Effect of CBC-Derived Inflammatory Indicators in Predicting Chronic Kidney Disease Risk in Hypertrophic Cardiomyopathy Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants Enrollment
2.2. Data Collection and Grouping
2.3. Statistical Analysis
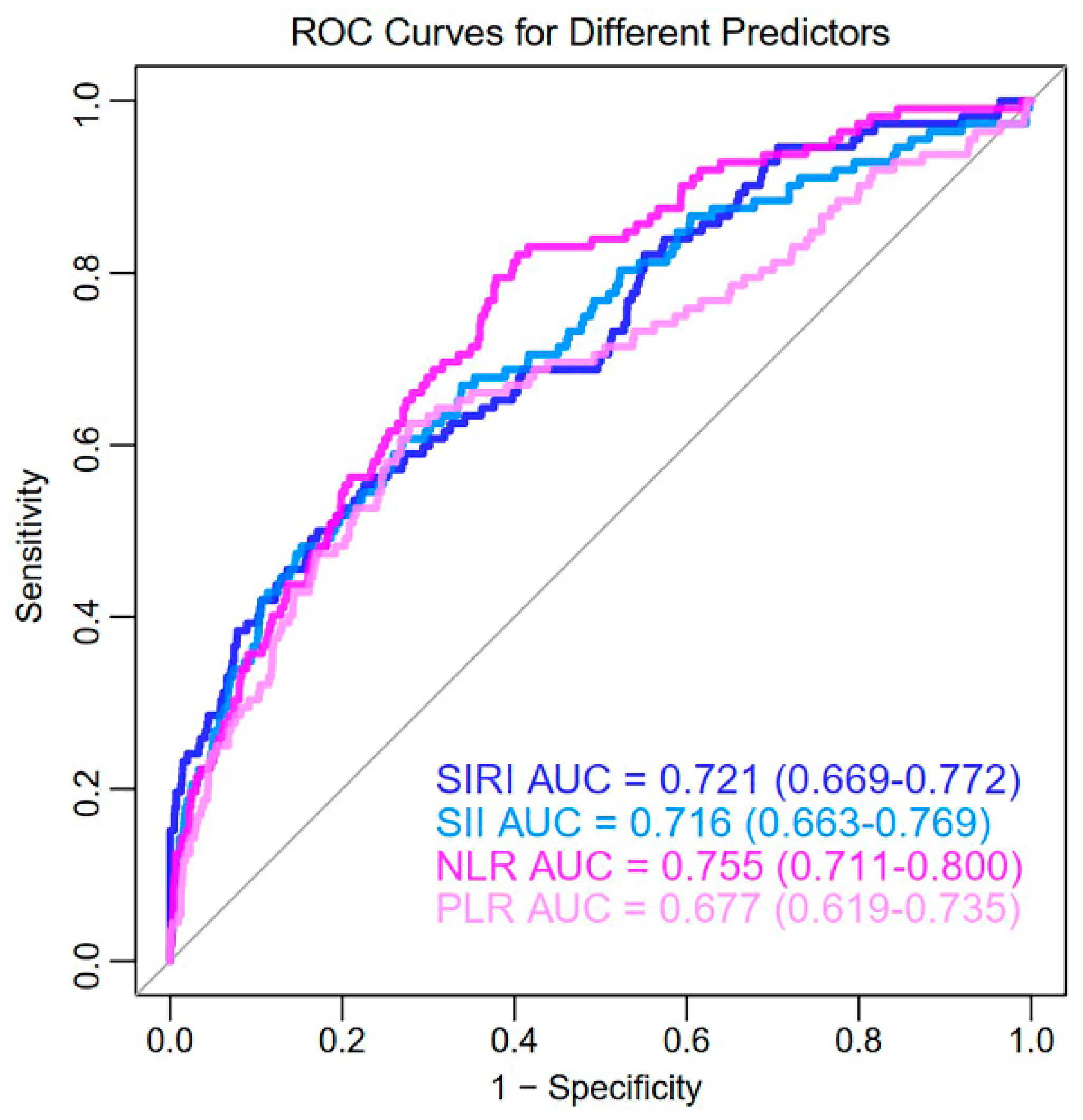
3. Results
3.1. Baseline Characteristics
3.2. Univariate Predictive Effect of CBC-Derived Inflammatory Indicators
3.3. LASSO-Logistic Regression and Multivariate Logistic Regression Analysis
3.4. Construction of Nomogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCM | Hypertrophic cardiomyopathy |
| CKD | Chronic kidney disease |
| LV | Left ventricular/ventricle |
| CBC | Complete blood cell counts |
| SIRI | Systemic inflammatory response index |
| SII | Systemic immune inflammation index |
| NLR | Neutrophil–lymphocyte ratio |
| PLR | Platelet–lymphocyte ratio |
| ROC | Receiver operating characteristic |
| AUC | Area under curve |
| CI | Confidence interval |
| LASSO | Least absolute shrinkage and selection operator |
| Pro-BNP | Pro-brain natriuretic peptide |
| Hb | Hemoglobin |
References
- Mushtaq, S.; Chiesa, M.; Novelli, V.; Sommariva, E.; Biondi, M.L.; Manzoni, M.; Florio, A.; Lampus, M.L.; Avallone, C.; Zocchi, C.; et al. Role of advanced CMR features in identifying a positive genotype of hypertrophic cardiomyopathy. Int. J. Cardiol. 2024, 417, 132554. [Google Scholar] [CrossRef] [PubMed]
- Möbius-Winkler, M.N.; Laufs, U.; Lenk, K. The diagnosis and treatment of hypertrophic cardiomyopathy. Dtsch. Aerzteblatt 2024, 121, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Buonincontri, V.; Viggiano, D.; Gigliotti, G. The brain extracellular space in chronic kidney disease. Behav. Brain Res. 2025, 476, 115271. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Monda, E.; Palmiero, G.; Rubino, M.; Verrillo, F.; Amodio, F.; Di Fraia, F.; Pacileo, R.; Fimiani, F.; Esposito, A.; Cirillo, A.; et al. Molecular Basis of Inflammation in the Pathogenesis of Cardiomyopathies. Int. J. Mol. Sci. 2020, 21, 6462. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Guo, B.; Liu, X.; Si, Q.; Zhang, D.; Li, M.; Li, X.; Zhao, Y.; Hu, F.; Zhang, M.; Liu, Y.; et al. Associations of CBC-Derived inflammatory indicators with sarcopenia and mortality in adults: Evidence from Nhanes 1999 ∼ 2006. BMC Geriatr. 2024, 24, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Bai, G.; Gao, Y.; Guo, Z.; Chen, X.; Liu, T.; Li, G. The Systemic Immune Inflammatory Response Index Can Predict the Clinical Prognosis of Patients with Initially Diagnosed Coronary Artery Disease. J. Inflamm. Res. 2023, ume 16, 5069–5082. [Google Scholar] [CrossRef]
- Huang, P.; Mai, Y.; Zhao, J.; Yi, Y.; Wen, Y. Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: Observational study of 40,937 adults. Inflamm. Res. 2024, 73, 655–667. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Hensley, N.; Dietrich, J.; Nyhan, D.; Mitter, N.; Yee, M.-S.; Brady, M. Hypertrophic cardiomyopathy: A review. Anesth. Analg. 2015, 120, 554–569. [Google Scholar] [CrossRef]
- Becker, R.C.; Owens, A.P.; Sadayappan, S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J. Thromb. Thrombolysis 2020, 49, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Huang, M.; Song, C.; Nie, C.; Zheng, X.; Wang, S.; Huang, X. Systemic inflammation is associated with myocardial fibrosis in patients with obstructive hypertrophic cardiomyopathy. ESC Hear. Fail. 2025, 12, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Kuusisto, J.; Kärjä, V.; Sipola, P.; Kholová, I.; Peuhkurinen, K.; Jääskeläinen, P.; Naukkarinen, A.; Ylä-Herttuala, S.; Punnonen, K.; Laakso, M. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 2012, 98, 1007–1013. [Google Scholar] [CrossRef]
- Ozyilmaz, S.; Akgul, O.; Uyarel, H.; Pusuroglu, H.; Gul, M.; Satilmisoglu, M.H.; Bolat, I.; Ozyilmaz, I.; Uçar, H.; Yildirim, A.; et al. The importance of the neutrophil-to-lymphocyte ratio in patients with hypertrophic cardiomyopathy. Rev. Port. De Cardiol. 2017, 36, 239–246. [Google Scholar] [CrossRef]
- Zhu, L.; Zou, Y.; Wang, Y.; Luo, X.; Sun, K.; Wang, H.; Jia, L.; Liu, Y.; Zou, J.; Yuan, Z.; et al. Prognostic Significance of Plasma High-Sensitivity C-Reactive Protein in Patients with Hypertrophic Cardiomyopathy. J. Am. Hear. Assoc. 2017, 6, e004529. [Google Scholar] [CrossRef]
- Nagai, T.; Anzai, T.; Kaneko, H.; Mano, Y.; Anzai, A.; Maekawa, Y.; Takahashi, T.; Meguro, T.; Yoshikawa, T.; Fukuda, K. C-Reactive Protein Overexpression Exacerbates Pressure Overload–Induced Cardiac Remodeling Through Enhanced Inflammatory Response. Hypertension 2011, 57, 208–215. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.Y.; Huang, X.R.; Wu, Y.; Chung, A.C.; Wu, E.X.; Szalai, A.J.; Wong, B.C.; Lau, C.-P.; Lan, H.Y. C-Reactive Protein Promotes Cardiac Fibrosis and Inflammation in Angiotensin II–Induced Hypertensive Cardiac Disease. Hypertension 2010, 55, 953–960. [Google Scholar] [CrossRef]
- Jellis, C.; Martin, J.; Narula, J.; Marwick, T.H. Assessment of Nonischemic Myocardial Fibrosis. J. Am. Coll. Cardiology 2010, 56, 89–97. [Google Scholar] [CrossRef]
- Zach, D.K.; Schwegel, N.; Santner, V.; Winkelbauer, L.; Hoeller, V.; Kolesnik, E.; Gollmer, J.; Seggewiss, H.; Batzner, A.; Perl, S.; et al. Low-grade systemic inflammation and left ventricular dysfunction in hypertensive compared to non-hypertensive hypertrophic cardiomyopathy. Int. J. Cardiol. 2023, 399, 131661. [Google Scholar] [CrossRef] [PubMed]
- Fonfara, S.; Kitz, S.; Monteith, G.; Hahn, S.; Kipar, A. Myocardial transcription of inflammatory and remodeling markers in cats with hypertrophic cardiomyopathy and systemic diseases associated with an inflammatory phenotype. Res. Veter. Sci. 2021, 136, 484–494. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. Mechanisms and Treatment of CKD. J. Am. Soc. Nephrol. 2012, 23, 1917–1928. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Vetrano, E.; Galiero, R.; Salvatore, T.; Docimo, G.; Epifani, R.; Alfano, M.; Sardu, C.; Marfella, R.; Rinaldi, L.; et al. Cardiac Hypertrophy: From Pathophysiological Mechanisms to Heart Failure Development. Rev. Cardiovasc. Med. 2022, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Dearani, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Management of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 390–414. [Google Scholar] [CrossRef]
- Huang, F.-Y.; Zhang, J.-L.; Huang, B.-T.; Peng, Y.; Chen, S.-J.; Chen, M. Renal function as a predictor of outcomes in patients with hypertrophic cardiomyopathy: A cohort study of a hospitalized population. Clin. Chim. Acta 2021, 512, 92–99. [Google Scholar] [CrossRef]
- Banerjee, D.; Rosano, G.; Herzog, C.A. Management of Heart Failure Patient with CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 1131–1139. [Google Scholar] [CrossRef]
- Lee, H.; Han, K.; Park, J.-B.; Hwang, I.-C.; Yoon, Y.E.; Park, H.E.; Choi, S.-Y.; Kim, Y.-J.; Cho, G.-Y.; Kim, H.-K.; et al. Risk of end-stage renal disease in patients with hypertrophic cardiomyopathy: A nationwide population-based cohort study. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Cerasola, G.; Nardi, E.; Palermo, A.; Mulè, G.; Cottone, S. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: A review. J. Nephrol. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Inflammatory Related Cardiovascular Diseases: From Molecular Mechanisms to Therapeutic Targets. Curr. Pharm. Des. 2020, 26, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Li, W.-C.; Xia, S.-H.; Xiang, T.; Tang, C.; Luo, J.-L.; Lin, M.-J.; Xia, X.-W.; Wang, W.-B. Predictive Value of the Systemic Immune Inflammation Index for Adverse Outcomes in Patients with Acute Ischemic Stroke. Front. Neurol. 2022, 13, 836595. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and Risk of All-Cause Mortality and Cardiovascular Mortality: A 20-Year Follow-Up Cohort Study of 42,875 US Adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef] [PubMed]
- Bronze-Da-Rocha, E.; Santos-Silva, A. Neutrophil Elastase Inhibitors and Chronic Kidney Disease. Int. J. Biol. Sci. 2018, 14, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, D.H.; Lee, H.W.; Kim, S.G.; Kim, Y.K.; Kim, J.-K. Role of increased neutrophil extracellular trap formation on acute kidney injury in COVID-19 patients. Front. Immunol. 2023, 14, 1122510. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, L.; Liu, F.; Lin, X.; Li, H.; Xu, X. Relationship between Left Ventricular Outflow Tract Pressure Gradient and Hemoglobin in Patients with Hypertrophic Cardiomyopathy. Acta Cardiol. Sin. 2020, 36, 343–350. [Google Scholar] [CrossRef]



| Items | Total (n = 1795) | CKD Group (n = 112) | Non-CKD Group (n = 1683) | p-Value |
|---|---|---|---|---|
| Age (years) | 61 (51, 69) | 61 (49, 70) | 61 (51, 68) | 0.901 |
| Gender (male, %) | 1184 (66.0) | 85 (75.9) | 1099 (65.3) | 0.022 |
| BMI (kg/m2) | 24.95 ± 3.51 | 25.09 ± 4.05 | 24.94 ± 3.47 | 0.723 |
| Smoking (%) | 723 (40.3) | 51 (45.5) | 672 (39.9) | 0.241 |
| Drinking (%) | 217 (12.1) | 15 (13.4) | 202 (12.0) | 0.662 |
| Comorbidities | ||||
| Hypertension (%) | 835 (46.5) | 58 (51.8) | 777 (46.2) | 0.248 |
| Diabetes (%) | 250 (13.9) | 22 (19.6) | 228 (13.5) | 0.071 |
| CAD (%) | 92 (5.1) | 12 (10.7) | 80 (4.8) | 0.006 |
| Biochemical results | ||||
| Hb (g/L) | 140 (127, 152) | 129 (108, 145) | 141 (128, 153) | <0.001 |
| WBC (109/L) | 6.53 (5.28, 8.42) | 7.42 (5.67, 10.88) | 6.46 (5.27, 8.31) | <0.001 |
| NEUT (109/L) | 4.07 (3.23, 5.38) | 5.41 (3.73, 7.41) | 4.04 (3.21, 5.29) | <0.001 |
| MONO (109/L) | 2.90 (0.37, 6.00) | 5.10 (0.69, 6.88) | 1.07 (0.37, 5.90) | <0.001 |
| LYMPH (109/L) | 1.50 (1.13, 1.91) | 1.14 (0.77, 1.53) | 1.52 (1.16, 1.93) | <0.001 |
| PLT (109/L) | 188 (152, 230) | 182 (147, 245) | 188 (153, 229) | 0.815 |
| ALT (U/L) | 22.00 (15.50, 34.00) | 20.00 (14.00, 30.75) | 22.40 (16.00, 34.00) | 0.030 |
| AST (U/L) | 24.00 (19.70, 31.00) | 22.00 (17.00, 28.75) | 24.00 (20.00, 31.00) | 0.003 |
| Albumin (g/L) | 39.70 (36.70, 42.70) | 36.8 (32.65, 41.93) | 39.90 (37.00, 42.70) | <0.001 |
| eGFR (mL/min/1.73 m2) | 96.13 (82.56, 106.71) | 43.48 (22.37, 68.36) | 97.11 (85.89, 107.57) | <0.001 |
| Cr (umol/L) | 68 (55, 80) | 127 (96, 227) | 67 (55, 78) | <0.001 |
| BUN (mmol/L) | 5.96 (4.94, 7.31) | 9.53 (6.88, 15.08) | 5.89 (4.88, 7.15) | <0.001 |
| HDL (mmol/L) | 0.97 (0.81, 1.14) | 0.90 (0.74, 1.11) | 0.97 (0.82, 1.14) | 0.025 |
| LDL (mmol/L) | 2.17 (1.66, 2.75) | 1.99 (1.50, 2.65) | 2.18 (1.67, 2.76) | 0.104 |
| TG (mmol/L) | 1.29 (0.93, 1.81) | 1.37 (0.87, 1.81) | 1.29 (0.93, 1.81) | 0.775 |
| TC (mmol/L) | 3.83 (3.22, 4.49) | 3.76 (3.19, 4.36) | 3.84 (3.24, 4.52) | 0.467 |
| Pro-BNP (pg/mL) | 1141 (362, 2887) | 2931 (999, 7135) | 1069 (342, 2710) | <0.001 |
| LDH (U/L) | 225 (198, 263) | 243 (198, 287) | 225 (197, 261) | 0.069 |
| CK-MB (U/L) | 13.69 (10.00, 18.09) | 14.85 (10.85, 20.90) | 13.50 (10.00, 18.00) | 0.049 |
| CK (U/L) | 27.80 (12.34, 79.00) | 33.00 (13.78, 65.00) | 27.10 (12.21, 79.00) | 0.873 |
| Echocardiology | ||||
| LVEDD (mm) | 48 (45, 52) | 48 (44, 52) | 48 (45, 52) | 0.770 |
| LVESD (mm) | 29 (26, 32) | 30 (26, 32) | 29 (26, 32) | 0.079 |
| IVST (mm) | 14 (11, 18) | 15 (11, 18) | 14 (11, 18) | 0.139 |
| LVEF (%) | 68 (63, 73) | 66 (58, 71) | 68 (63, 73) | 0.001 |
| CBC-Derived inflammatory indicators | ||||
| SIRI | 6.68 (0.99, 16.66) | 19.27 (3.25, 38.11) | 5.96 (0.96, 15.86) | <0.001 |
| SII | 501.15 (345.31, 766.65) | 855.74 (497.43, 1609.18) | 488.21 (340.61, 736.79) | <0.001 |
| NLR | 2.68 (1.96, 3.83) | 4.26 (3.14, 7.48) | 2.61 (1.93, 3.70) | <0.001 |
| PLR | 125.00 (94.25, 166.67) | 174.37 (112.68, 259.18) | 123.33 (93.55, 163.00) | <0.001 |
| Items | LASSO-Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|
| Assignment | Coefficient | OR (95% CI) | p-Value | |
| Gender (Male, %) | Male = 1; Female = 0 | 0.5988 | 2.622 (1.565–4.393) | <0.001 |
| CAD (%) | Yes = 1; No = 0 | |||
| Hb (g/L) | Continuous variable (reference range: 115–150) | −0.6793 | 0.972 (0.962–0.981) | <0.001 |
| WBC (109/L) | Continuous variable (reference range: 3.5–5.5) | |||
| NEUT (109/L) | Continuous variable (reference range: 40–75) | |||
| MONO (109/L) | Continuous variable (reference range: 0.1–0.6) | |||
| LYMPH (109/L) | Continuous variable (reference range: 1.1–3.2) | |||
| ALT (U/L) | Continuous variable (reference range: 7–40) | |||
| AST (U/L) | Continuous variable (reference range: 13–45) | |||
| Albumin (g/L) | Continuous variable (reference range: 40–55) | |||
| HDL (mmol/L) | Continuous variable (reference range: 1.16–1.42) | |||
| Pro-BNP (pg/mL) | Continuous variable (reference range for healthy populations: 0–125) | 0.2029 | 1.000 (1.000–1.000) | <0.001 |
| CK-MB (U/L) | Continuous variable (reference range: 0–24) | |||
| LVEF (%) | Continuous variable | −0.1696 | 0.983 (0.964–1.003) | 0.095 |
| SIRI | Continuous variable | 0.5143 | 1.037 (1.026–1.049) | <0.001 |
| SII | Continuous variable | 0.2917 | 1.000 (1.000–1.001) | 0.003 |
| NLR | Continuous variable | |||
| PLR | Continuous variable | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Yan, L.; Liu, Y.; Chen, S.; Lan, B.; Liu, R.; Xin, J.; Shi, T.; Yang, X. Effect of CBC-Derived Inflammatory Indicators in Predicting Chronic Kidney Disease Risk in Hypertrophic Cardiomyopathy Patients. Biomedicines 2025, 13, 997. https://doi.org/10.3390/biomedicines13040997
Zhao C, Yan L, Liu Y, Chen S, Lan B, Liu R, Xin J, Shi T, Yang X. Effect of CBC-Derived Inflammatory Indicators in Predicting Chronic Kidney Disease Risk in Hypertrophic Cardiomyopathy Patients. Biomedicines. 2025; 13(4):997. https://doi.org/10.3390/biomedicines13040997
Chicago/Turabian StyleZhao, Changying, Luqin Yan, Yong Liu, Siyuan Chen, Beidi Lan, Ruohan Liu, Jinqi Xin, Tao Shi, and Xiaohong Yang. 2025. "Effect of CBC-Derived Inflammatory Indicators in Predicting Chronic Kidney Disease Risk in Hypertrophic Cardiomyopathy Patients" Biomedicines 13, no. 4: 997. https://doi.org/10.3390/biomedicines13040997
APA StyleZhao, C., Yan, L., Liu, Y., Chen, S., Lan, B., Liu, R., Xin, J., Shi, T., & Yang, X. (2025). Effect of CBC-Derived Inflammatory Indicators in Predicting Chronic Kidney Disease Risk in Hypertrophic Cardiomyopathy Patients. Biomedicines, 13(4), 997. https://doi.org/10.3390/biomedicines13040997







