosis of Amyotrophic Lateral Sclerosis: A Population-Based Cohort Study. Neuroepidemiology 2015, 44, 91–98. [Google Scholar] [CrossRef]
- Tipton, P.W.; Ertekin-Taner, N. Mild Cognitive Impairment and Alzheimer Disease. In Mayo Clinic Neurology Board Review, 2nd ed.; Oxford University Press: New York, NY, USA, 2021; pp. 662–671. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Christie, G.J.; Cosco, T.D.; Moreno, S. Neuroimaging and analytical methods for studying the pathways from mild cognitive impairment to Alzheimer’s disease: Protocol for a rapid systematic review. Syst. Rev. 2020, 9, 71. [Google Scholar] [CrossRef]
- Janoutová, J.; Šerý, O.; Hosák, L.; Janout, V. Is Mild Cognitive Impairment a Precursor of Alzheimer’s Disease? Short Review. Cent. Eur. J. Public Health 2015, 23, 365–367. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. Contin. Lifelong Learn. Neurol. 2016, 22, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Petersen, R.C.; Parisi, J.E.; Ivnik, R.J.; Kokmen, E.; Tangalos, E.G.; Waring, S. Definition, course, and outcome of mild cognitive impairment. Aging Neuropsychol. Cogn. 1996, 3, 141–147. [Google Scholar] [CrossRef]
- Sobreira, G.; Aleixo, M.A.; Moreia, C.; Oliveira, J. Depression and mild cognitive impairment—Comorbidity and/or continuum? Eur. Psychiatry 2016, 33, S190–S191. [Google Scholar] [CrossRef]
- Reischies, F.M.; Neu, P. Comorbidity of mild cognitive disorder and depression—A neuropsychological analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2000, 250, 186–193. [Google Scholar] [CrossRef]
- Mayor, S. One in three with mild cognitive impairment has depression, review finds. BMJ 2016, 355, i6387. [Google Scholar] [CrossRef]
- Ismail, Z.; Elbayoumi, H.; Fischer, C.E.; Hogan, D.B.; Millikin, C.P.; Schweizer, T.; Mortby, M.E.; Smith, E.E.; Patten, S.B.; Fiest, K.M. Prevalence of Depression in Patients with Mild Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017, 74, 58–67. [Google Scholar] [CrossRef]
- Bazin, N.; Bratu, L. Depression in the elderly: Prodroma or risk factor for dementia? A critical review of the literature. Gériatr. Psychol. Neuropsychiatr. Vieil. 2014, 12, 289–297. [Google Scholar] [CrossRef]
- Kim, H.K.; Nunes, P.V.; Oliveira, K.C.; Young, L.T.; Lafer, B. Neuropathological relationship between major depression and dementia: A hypothetical model and review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 67, 51–57. [Google Scholar] [CrossRef]
- Sotiropoulos, I. The neurodegenerative potential of chronic stress: A link between depression and Alzheimer’s disease. Adv. Exp. Med. Biol. 2015, 822, 221–222. [Google Scholar] [CrossRef]
- Schweitzer, I.; Tuckwell, V.; O’Brien, J.; Ames, D. Is late onset depression a prodrome to dementia? Int. J. Geriatr. Psychiatry 2002, 17, 997–1005. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef]
- Bennett, S.; Thomas, A.J. Depression and dementia: Cause, consequence or coincidence? Maturitas 2014, 79, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Piras, F.; Banaj, N.; Porcari, D.E.; Piras, F.; Spalletta, G. Later life depression as risk factor for developing dementia: Epidemiological evidence, predictive models, preventive strategies and future trends. Minerva Medica 2021, 112, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Brommelhoff, J.A.; Gatz, M.; Johansson, B.; McArdle, J.J.; Fratiglioni, L.; Pedersen, N.L. Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol. Aging 2009, 24, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kessing, L.V. Depression and the risk for dementia. Curr. Opin. Psychiatry 2012, 25, 457–461. [Google Scholar] [CrossRef]
- Rubin, R. Exploring the Relationship Between Depression and Dementia. JAMA 2018, 320, 961–962. [Google Scholar] [CrossRef]
- Botto, R.; Callai, N.; Cermelli, A.; Causarano, L.; Rainero, I. Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol. Sci. 2022, 43, 4107–4124. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467. [Google Scholar] [CrossRef]
- Elicit: The AI Research Assistant. Available online: https://elicit.com/?via=startfree&gad_source=1&gclid=Cj0KCQiA57G5BhDUARIsACgCYnyb76ME0U7vP-w2pnNrwVYlITcvNmUd3imDqqbKngWnLn4zsH5OFn8aAp5iEALw_wcB (accessed on 26 July 2024).
- Connected Papers|Find and Explore Academic Papers. Available online: https://www.connectedpapers.com/ (accessed on 4 August 2024).
- Open Science Framework (OSF). Center for Open Science. Available online: https://osf.io (accessed on 9 March 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fujishiro, H. Late-Life Depression and Lewy Body Disease. Am. J. Geriatr. Psychiatry 2019, 27, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K.; Byers, A.L.; McCormick, M.; Schaefer, C.; Whitmer, R.A. Midlife vs. late-life depressive symptoms and risk of dementia: Differential effects for Alzheimer disease and vascular dementia. Arch. Gen. Psychiatry 2012, 69, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Asmer, M.S.; Kirkham, J.; Newton, H.; Ismail, Z.; Elbayoumi, H.; Leung, R.H.; Seitz, D.P. Meta-Analysis of the Prevalence of Major Depressive Disorder Among Older Adults With Dementia. J. Clin. Psychiatry 2018, 79, 17r11772. [Google Scholar] [CrossRef]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Puentes, R.C.; Habeych, M.E. Subtypes of depression among patients with Alzheimer’s disease and other dementias. Alzheimer’s Dement. 2010, 6, 63–69. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.B.; Lee, T.J.; Lee, D.Y.; Jhoo, J.H.; Youn, J.C.; Choo, I.H.; Choi, E.A.; Jeong, J.W.; Choe, J.Y.; et al. Depression in Vascular Dementia Is Quantitatively and Qualitatively Different from Depression in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2007, 23, 67–73. [Google Scholar] [CrossRef]
- O’Brien, J. Behavioral Symptoms in Vascular Cognitive Impairment and Vascular Dementia. Int. Psychogeriatr. 2003, 15, 133–138. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Vascular Disease, Depression, and Dementia. J. Am. Geriatr. Soc. 2003, 51, 1178–1180. [Google Scholar] [CrossRef]
- Groves, W.C.; Brandt, J.; Steinberg, M.; Warren, A.; Rosenblatt, A.; Baker, A.; Lyketsos, C.G. Vascular Dementia and Alzheimer’s Disease: Is There a Difference?: A Comparison of Symptoms by Disease Duration. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 305–315. [Google Scholar] [CrossRef]
- Hargrave, R.; Reed, B.; Mungas, D. Depressive Syndromes and Functional Disability in Dementia. J. Geriatr. Psychiatry Neurol. 2000, 13, 72–77. [Google Scholar] [CrossRef]
- Newman, S.C. The prevalence of depression in Alzheimer’s disease and vascular dementia in a population sample. J. Affect. Disord. 1999, 52, 169–176. [Google Scholar] [CrossRef]
- Ballard, C.; Bannister, C.; Solis, M.; Oyebode, F.; Wilcock, G. The prevalence, associations and symptoms of depression amongst dementia sufferers. J. Affect. Disord. 1996, 36, 135–144. [Google Scholar] [CrossRef]
- Cooper, J.K.; Mungas, D. Risk Factor and Behavioral Differences Between Vascular and Alzheimer’s Dementias: The Pathway to End-Stage Disease. J. Geriatr. Psychiatry Neurol. 1993, 6, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Sultzer, D.L.; Levin, H.S.; Mahler, M.E.; High, W.M.; Cummings, J.L. A comparison of psychiatric symptoms in vascular dementia and Alzheimer’s disease. Am. J. Psychiatry 1993, 150, 1806–1812. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Yang, J. Association between depression and macrovascular disease: A mini review. Front. Psychiatry 2023, 14, 1215173. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Epel, E.S.; Reus, V.I.; Mellon, S.H. Depression gets old fast: Do stress and depression accelerate cell aging? Depress. Anxiety 2010, 27, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F. History of Depression as a Risk Factor for Dementia: An Updated Review. Aust. New Zealand J. Psychiatry 2001, 35, 776–781. [Google Scholar] [CrossRef]
- Ownby, R.L.; Crocco, E.; Acevedo, A.; John, V.; Loewenstein, D. Depression and Risk for Alzheimer Disease: Systematic Review, Meta-analysis, and Metaregression Analysis. Arch. Gen. Psychiatry 2006, 63, 530–538. [Google Scholar] [CrossRef]
- Gatz, J.L.; Tyas, S.L.; St John, P.; Montgomery, P. Do Depressive Symptoms Predict Alzheimer’s Disease and Dementia? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 744–747. [Google Scholar] [CrossRef]
- Gallagher, D.; Kiss, A.; Lanctot, K.L.; Herrmann, N. Toward Prevention of Mild Cognitive Impairment in Older Adults with Depression: An Observational Study of Potentially Modifiable Risk Factors. J. Clin. Psychiatry 2018, 80, 18m12331. [Google Scholar] [CrossRef]
- Lara, E.; Koyanagi, A.; Domènech-Abella, J.; Miret, M.; Ayuso-Mateos, J.L.; Haro, J.M. The Impact of Depression on the Development of Mild Cognitive Impairment over 3 Years of Follow-Up: A Population-Based Study. Dement. Geriatr. Cogn. Disord. 2017, 43, 155–169. [Google Scholar] [CrossRef]
- Mourao, R.J.; Mansur, G.; Malloy-Diniz, L.F.; Castro Costa, E.; Diniz, B.S. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: Systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2016, 31, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Ma, L. Depression, Anxiety, and Apathy in Mild Cognitive Impairment: Current Perspectives. Front. Aging Neurosci. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mussele, S.; Fransen, E.; Struyfs, H.; Luyckx, J.; Mariën, P.; Saerens, J.; Somers, N.; Goeman, J.; De Deyn, P.P.; Engelborghs, S. Depression in Mild Cognitive Impairment is associated with Progression to Alzheimer’s Disease: A Longitudinal Study. J. Alzheimer’s Dis. 2014, 42, 1239–1250. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, H.; Joo, J.H.; Jang, S.-I.; Park, E.-C. Association between depression and risk of Parkinson’s disease in South Korean adults. J. Affect. Disord. 2021, 292, 75–80. [Google Scholar] [CrossRef]
- Gustafsson, H.; Nordström, A.; Nordström, P. Depression and subsequent risk of Parkinson disease: A nationwide cohort study. Neurology 2015, 84, 2422–2429. [Google Scholar] [CrossRef]
- Leentjens, A.F. Depression—Risk factor or early symptom in Parkinson disease? Nat. Rev. Neurol. 2015, 11, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, L.; Brayne, C. A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol. Scand. 2006, 113, 211–220. [Google Scholar] [CrossRef]
- Fang, F.; Xu, Q.; Park, Y.; Huang, X.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Kamel, F.; Chen, H. Depression and the subsequent risk of Parkinson’s disease in the NIH-AARP Diet and Health Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 1157–1162. [Google Scholar] [CrossRef]
- Schuurman, A.G.; Van Den Akker, M.; Ensinck, K.T.J.L.; Metsemakers, J.F.M.; Knottnerus, J.A.; Leentjens, A.F.G.; Buntinx, F. Increased risk of Parkinson’s disease after depression: A retrospective cohort study. Neurology 2002, 58, 1501–1504. [Google Scholar] [CrossRef]
- Shen, C.-C.; Tsai, S.-J.; Perng, C.-L.; Kuo, B.I.-T.; Yang, A.C. Risk of Parkinson disease after depression: A nationwide population-based study. Neurology 2013, 81, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mao, S.; Xiang, D.; Fang, C. Association between depression and the subsequent risk of Parkinson’s disease: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 186–192. [Google Scholar] [CrossRef]
- Dallé, E.; Mabandla, M.V. Early Life Stress, Depression And Parkinson’s Disease: A New Approach. Mol. Brain 2018, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Boot, B.P.; Orr, C.F.; Ahlskog, J.E.; Ferman, T.J.; Roberts, R.; Pankratz, V.S.; Dickson, D.W.; Parisi, J.; Aakre, J.A.; Geda, Y.E.; et al. Risk factors for dementia with Lewy bodies: A case-control study. Neurology 2013, 81, 833–840. [Google Scholar] [CrossRef]
- Ishiguro, M.; Baba, H.; Maeshima, H.; Shimano, T.; Inoue, M.; Ichikawa, T.; Yasuda, S.; Shukuzawa, H.; Suzuki, T.; Arai, H. Increased Serum Levels of α-Synuclein in Patients with Major Depressive Disorder. Am. J. Geriatr. Psychiatry 2019, 27, 280–286. [Google Scholar] [CrossRef]
- Patterson, L.; Rushton, S.P.; Attems, J.; Thomas, A.J.; Morris, C.M. Degeneration of dopaminergic circuitry influences depressive symptoms in Lewy body disorders. Brain Pathol. 2019, 29, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Saari, L.; Heiskanen, L.; Gardberg, M.; Kaasinen, V. Depression and Nigral Neuron Density in Lewy Body Spectrum Diseases. Ann. Neurol. 2021, 89, 1046–1050. [Google Scholar] [CrossRef]
- Turner, M.R.; Goldacre, R.; Talbot, K.; Goldacre, M.J. Psychiatric disorders prior to amyotrophic lateral sclerosis. Ann. Neurol. 2016, 80, 935–938. [Google Scholar] [CrossRef]
- Mossello, E.; Boncinelli, M.; Caleri, V.; Cavallini, M.C.; Palermo, E.; Di Bari, M.; Tilli, S.; Sarcone, E.; Simoni, D.; Biagini, C.A.; et al. Is Antidepressant Treatment Associated with Reduced Cognitive Decline in Alzheimer’s Disease? Dement. Geriatr. Cogn. Disord. 2008, 25, 372–379. [Google Scholar] [CrossRef]
- Saczynski, J.S.; Beiser, A.; Seshadri, S.; Auerbach, S.; Wolf, P.A.; Au, R. Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology 2010, 75, 35–41. [Google Scholar] [CrossRef]
- Chen, R.; Hu, Z.; Wei, L.; Qin, X.; McCracken, C.; Copeland, J.R. Severity of depression and risk for subsequent dementia: Cohort studies in China and the UK. Br. J. Psychiatry 2008, 193, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Dotson, V.M.; Beydoun, M.A.; Zonderman, A.B. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010, 75, 27–34. [Google Scholar] [CrossRef]
- Wilson, R.S.; Barnes, L.L.; Mendes De Leon, C.F.; Aggarwal, N.T.; Schneider, J.S.; Bach, J.; Pilat, J.; Beckett, L.A.; Arnold, S.E.; Evans, D.A.; et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002, 59, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.L.; Covinsky, K.E.; Barnes, D.E.; Yaffe, K. Dysthymia and Depression Increase Risk of Dementia and Mortality Among Older Veterans. Am. J. Geriatr. Psychiatry 2012, 20, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Lara, E.; Martín-María, N.; Miret, M.; Olaya, B.; Haro, J.M.; Ayuso-Mateos, J.L. Is there a combined effect of depression and cognitive reserve on cognitive function? Findings from a population-based study. Psychol. Health 2022, 37, 1132–1147. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Wei, N.; Sun, D.; Cao, F. Depression, cognitive reserve markers, and dementia risk in the general population. Aging Ment. Health 2022, 26, 2006–2013. [Google Scholar] [CrossRef]
- Kawakami, I.; Iga, J.; Takahashi, S.; Lin, Y.; Fujishiro, H. Towards an understanding of the pathological basis of senile depression and incident dementia: Implications for treatment. Psychiatry Clin. Neurosci. 2022, 76, 620–632. [Google Scholar] [CrossRef]
- You, Y.; Li, J.; Zhang, Y.; Li, X.; Li, X.; Ma, X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: Insights from population-based and mice studies. CNS Neurosci. Ther. 2024, 30, e14783. [Google Scholar] [CrossRef]
- Leonard, B.E.; Myint, A. Inflammation and depression: Is there a causal connection with dementia? Neurotox. Res. 2006, 10, 149–160. [Google Scholar] [CrossRef]
- Rodrigues, R.; Petersen, R.B.; Perry, G. Parallels Between Major Depressive Disorder and Alzheimer’s Disease: Role of Oxidative Stress and Genetic Vulnerability. Cell. Mol. Neurobiol. 2014, 34, 925–949. [Google Scholar] [CrossRef]
- Réus, G.Z.; Titus, S.E.; Abelaira, H.M.; Freitas, S.M.; Tuon, T.; Quevedo, J.; Budni, J. Neurochemical correlation between major depressive disorder and neurodegenerative diseases. Life Sci. 2016, 158, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tiemeier, H. Biological risk factors for late life depression. Eur. J. Epidemiol. 2003, 18, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Carvalho, L.A.; Pariante, C.M. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011, 36, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Kennedy, J.A. Depressive pseudodementia—how ‘pseudo’ is it really? Old Age Psychiatr. 2015, 62, 30–37. [Google Scholar]
- Barjau Romero, J.M.; Guerro-Prado, D.; Viloria Jiménez, A.; Vega-Piñero, M.; Chinchilla Moreno, A. Depressive pseudodementia: Diagnostic frontiers. Actas Esp. De Psiquiatr. 2002, 30, 43–53. [Google Scholar]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimäki, M.; Sabia, S. Trajectories of Depressive Symptoms Before Diagnosis of Dementia: A 28-Year Follow-up Study. JAMA Psychiatry 2017, 74, 712–718. [Google Scholar] [CrossRef]
- Almeida, O.P.; Hankey, G.J.; Yeap, B.B.; Golledge, J.; Flicker, L. Depression as a modifiable factor to decrease the risk of dementia. Transl. Psychiatry 2017, 7, e1117. [Google Scholar] [CrossRef]
- Bareeqa, S.B.; Samar, S.S.; Kamal, S.; Masood, Y.; Allahyar Ahmed, S.I.; Hayat, G. Prodromal Depression and Subsequent Risk of Developing Parkinson’s Disease: A Systematic Review with Meta-Analysis. Neurodegener. Dis. Manag. 2022, 12, 155–164. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Almeida, O.P. Management of Depression in Patients with Dementia: Is Pharmacological Treatment Justified? Drugs Aging 2017, 34, 89–95. [Google Scholar] [CrossRef]
- Cummings, J.L. Depression in vascular dementia. Hillside J. Clin. Psychiatry 1988, 10, 209–231. [Google Scholar]
- Alexopoulos, G.S.; Bruce, M.L.; Silbersweig, D.; Kalayam, B.; Stern, E. Vascular depression: A new view of late-onset depression. Dialogues Clin. Neurosci. 1999, 1, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, P.; Olesen, P.J.; Simoni, M.; Pantoni, L.; Östling, S.; Kern, S.; Guo, X.; Skoog, I. White matter lesions and temporal lobe atrophy related to incidence of both dementia and major depression in 70-year-olds followed over 10 years. Eur. J. Neurol. 2015, 22, 781-e50. [Google Scholar] [CrossRef]
- Mirza, S.S.; Ikram, M.A.; Bos, D.; Mihaescu, R.; Hofman, A.; Tiemeier, H. Mild cognitive impairment and risk of depression and anxiety: A population-based study. Alzheimer’s Dement. 2017, 13, 130–139. [Google Scholar] [CrossRef]
- Steffens, D.C. Depressive Symptoms and Mild Cognitive Impairment in the Elderly: An Ominous Combination. Biol. Psychiatry 2012, 71, 762–764. [Google Scholar] [CrossRef]
- Kostić, V.S.; Djuričić, B.M.; Čovičković-Šternić, N.; Bumbaširević, L.; Nikolić, M.; Mršulja, B.B. Depression and parkinson’s disease: Possible role of serotonergic mechanisms. J. Neurol. 1987, 234, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Huey, E.D.; Putnam, K.T.; Grafman, J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 2006, 66, 17–22. [Google Scholar] [CrossRef]
- Tarakita, N.; Nishijima, H.; Yasui-Furukori, N. Levodopa-responsive depression associated with corticobasal degeneration: A case report. Neuropsychiatr. Dis. Treat. 2017, 13, 1107–1110. [Google Scholar] [CrossRef]
- Hillemacher, T.; Grässel, E.; Tigges, S.; Bleich, S.; Neundörfer, B.; Kornhuber, J.; Hecht, M. Depression and bulbar involvement in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Caga, J.; Ramsey, E.; Hogden, A.; Mioshi, E.; Kiernan, M.C. A longer diagnostic interval is a risk for depression in amyotrophic lateral sclerosis. Palliat. Support. Care 2015, 13, 1019–1024. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Resolving a paradox: Antidepressants, neuroinflammation, and neurodegeneration. Explor. Neuroprot. Ther. 2024, 4, 11–37. [Google Scholar] [CrossRef]
- Lozupone, M.; La Montagna, M.; D’Urso, F.; Piccininni, C.; Rinaldi, A.; Beghi, M.; Cornaggia, C.M.; Sardone, R.; Solfrizzi, V.; Daniele, A.; et al. The Challenge of Antidepressant Therapeutics in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020, 1260, 267–281. [Google Scholar] [CrossRef]
- Yip, A.G.; Brayne, C.; Matthews, F.E. Risk factors for incident dementia in England and Wales: The Medical Research Council Cognitive Function and Ageing Study. A population-based nested case–control study. Age Ageing 2006, 35, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Heun, R.; Hein, S. Risk factors of major depression in the elderly. Eur. Psychiatry 2005, 20, 199–204. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Burden of Mental Disorders and the Need for a Comprehensive, Coordinated Response from Health and Social Sectors at the Country Level: Report by the Secretariat. 2012. Available online: https://apps.who.int/iris/handle/10665/78898 (accessed on 22 August 2024).
- Fabiano, N.; Gupta, A.; Bhambra, N.; Luu, B.; Wong, S.; Maaz, M.; Fiedorowicz, J.G.; Smith, A.L.; Solmi, M. How to optimize the systematic review process using AI tools. JCPP Adv. 2024, 4, e12234. [Google Scholar] [CrossRef]
- Bolaños, F.; Salatino, A.; Osborne, F.; Motta, E. Artificial intelligence for literature reviews: Opportunities and challenges. arXiv 2024. [Google Scholar] [CrossRef]
- Burns, J.K.; Etherington, C.; Cheng-Boivin, O.; Boet, S. Using an artificial intelligence tool can be as accurate as human assessors in level one screening for a systematic review. Health Inf. Libr. J. 2024, 41, 136–148. [Google Scholar] [CrossRef]
- van Dijk, S.H.B.; Brusse-Keizer, M.G.J.; Bucsán, C.C.; van der Palen, J.; Doggen, C.J.M.; Lenferink, A. Artificial intelligence in systematic reviews: Promising when appropriately used. BMJ Open 2023, 13, e072254. [Google Scholar] [CrossRef]
- de la Torre-López, J.; Ramírez, A.; Romero, J.R. Artificial intelligence to automate the systematic review of scientific literature. Computing 2023, 105, 2171–2194. [Google Scholar] [CrossRef]
- Quattropani, M.C.; Lenzo, V.; Armieri, V.; Filastro, A. L’origine della depressione nella malattia di Alzheimer: Una revisione della letteratura. Riv. Di Psichiatr. 2018, 53, 18–30. [Google Scholar] [CrossRef]
- Ganguli, M. Depression, cognitive impairment and dementia: Why should clinicians care about the web of causation? Indian J. Psychiatry 2009, 51 (Suppl. S1), S29–S34. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, D.; Ingenito, A.; von Gal, A.; De Pandis, M.F.; Piccardi, L. Relationship Between Depression and Neurodegeneration: Risk Factor, Prodrome, Consequence, or Something Else? A Scoping Review. Biomedicines 2025, 13, 1023. https://doi.org/10.3390/biomedicines13051023
Papa D, Ingenito A, von Gal A, De Pandis MF, Piccardi L. Relationship Between Depression and Neurodegeneration: Risk Factor, Prodrome, Consequence, or Something Else? A Scoping Review. Biomedicines. 2025; 13(5):1023. https://doi.org/10.3390/biomedicines13051023
Chicago/Turabian StylePapa, Dario, Alessandro Ingenito, Alessandro von Gal, Maria Francesca De Pandis, and Laura Piccardi. 2025. "Relationship Between Depression and Neurodegeneration: Risk Factor, Prodrome, Consequence, or Something Else? A Scoping Review" Biomedicines 13, no. 5: 1023. https://doi.org/10.3390/biomedicines13051023
APA StylePapa, D., Ingenito, A., von Gal, A., De Pandis, M. F., & Piccardi, L. (2025). Relationship Between Depression and Neurodegeneration: Risk Factor, Prodrome, Consequence, or Something Else? A Scoping Review. Biomedicines, 13(5), 1023. https://doi.org/10.3390/biomedicines13051023







