Modular-Based Synergetic Mechanisms of Jasminoidin and Ursodeoxycholic Acid in Cerebral Ischemia Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Expression Dataset
2.2. Establishing and Identifying of Co-Expression Modules
2.3. Identification of Drug Response On_Module and Sy-Module
2.4. Analysis of Differentially Expressed Genes (DEGs)
2.5. Synergistic Prediction Analysis
2.6. Driver Gene Identification Based on Network Proximity Indices
2.7. Functional Annotation Gene Analysis
2.8. Animal Model and Drug Administration
2.9. Evaluation of the Neurological Deficit Score and Infarct Volume
2.10. Western Blotting
2.11. Literature Validation
2.12. Statistical Analysis
3. Results
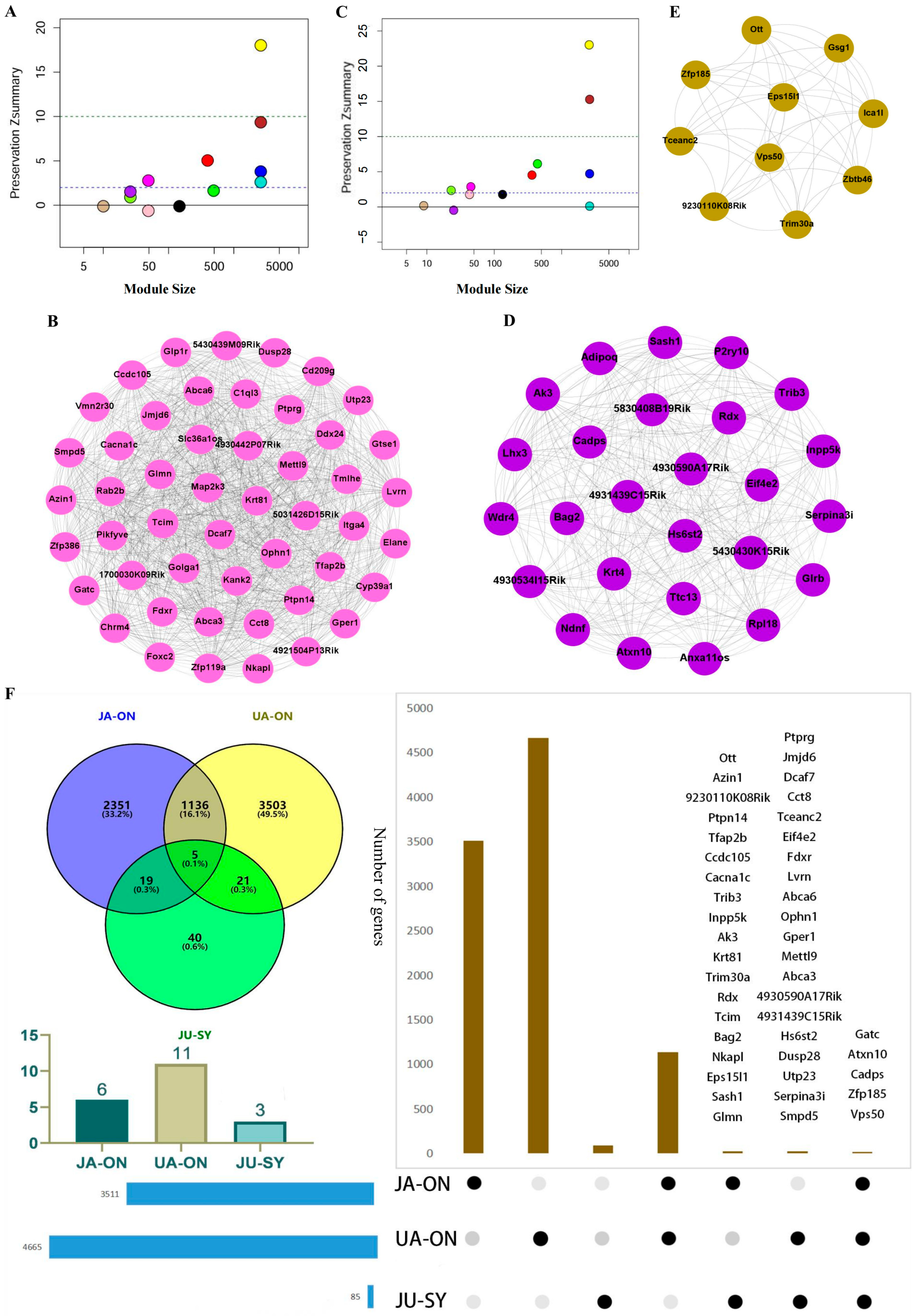
3.1. Co-Expression Modules of Three Drug Groups
3.2. The Conserved and On_Modules of JA, UA, and JU
3.3. The Synergistic Modules of JU
3.4. The Overlap of On_Module Genes and Differentially Expressed Genes
3.5. Network Synergistic Map Between Different Levels of Modules
3.6. Identification of Driver Genes and Literature Verification
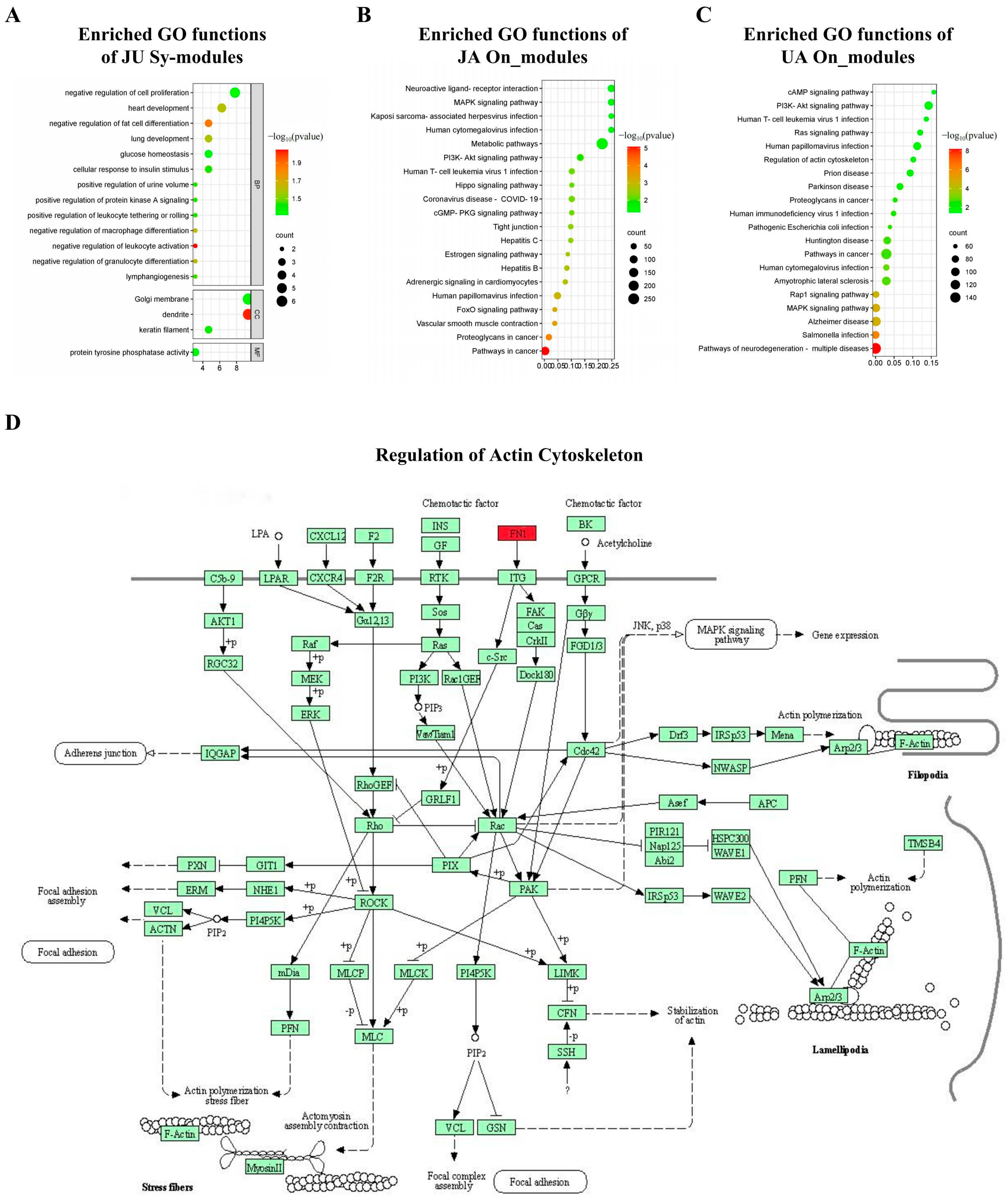
3.7. Significant Biological Functions
3.8. Protein Expression of Driver Genes and JU Sy_Modules’ Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Yadava, S.; Reddy, D.H.; Nakka, V.P.; Anusha, V.L.; Dumala, N.; Viswanadh, M.K.; Chakravarthi, G.; Nalluri, B.N.; Ramakrishna, K. Unravelling neuroregenerative and neuroprotective roles of Wnt/β-catenin pathway in ischemic stroke: Insights into molecular mechanisms. Neuroscience 2025, 565, 527–547. [Google Scholar] [CrossRef]
- Li, W.; Liu, X. Progress in the therapeutic time window for acute ischemic stroke. Stroke Vasc. Neurol. 2018, 13, 364–371. (In Chinese) [Google Scholar]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adevoe, O.M.; Bambakidis, N.C.; Becker, K. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Song, K.; Guan, M.; Li, W.; Jing, Z.; Xie, X.; Shi, C.; Liang, J.; Qiao, H.; Huang, L. Acute ischemic stroke patients with diffusion-weighted imaging-Alberta Stroke Program Early Computed Tomography Score ≤ 5 can benefit from endovascular treatment: A single-center experience and literature review. Neuroradiology 2019, 61, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Easton, J.D.; Farrant, M.; Barsan, W.; Conwit, R.A.; Elm, J.J.; Kim, A.S.; Lindblad, A.S.; Palesch, Y.Y. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N. Engl. J. Med. 2018, 379, 215–225. [Google Scholar] [CrossRef]
- Wu, C.; Sun, C.; Wang, L.; Lian, Y.; Xie, N.; Huang, S.; Zhao, W.; Ren, M.; Wu, D.; Ding, J.; et al. Low-dose tirofiban treatment improves neurological deterioration outcome after intravenous thrombolysis. Stroke 2019, 50, 3481–3487. [Google Scholar] [CrossRef]
- Wang, L.; Peng, B.; Zhang, H.; Wang, Y.; Liu, M.; Shan, C.; Cao, L.; Wang, L.; Xie, W.; Wang, P.; et al. Brief report on stroke prevention and treatment in China, 2020. Chin. J. Cerebrovasc. Dis. 2022, 19, 136–144. (In Chinese) [Google Scholar]
- Ma, G.; Li, S.; Jia, B.; Mo, D.; Ma, N.; Gao, F.; Huo, X.; Luo, G.; Wang, A.; Pan, Y.; et al. Safety and efficacy of low-dose tirofiban combined with intravenous thrombolysis and mechanical thrombectomy in acute ischemic stroke: A matched-control analysis from a nationwide registry. Front. Neurol. 2021, 12, 666919. [Google Scholar] [CrossRef]
- Simats, A.; Ramiro, L.; Valls, R.; de Ramón, H.; García-Rodríguez, P.; Orset, C.; Artigas, L.; Sardon, T.; Rosell, A.; Montaner, J. Ceruletide and alpha-1 antitrypsin as a novel combination therapy for ischemic stroke. Neurotherapeutics 2022, 19, 513–527. [Google Scholar] [CrossRef]
- Wang, F.J.; Wang, S.X.; Chai, L.J.; Zhang, Y.; Guo, H.; Hu, L.M. Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol. Sin. 2018, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Shu, Y.; Wang, Y.; Jia, D.; Han, Z.; Shi, B.; Chen, J.; Yang, J.; Qin, Z.; Huang, S. Combination treatment of Danggui Buxue Decoction and endothelial progenitor cells can enhance angiogenesis in rats with focal cerebral ischemia and hyperlipidemia. J. Ethnopharmacol. 2023, 314, 116563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.Y. Modular pharmacology: Deciphering the interacting structural organization of the targeted networks. Drug Discov. Today 2013, 18, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Chihara, N.; Madi, A.; Kondo, T.; Zhang, H.; Acharya, N.; Singer, M.; Nyman, J.; Marjanovic, N.D.; Kowalczyk, M.S.; Wang, C.; et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018, 558, 454–459. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Zhang, Y.; Wang, P.; Yu, Y.; Kang, R.; Wu, H.; Zhang, X.; Wang, Z.; Wang, Y. Quantitative identification of compounds-dependent on-modules and differential allosteric modules from homologous ischemic networks. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 575–584. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar]
- Sun, Y.Y.; Zhu, H.J.; Zhao, R.Y.; Zhou, S.Y.; Wang, M.Q.; Yang, Y.; Guo, Z.N. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023, 66, 102852. [Google Scholar] [CrossRef]
- Cai, W.; Hu, M.; Li, C.; Wu, R.; Lu, D.; Xie, C.; Zhang, W.; Li, T.; Shen, S.; Huang, H.; et al. FOXP3+ macrophage represses acute ischemic stroke-induced neural inflammation. Autophagy 2022, 19, 1144–1163. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Wang, F.; Feng, J.; Yu, B.; Wang, B.; Gao, Q.; Wang, T.-Y.; Hu, B.; Gao, X.; Chen, J.-F.; et al. Prolonged myelin deficits contribute to neuron loss and functional impairments after ischaemic stroke. Brain 2024, 147, 1294–1311. [Google Scholar] [CrossRef]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.M.; Guney, E.; Egea, J.; López, M.G.; Baumbach, J.; et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Xu, T.; Zhang, S.; Liu, S.; Zhai, C.; Wang, Z.; Mu, J.; Li, C.; Cheng, F.; et al. An integrative pharmacology-based analysis of refined qingkailing injection against cerebral ischemic stroke: A novel combination of baicalin, geniposide, cholic acid, and hyodeoxycholic acid. Front. Pharmacol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Spellman, S.R.; Solá, S.; Grande, A.W.; Linehan-Stieers, C.; Low, W.C.; Steer, C.J. Neuroprotection by a bile acid in an acute stroke model in the rat. J. Cereb. Blood Flow Metab. 2002, 22, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Steer, C.J. The therapeutic effects of ursodeoxycholic acid as an anti-apoptotic agent. Expert Opin. Investig. Drugs 2001, 10, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, R.; Zhang, M.; Wang, Y.; Wang, T.; Wang, Y.; Shang, X.; Peng, J. A network-based method for brain disease gene prediction by integrating brain connectome and molecular network. Briefings Bioinform. 2021, 23, bbab459. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Zhang, Y.; Liu, D.; Zhang, H.; Wang, X. PPDTS: Predicting potential drug–target interactions based on network similarity. IET Syst. Biol. 2021, 16, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B.; Jiang, D.; Wei, Y.; Zhang, N. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae. BMC Bioinform. 2010, 11, S6. [Google Scholar] [CrossRef]
- Gan, X.; Shu, Z.; Wang, X.; Yan, D.; Li, J.; Ofaim, S.; Albert, R.; Li, X.; Liu, B.; Zhou, X.; et al. Network medicine framework reveals generic herb-symptom effectiveness of traditional Chinese medicine. Sci. Adv. 2023, 9, eadh0215. [Google Scholar] [CrossRef]
- Wang, Z.; Jing, Z.-W.; Zhou, C.-X.; Zhang, L.; Cheng, J.; Zhang, Z.-J.; Liu, J.; Xu, C.-S.; Li, P.-T.; Wang, Y.-Y. Fusion of core pathways reveals a horizontal synergistic mechanism underlying combination therapy. Eur. J. Pharmacol. 2011, 667, 278–286. [Google Scholar] [CrossRef]
- Li, B.; Yu, Y.; Zhang, Y.; Liu, J.; Li, H.; Dang, H.; Guo, S.; Wang, L.; Wu, H.; Wang, Z.; et al. Vertical and horizontal convergences of targeting pathways in combination therapy with baicalin and jasminoidin for cerebral ischemia. CNS Neurol. Disord. Drug Targets 2016, 15, 740–750. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Zhang, B.; Horvath, S. Defining clusters from a hierarchical cluster tree: The Dynamic Tree Cut package for R. Bioinformatics 2008, 24, 719–720. [Google Scholar] [CrossRef]
- Langfelder, P.; Luo, R.; Oldham, M.C.; Horvath, S. Is my network module preserved and reproducible? PLoS Comput. Biol. 2011, 7, e1001057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, S.; Lei, E.P. DiffChIPL: A differential peak analysis method for high-throughput sequencing data with biological replicates based on limma. Bioinformatics 2022, 38, 4062–4069. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Kovacs, I.A.; Barabási, A.-L. Network-based prediction of drug combinations. Nat. Commun. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Chen, L.; Jafari, M.; Tang, J. Network-based modeling of herb combinations in traditional Chinese medicine. Brief Bioinform. 2021, 22, bbab106. [Google Scholar] [CrossRef]
- Maiorino, E.; Baek, S.H.; Guo, F.; Zhou, X.; Kothari, P.H.; Silverman, E.K.; Barabási, A.-L.; Weiss, S.T.; Raby, B.A.; Sharma, A. Author Correction: Discovering the genes mediating the interactions between chronic respiratory diseases in the human interactome. Nat. Commun. 2021, 12, 2434. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Gu, H.; Yu, Y.; Wang, P.; Liu, J.; Zhang, Y.; Chen, Y.; Niu, Q.; Wang, B.; et al. Modular Screening Reveals Driver Induced Additive Mechanisms of Baicalin and Jasminoidin on Cerebral Ischemia Therapy. Front. Cardiovasc. Med. 2022, 9, 813983. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, W.S.; Feng, C.Q. Progress in the absorption, distribution, metabolism, and excretion processes of geniposide and its hepatotoxicity. Chin. J. Pharmacol. Toxicol. 2022, 36, 552–558. (In Chinese) [Google Scholar]
- Li, X.Y.; Su, F.F.; Jiang, C.; Zhang, W.; Wang, F.; Zhu, Q.; Yang, G. Overview of the evolution of bear bile efficacy and component research. Chin. J. Chin. Mater. Med. 2022, 47, 4846–4853. (In Chinese) [Google Scholar]
- Chen, Y.; Zhou, C.; Yu, Y.; Liu, J.; Jing, Z.; Lv, A.; Meng, F.; Wang, Z.; Wang, Y. Variations in target gene expression and pathway profiles in the mouse hippocampus following treatment with different effective compounds for ischemia–reperfusion injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 797–806. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, C.; Zhang, Z.; Wang, L.; Jing, Z.; Wang, Z. Synergistic mechanism of gene expression and pathways between jasminoidin and ursodeoxycholic acid in treating focal cerebral ischemia-reperfusion injury. CNS Neurosci. Ther. 2012, 18, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L. Study on the Mechanism of Congenital Heart Disease Caused by RCAN1.4 and VPS50 Loss of Function. Ph.D. Thesis, Chongqing Medical University, Chongqing, China, 2018. (In Chinese). [Google Scholar]
- Hasan, A.; Furtado, G.V.; Miglorini, E.; Mergener, R.; Massuyama, B.; Barsottini, O.; Pedroso, J.L.; Teive, H.G.; Saraiva-Pereira, M.L.; Ashizawa, T.; et al. The impact of interrupted ATXN10 expansions on clinical findings of spinocerebellar ataxia type 10. J. Neurol. 2025, 272, 261. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, H.; Katsuzawa, T.; Saruta, C.; Sadakata, T.; Kobayashi, S.; Sato, Y.; Sato, A.; Sano, Y.; Maezawa, S.; Shinoda, Y.; et al. Calcium-dependent activator protein for secretion 2 is involved in dopamine release in mouse midbrain neurons. Front. Mol. Neurosci. 2024, 17, 1444629. [Google Scholar] [CrossRef]
- Nobelmann, B.; Lengeler, J.W. Molecular analysis of the gat genes from Escherichia coli and of their roles in galactitol transport and metabolism. J. Bacteriol. 1996, 178, 6790–6795. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Tandler, B.; Hoppel, C.L. Mitochondrial dysfunction and myocardial ischemia-reperfusion: Implications for novel therapies. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 535–565. [Google Scholar] [CrossRef]
- Maneechote, C.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J. Cell. Mol. Med. 2017, 21, 2643–2653. [Google Scholar] [CrossRef]
- Sun, G.; Qin, W.; Wang, Q.; Sun, X.; Chen, H.; Li, J.; Sun, L.; Shi, F.; Zhang, G.; Wang, M. Selective-cerebral-hypothermia-induced neuroprotection against-focal cerebral ischemia/reperfusion injury is associated with an increase in SUMO2/3 conjugation. Brain Res. 2021, 1756, 147311. [Google Scholar] [CrossRef]
- Peng, K.; Yang, L.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Cui, Z.; Ao, L.; Liu, J.; et al. The interaction of mitochondrial biogenesis and fission/fusion mediated by PGC-1α regulates rotenone-induced dopaminergic neurotoxicity. Mol. Neurobiol. 2016, 54, 3783–3797. [Google Scholar] [CrossRef]
- Salehpour, F.; Farajdokht, F.; Mahmoudi, J.; Erfani, M.; Farhoudi, M.; Karimi, P.; Rasta, S.H.; Sadigh-Eteghad, S.; Hamblin, M.R.; Gjedde, A. Photobiomodulation and coenzyme Q10 treatments attenuate cognitive impairment associated with model of transient global brain ischemia in artificially aged mice. Front. Cell. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Antero, S.; Anu, K.; Kai, K. Hypoxia/ischemia activate processing of amyloid precursor protein: Impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2017, 140, 536–549. [Google Scholar]
- Li, W.; Liu, D.; Xu, J.; Zha, J.; Wang, C.; An, J.; Xie, Z.; Qiao, S. Astrocyte-derived TNF-α-activated platelets promote cerebral ischemia/reperfusion injury by regulating the RIP1/RIP3/AKT signaling pathway. Mol. Neurobiol. 2022, 59, 5734–5749. [Google Scholar] [CrossRef]
- Abeysinghe, H.C.S.; Phillips, E.L.; Chin-Cheng, H.; Beart, P.M.; Roulston, C.L. Modulating astrocyte transition after stroke to promote brain rescue and functional recovery: Emerging Targets Include Rho Kinase. Int. J. Mol. Sci. 2016, 17, 288. [Google Scholar] [CrossRef]
- Ardelt, A.A.; Carpenter, R.S.; Iwuchukwu, I.; Zhang, A.; Lin, W.; Kosciuczuk, E.; Hinkson, C.; Rebeiz, T.; Reitz, S.; King, P.H. Transgenic expression of HuR increases vasogenic edema and impedes functional recovery in rodent ischemic stroke. Neurosci. Lett. 2017, 661, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.; Floyd, C.L.; Patel, J.; Mohaimany-Aponte, A.; King, P.H. Astrocytic expression of the RNA regulator HuR accentuates spinal cord injury in the acute phase. Neurosci. Lett. 2017, 651, 140–145. [Google Scholar] [CrossRef]
- Luo, H.-C.; Yi, T.-Z.; Huang, F.-G.; Wei, Y.; Luo, X.-P.; Luo, Q.-S. Role of long noncoding RNA MEG3/miR-378/GRB2 axis in neuronal autophagy and neurological functional impairment in ischemic stroke. J. Biol. Chem. 2020, 295, 14125–14139. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, Y.; Yu, H.; Liu, S.; Xiao, P.; Jia, Z.; Zhang, Z. The Role of Cullin 3 in Cerebral Ischemia–Reperfusion Injury. Neuroscience 2023, 514, 14–24. [Google Scholar] [CrossRef]
- Cheah, P.-S.; Ramshaw, H.S.; Thomas, P.Q.; Toyo-Oka, K.; Xu, X.; Martin, S.; Coyle, P.; Guthridge, M.A.; Stomski, F.; Buuse, M.v.D.; et al. Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. Mol. Psychiatry 2011, 17, 451–466. [Google Scholar] [CrossRef]
- Almeida, A.; Sánchez-Morán, I.; Rodríguez, C. Mitochondrial–nuclear p53 trafficking controls neuronal susceptibility in stroke. IUBMB Life 2021, 73, 582–591. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Y.; Qian, J.; Xiao, B.G.; Lu, C.Z. Fibronectin and neuroprotective effect of granulocyte colony-stimulating factor in focal cerebral ischemia. Brain Res. 2006, 1098, 161–169. [Google Scholar]
- Zhang, H.; Zhao, L.; Yang, J.; Zhou, S.; Liu, J.; Wang, C.; Wang, B. Research on mechanism of combination of Scutellaria baicalensis Georgi and gardenia in treatment of ischemic stroke based on network pharmacology. Chin. Pharmacol. Bull. 2022, 38, 436–445. (In Chinese) [Google Scholar]
- Patel, S.; Khan, M.B.; Kumar, S.; Vyavahare, S.; Mendhe, B.; Lee, T.J.; Cai, J.; Isales, C.M.; Liu, Y.; Hess, D.C.; et al. The impact of ischemic stroke on bone marrow microenvironment and extracellular vesicles: A study on inflammatory and molecular changes. Exp. Neurol. 2024, 379, 114867. [Google Scholar] [CrossRef]
- Ding, Y.; Lang, Y.; Zhang, H.; Li, Y.; Liu, X.; Li, M. Candesartan Reduces Neuronal Apoptosis Caused by Ischemic Stroke via Regulating the FFAR1/ITGA4 Pathway. Mediat. Inflamm. 2022, 2022, 2356507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yin, P.; Bai, X.; Yang, L.; Zhang, J.; Xu, S. Proinflammatory and Anti-inflammatory Genes in Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4220–4233. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.; Park, J.; Kang, K.; Lee, S.J.; Kim, J.G.; Cha, J.; Kim, D.; Han, M.; Kang, J.; et al. Prevalence of mutations in Mendelian stroke genes in early onset stroke patients. Ann. Neurol. 2023, 93, 768–782. [Google Scholar] [CrossRef]
- Benson, A.R.; Gleich, D.F.; Leskovec, J. Higher-order organization of complex networks. Science 2016, 353, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Choobdar, S.; Ahsen, M.E.; Crawford, J.; Tomasoni, M.; Fang, T.; Lamparter, D.; Lin, J.; Hescott, B.; Hu, X.; Marbach, D.; et al. Assessment of network module identification across complex diseases. Nat. Methods 2019, 16, 843–852. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Zhang, B.; Zhu, L.; Cai, H. Evaluation of gene–drug common module identification methods using pharmacogenomics data. Briefings Bioinform. 2020, 22, bbaa087. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Atakishizada, S.; Valiyeva, M.; Ardalan, M.; Khalilov, R.; Kavetskyy, T. Podocytopathy: The role of actin cytoskeleton. Biomed. Pharmacother. 2022, 156, 113920. [Google Scholar] [CrossRef]
- Das, S.; Zhang, Z.; Kalvakota, S.; Soto, R.; Phillips, M.L.; Terman, J.R.; Reisler, E. Parallel actin monomers in the 8S complex of actin–INF2. J. Biomol. Struct. Dyn. 2022, 41, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, T.; Hu, Y.; Wang, J.; Wang, Y.; Zhang, Y.; Li, F.; Liu, W.; Sun, Y.; Yu, B.; et al. NMMHC IIA triggers neuronal autophagic cell death by promoting F-actin-dependent ATG9A trafficking in cerebral ischemia/reperfusion. Cell Death Dis. 2020, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Yu, X.; Yan, X.-J.; Lei, F.; Chai, Y.-S.; Jiang, J.-F.; Yuan, Z.-Y.; Xing, D.-M.; Du, L.-J. TRPM8 in the negative regulation of TNFα expression during cold stress. Sci. Rep. 2017, 7, 45155. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Lv, Y.; Wu, X.; Gui, L.; Zhang, Y.; Qiu, J.; Song, G.; Yao, W.; Wan, L.; et al. Cdh1 overexpression improves emotion and cognitive-related behaviors via regulating hippocampal neuroplasticity in global cerebral ischemia rats. Neurochem. Int. 2019, 124, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, J.; Gong, J.; Shen, J.; Ye, D.; Cheng, D.; Xie, Z.; Zeng, J.; Xu, K.; Shen, J.; et al. PTP1B inhibitor alleviates deleterious microglial activation and neuronal injury after ischemic stroke by modulating the ER stress-autophagy axis via PERK signaling in microglia. Aging 2021, 13, 3405–3427. [Google Scholar] [CrossRef]
- Vafiadaki, E.; Arvanitis, D.A.; Eliopoulos, A.G.; Kranias, E.G.; Sanoudou, D. The cardioprotective PKA-mediated Hsp20 phosphorylation modulates protein associations regulating cytoskeletal dynamics. Int. J. Mol. Sci. 2020, 21, 9572. [Google Scholar] [CrossRef]
- Yadunandanan Nair, N.; Samuel, V.; Ramesh, L.; Marib, A.; David, D.T.; Sundararaman, A. Actin cytoskeleton in angiogenesis. Biol. Open 2022, 11, bio058899. [Google Scholar]
- Guo, C.-Y.; Xiong, T.-Q.; Tan, B.-H.; Gui, Y.; Ye, N.; Li, S.-L.; Li, Y.-C. The temporal and spatial changes of actin cytoskeleton in the hippocampal CA1 neurons following transient global ischemia. Brain Res. 2019, 1720, 146297. [Google Scholar] [CrossRef]
- Xue, Y.; He, J.-T.; Zhang, K.-K.; Chen, L.-J.; Wang, Q.; Xie, X.-L. Methamphetamine reduces expressions of tight junction proteins, rearranges F-actin cytoskeleton and increases the blood brain barrier permeability via the RhoA/ROCK-dependent pathway. Biochem. Biophys. Res. Commun. 2019, 509, 395–401. [Google Scholar] [CrossRef]
- Shang, G.; Zhang, W.; Jia, Y.; Ji, D.; Wei, E.; Gao, C.; Zeng, C.; Wang, C.; Liu, N.; Ge, P.; et al. GAS41 promotes ITGA4-mediated PI3K/Akt/mTOR signaling pathway and glioma tumorigenesis. Biochem. Pharmacol. 2025, 233, 116747. [Google Scholar] [CrossRef]
- You, W.; Knoops, K.; Berendschot, T.T.J.M.; Benedikter, B.J.; Webers, C.A.B.; Reutelingsperger, C.P.M.; Gorgels, T.G.M.F. PGC-1a mediated mitochondrial biogenesis promotes recovery and survival of neuronal cells from cellular degeneration. Cell Death Discov. 2024, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Shi, Y.; Zhang, J.; Peng, J.; Zhang, X.; Chen, K.; Chen, Y.; Liu, L. MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed. Pharmacother. 2019, 117, 109117. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Yang, B.; Tu, D.; Liang, R.; Shi, S. Propofol attenuates neuroinflammation and brain damage via modulating PI3K/Akt signaling pathway in rats of focal cerebral ischemia. Chin. Pharmacol. Bull. 2019, 35, 630–633. (In Chinese) [Google Scholar]
- Wang, C.; Wan, H.; Li, M.; Zhang, C. Celastrol attenuates ischemia/reperfusion-mediated memory dysfunction by downregulating AK005401/MAP3K12. Phytomedicine 2021, 82, 153441. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, Y.; Ma, Y.; Zhang, X.; Yang, L.; Shen, X.; Zhang, J.; Jing, L. Selenium attenuates ischemia/reperfusion injury-induced damage to the blood-brain barrier in hyperglycemia through PI3K/AKT/mTOR pathway-mediated autophagy inhibition. Int. J. Mol. Med. 2021, 48, 178. [Google Scholar] [CrossRef]
- Lan, H.; Zheng, Q.; Wang, K.; Li, C.; Xiong, T.; Shi, J.; Dong, N. Cinnamaldehyde protects donor heart from cold ischemia–reperfusion injury via the PI3K/AKT/mTOR pathway. Biomed. Pharmacother. 2023, 165, 114867. [Google Scholar] [CrossRef]
- Li, R.; Zheng, Y.; Zhang, J.; Zhou, Y.; Fan, X. Gomisin N attenuated cerebral ischemia-reperfusion injury through inhibition of autophagy by activating the PI3K/AKT/mTOR pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 110, 154644. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Li, X.; Jiang, Y.; Wang, C.; Zhang, Y.; Kong, Q.; Tian, C.; Dai, Y.; Zhao, W.; et al. Excess salt intake promotes M1 microglia polarization via a p38/MAPK/AR-dependent pathway after cerebral ischemia in mice. Int. Immunopharmacol. 2020, 81, 106176. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, M.; Min, L.; Jiang, K.; Jiang, Y. Cerebral ischemia-reperfusion is modulated by macrophage-stimulating 1 through the MAPK-ERK signaling pathway. J. Cell Physiol. 2020, 235, 7067–7080. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, M.; Betancourt, C.E.; Liu, L.; Sitikov, A.; Sladojevic, N.; Zhao, Q.; Zhang, J.H.; Liao, J.K.; Wu, R. Increase in blood-brain barrier (BBB) permeability is regulated by MMP3 via the ERK signaling pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6655122. [Google Scholar] [CrossRef]
- Li, G.; Duan, L.; Yang, F.; Yang, L.; Deng, Y.; Yu, Y.; Xu, Y.; Zhang, Y. Curcumin suppress inflammatory response in traumatic brain injury via p38/MAPK signaling pathway. Phytother. Res. 2022, 36, 1326–1337. [Google Scholar] [CrossRef] [PubMed]




| Module-a | Module-a | SAB |
|---|---|---|
| JA-DEGs | UA-DEGs | −0.266 |
| JA-DEGs | JU-DEGs | −0.183 |
| UA-DEGs | JU-DEGs | −0.149 |
| Driver Gene | The Shortest Distance | Flow Centrality | Literature |
|---|---|---|---|
| UBC | 5904 | 2,442,296.72 | - |
| SUMO2 | 8758 | 44,576.78 | [48,49,50] |
| NRF1 | 8445 | 156,372.58 | [51,52] |
| APP | 8420 | 171,885.22 | [53] |
| JUN | 8589 | 52,966.16 | [54] |
| ELAVL1 | 8601 | 89,125.44 | [55,56,57] |
| GRB2 | 8746 | 61,624.5 | [58] |
| CUL3 | 8757 | 31,379.5 | [59] |
| YWHAZ | 8828 | - | [60] |
| TP53 | 8841 | - | [61] |
| FN1 | - | 38,194.56 | [62] |
| PRKACA | - | 33,893.5 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Niu, Q.; Yu, Y.; Liu, J.; Zhang, S.; Zong, W.; Tian, S.; Wang, Z.; Li, B. Modular-Based Synergetic Mechanisms of Jasminoidin and Ursodeoxycholic Acid in Cerebral Ischemia Therapy. Biomedicines 2025, 13, 938. https://doi.org/10.3390/biomedicines13040938
Wang J, Niu Q, Yu Y, Liu J, Zhang S, Zong W, Tian S, Wang Z, Li B. Modular-Based Synergetic Mechanisms of Jasminoidin and Ursodeoxycholic Acid in Cerebral Ischemia Therapy. Biomedicines. 2025; 13(4):938. https://doi.org/10.3390/biomedicines13040938
Chicago/Turabian StyleWang, Jingai, Qikai Niu, Yanan Yu, Jun Liu, Siqi Zhang, Wenjing Zong, Siwei Tian, Zhong Wang, and Bing Li. 2025. "Modular-Based Synergetic Mechanisms of Jasminoidin and Ursodeoxycholic Acid in Cerebral Ischemia Therapy" Biomedicines 13, no. 4: 938. https://doi.org/10.3390/biomedicines13040938
APA StyleWang, J., Niu, Q., Yu, Y., Liu, J., Zhang, S., Zong, W., Tian, S., Wang, Z., & Li, B. (2025). Modular-Based Synergetic Mechanisms of Jasminoidin and Ursodeoxycholic Acid in Cerebral Ischemia Therapy. Biomedicines, 13(4), 938. https://doi.org/10.3390/biomedicines13040938






