Tauroursodeoxycholic Acid Induces Liver Regeneration and Alleviates Fibrosis Through GATA3 Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. CCK-8 Proliferation Assay
2.3. Cell Cycle Analysis
2.4. EdU Cell Proliferation Assay
2.5. Scratch Wound Healing Assay
2.6. Animal Husbandry
2.7. Animal Experiments
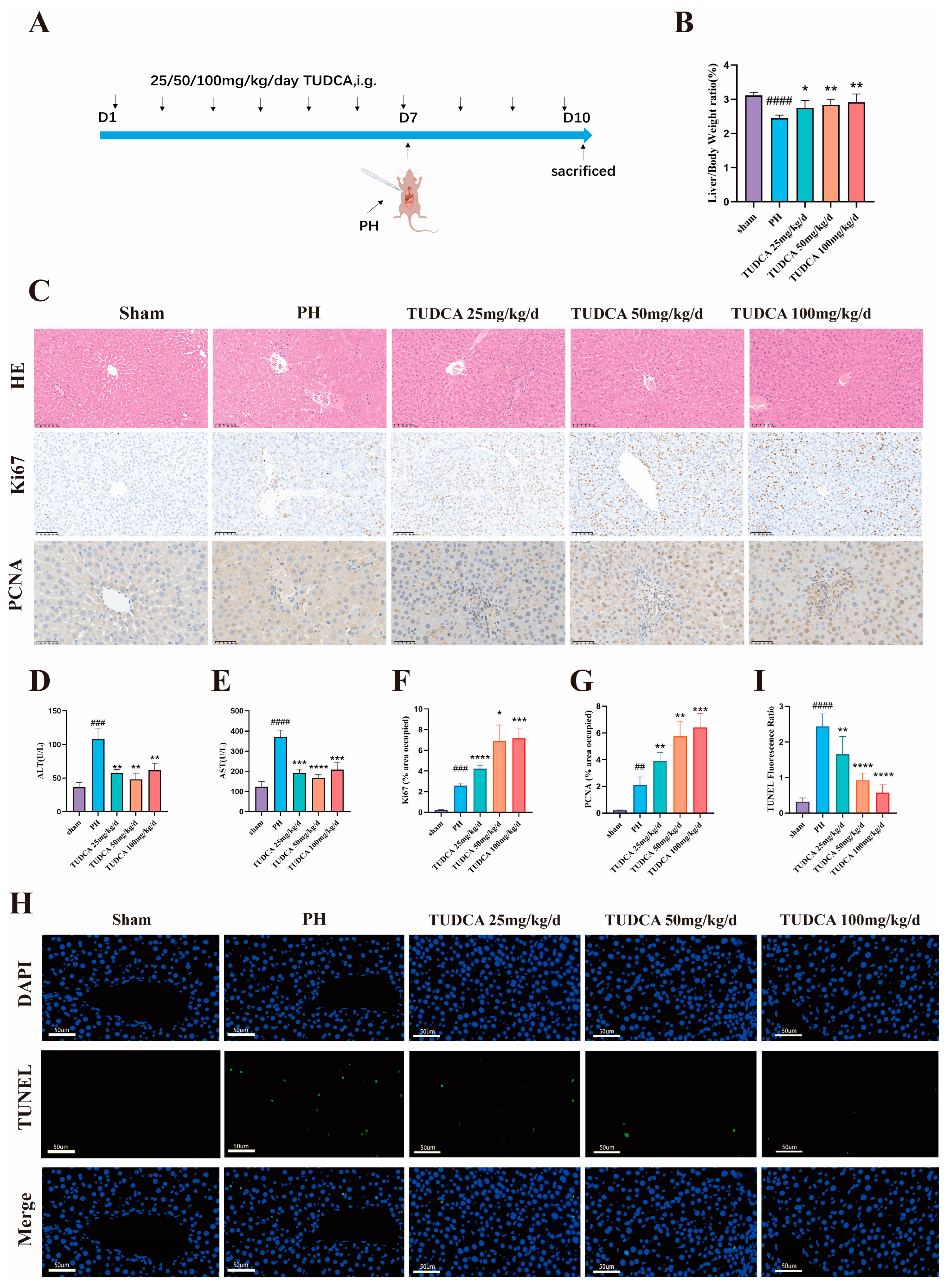
2.7.1. Effects of TUDCA on Liver Regeneration After 70% Hepatectomy
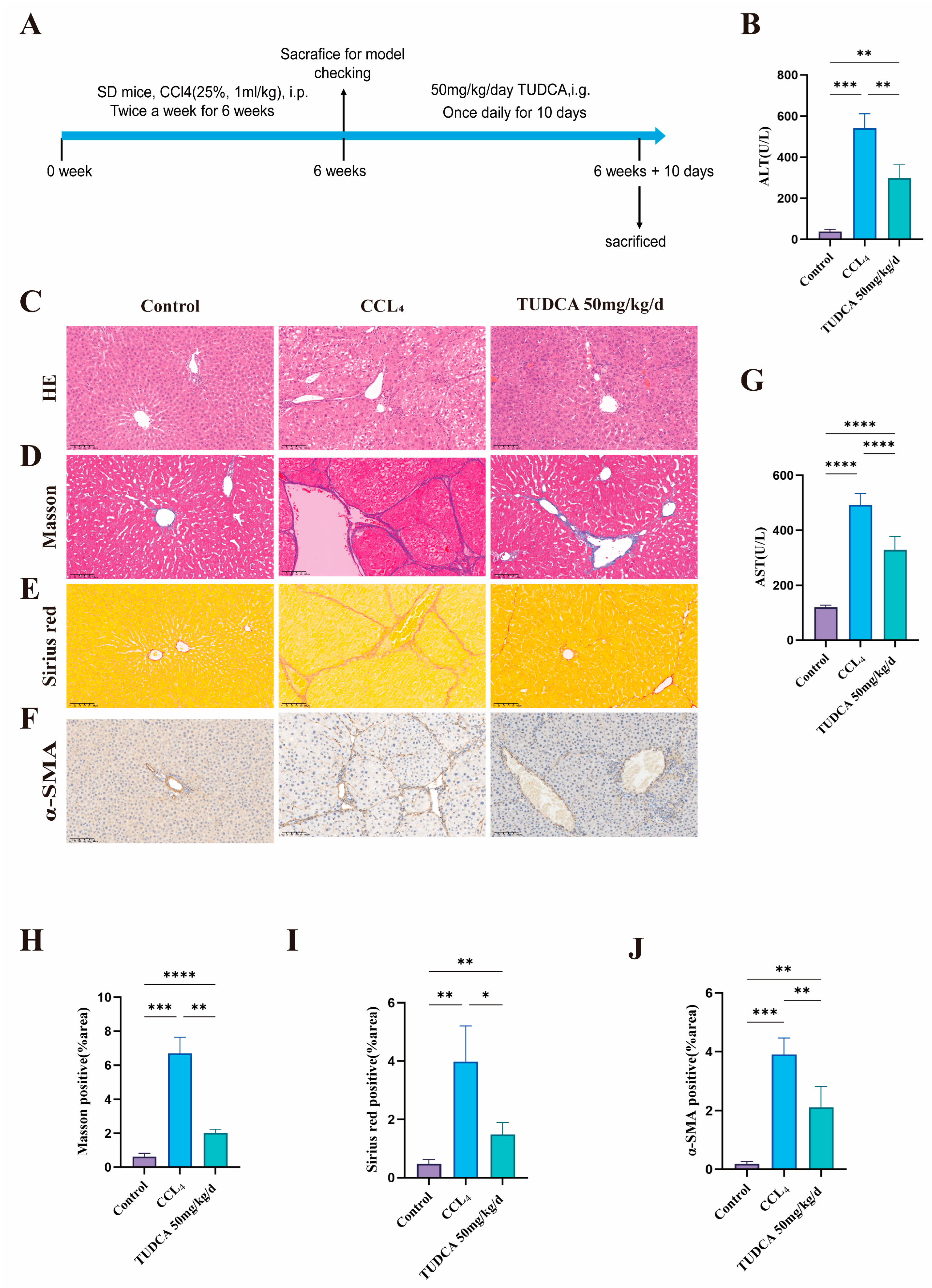
2.7.2. Alleviation of CCL4-Induced Liver Fibrosis by TUDCA
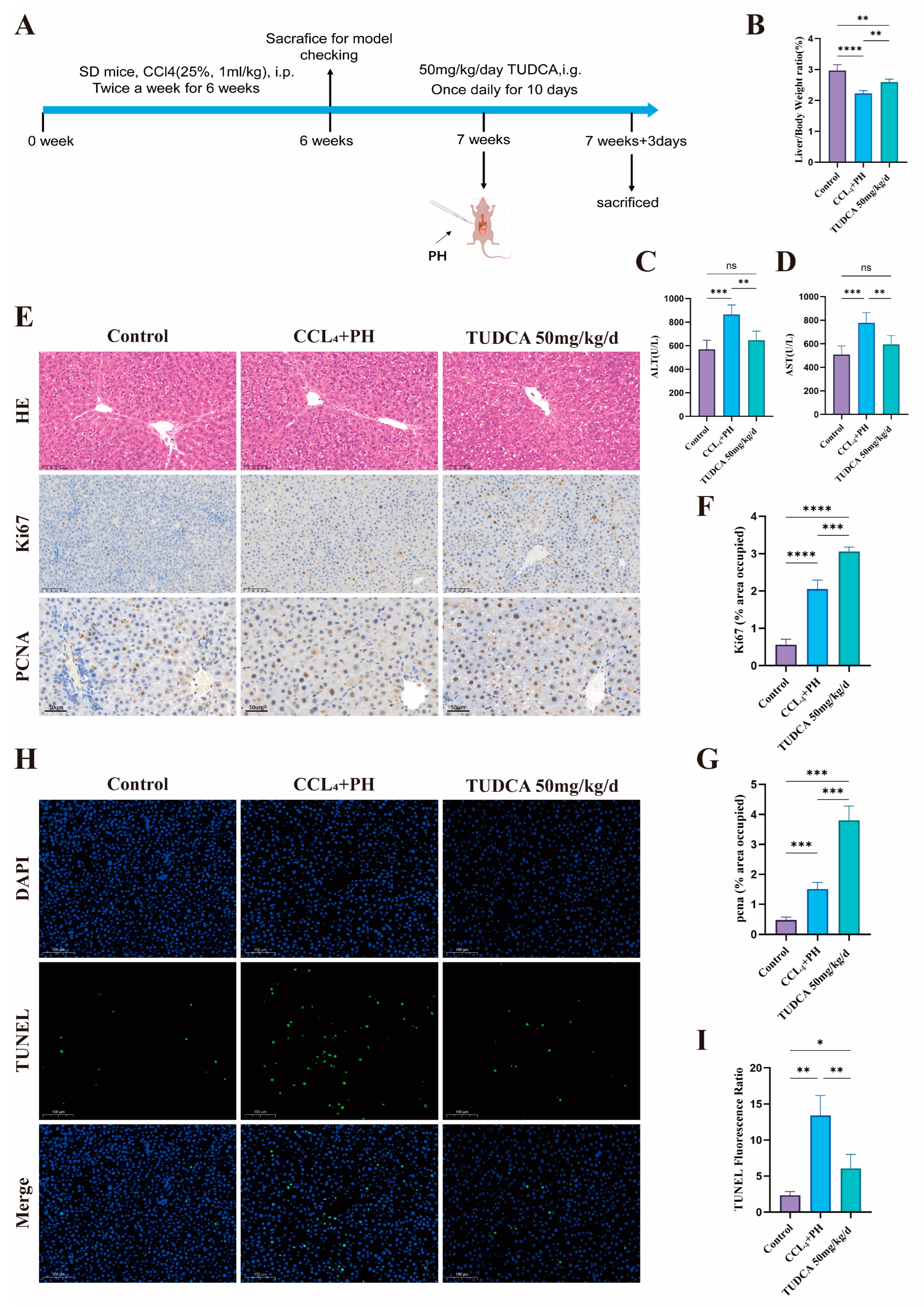
2.7.3. TUDCA Promotes Liver Regeneration After Hepatectomy in the Presence of CCL4-Induced Liver Fibrosis
2.8. Histological and Serum Biochemical Analysis
2.9. Immunohistochemistry
2.10. Immunofluorescence
2.11. RNA-seq and Data Analysis
2.12. RT-qPCR
2.13. Western Blotting
2.14. Statistical Analysis
3. Results
3.1. TUDCA Promotes the Proliferation and Migration of BRL-3A Cells
3.2. TUDCA Promotes Liver Regeneration in 70% Hepatectomized Rats
3.3. TUDCA Alleviates CCL4-Induced Liver Fibrosis
3.4. TUDCA Facilitates the Recovery of Liver Tissue Within a Fibrotic Environment Following Partial Liver Resection
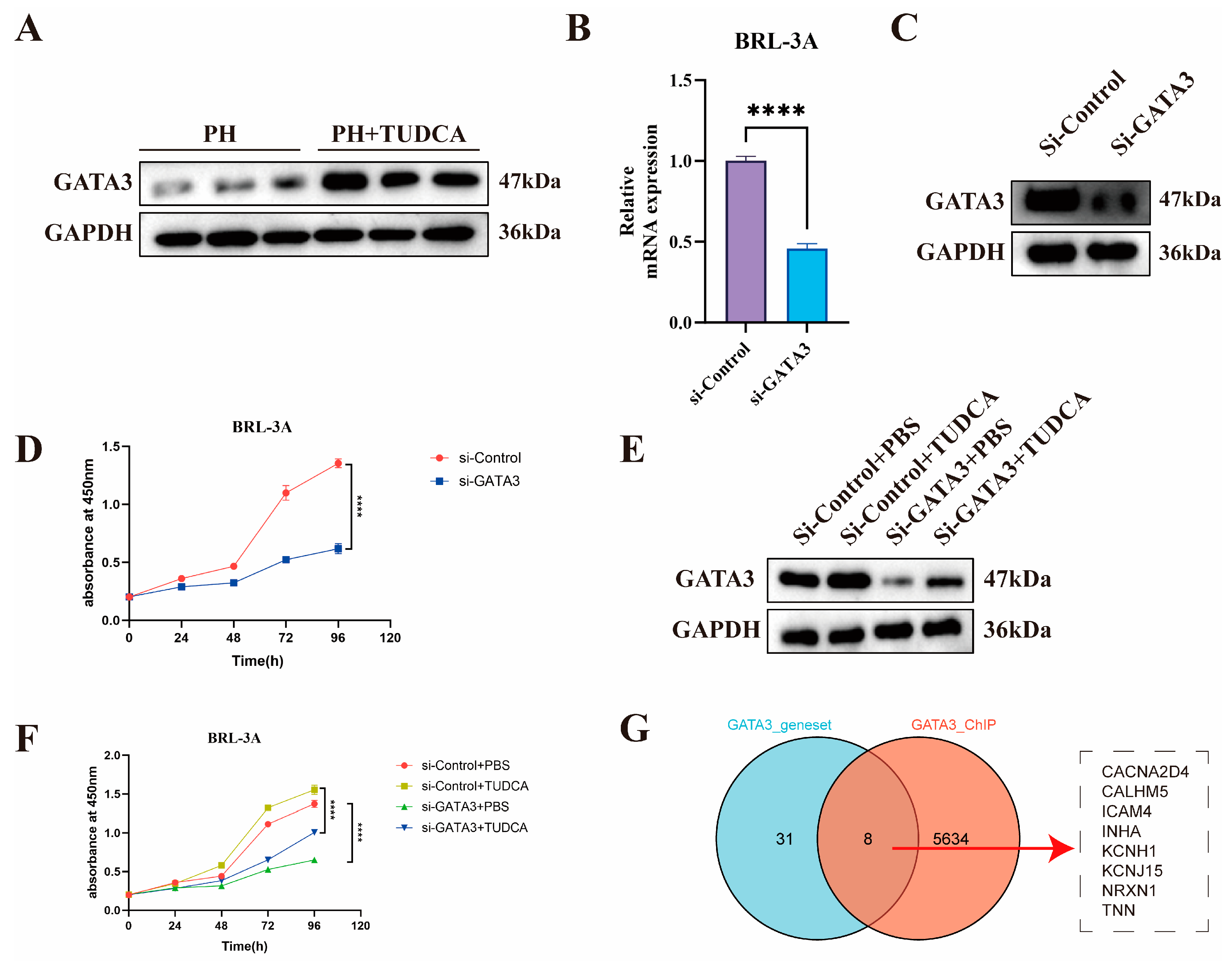
3.5. Mechanistic Study of TUDCA in Promoting Liver Proliferation
3.6. Knockdown of GATA3 Abolishes TUDCA-Induced Proliferation in BRL-3A Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Man, S.; Deng, Y.; Ma, Y.; Fu, J.; Bao, H.; Yu, C.; Lv, J.; Liu, H.; Wang, B.; Li, L. Prevalence of Liver Steatosis and Fibrosis in the General Population and Various High-Risk Populations: A Nationwide Study With 5.7 Million Adults in China. Gastroenterology 2023, 165, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Latif, M.U.; Schmidt, G.E.; Mercan, S.; Rahman, R.; Gibhardt, C.S.; Stejerean-Todoran, I.; Reutlinger, K.; Hessmann, E.; Singh, S.K.; Moeed, A.; et al. NFATc1 signaling drives chronic ER stress responses to promote NAFLD progression. Gut 2022, 71, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Luan, J.; Huang, T.; Deng, T.; Li, X.; Xiao, Z.; Zhan, J.; Luo, D.; Hou, Y.; Xu, L.; et al. Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J. Neuroinflamm. 2021, 18, 216. [Google Scholar] [CrossRef]
- Schoemaker, M.H.; Conde de la Rosa, L.; Buist-Homan, M.; Vrenken, T.E.; Havinga, R.; Poelstra, K.; Haisma, H.J.; Jansen, P.L.; Moshage, H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 2004, 39, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fu, D.; Sheng, Q.; Yu, S.; Bao, X.; Lv, Z. TUDCA attenuates intestinal injury and inhibits endoplasmic reticulum stress-mediated intestinal cell apoptosis in necrotizing enterocolitis. Int. Immunopharmacol. 2019, 74, 105665. [Google Scholar] [CrossRef]
- Pandolfi, P.P.; Roth, M.E.; Karis, A.; Leonard, M.W.; Dzierzak, E.; Grosveld, F.G.; Engel, J.D.; Lindenbaum, M.H. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat. Genet. 1995, 11, 40–44. [Google Scholar] [CrossRef]
- Kouros-Mehr, H.; Slorach, E.M.; Sternlicht, M.D.; Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 2006, 127, 1041–1055. [Google Scholar] [CrossRef]
- Bai, F.; Zheng, C.; Liu, X.; Chan, H.L.; Liu, S.; Ma, J.; Ren, S.; Zhu, W.G.; Pei, X.H. Loss of function of GATA3 induces basal-like mammary tumors. Theranostics 2022, 12, 720–733. [Google Scholar] [CrossRef]
- Miyamoto, H.; Izumi, K.; Yao, J.L.; Li, Y.; Yang, Q.; McMahon, L.A.; Gonzalez-Roibon, N.; Hicks, D.G.; Tacha, D.; Netto, G.J. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum. Pathol. 2012, 43, 2033–2040. [Google Scholar] [CrossRef]
- Wang, T.; Feldman, A.L.; Wada, D.A.; Lu, Y.; Polk, A.; Briski, R.; Ristow, K.; Habermann, T.M.; Thomas, D.; Ziesmer, S.C.; et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood 2014, 123, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Provot, S.; Werb, Z. GATA3 in development and cancer differentiation: Cells GATA have it! J. Cell Physiol. 2010, 222, 42–49. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, J.; Huang, H.; Liu, L.; Ren, L.; Hu, J.; Jiang, X.; Zheng, Y.; Xu, L.; Zhong, F.; et al. The m(6)A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis. Cell Death Dis. 2024, 15, 189. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Liu, L.; Yan, M.; Zhang, Q.; Song, X.; Lin, Y.; Zhu, D.; Wei, Y.; Fu, Z.; et al. Gly-tRF enhances LCSC-like properties and promotes HCC cells migration by targeting NDFIP2. Cancer Cell Int. 2021, 21, 502. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [CrossRef]
- Wei, J.; Yao, J.; Yang, C.; Mao, Y.; Zhu, D.; Xie, Y.; Liu, P.; Yan, M.; Ren, L.; Lin, Y.; et al. Heterogeneous matrix stiffness regulates the cancer stem-like cell phenotype in hepatocellular carcinoma. J. Transl. Med. 2022, 20, 555. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhou, Y.; Ji, H.; Wang, Y.; Wu, Q.; Bao, J.; Ye, F.; Shi, Y.; Bu, H. Loss of histone deacetylases 1 and 2 in hepatocytes impairs murine liver regeneration through Ki67 depletion. Hepatology 2013, 58, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, A.; Callejas, N.A.; Casado, M.; Boscá, L.; Martín-Sanz, P. Thioacetamide-induced liver regeneration involves the expression of cyclooxygenase 2 and nitric oxide synthase 2 in hepatocytes. J. Hepatol. 2004, 40, 963–970. [Google Scholar] [CrossRef]
- Vaquerizas, J.M.; Kummerfeld, S.K.; Teichmann, S.A.; Luscombe, N.M. A census of human transcription factors: Function, expression and evolution. Nat. Rev. Genet. 2009, 10, 252–263. [Google Scholar] [CrossRef]
- McNally, J.G.; Müller, W.G.; Walker, D.; Wolford, R.; Hager, G.L. The glucocorticoid receptor: Rapid exchange with regulatory sites in living cells. Science 2000, 287, 1262–1265. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Elizarraras, J.M.; Liao, Y.; Shi, Z.; Zhu, Q.; Pico, A.R.; Zhang, B. WebGestalt 2024: Faster gene set analysis and new support for metabolomics and multi-omics. Nucleic Acids Res. 2024, 52, W415–W421. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, H.; Wei, Y.; Liu, F.; Yang, Y.; Yu, H.; Fu, J.; Cui, X.; Yu, T.; Xu, Y.; et al. Balance of Gata3 and Ramp2 in hepatocytes regulates hepatic vascular reconstitution in postoperative liver regeneration. J. Hepatol. 2024, 80, 309–321. [Google Scholar] [CrossRef]
- Gattinoni, L.; Zhong, X.S.; Palmer, D.C.; Ji, Y.; Hinrichs, C.S.; Yu, Z.; Wrzesinski, C.; Boni, A.; Cassard, L.; Garvin, L.M.; et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009, 15, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Regard, J.B.; Zhong, Z.; Williams, B.O.; Yang, Y. Wnt signaling in bone development and disease: Making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012, 4, a007997. [Google Scholar] [CrossRef]
- Zheng, W.; Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Zen, Y.; Liberal, R.; Nakanuma, Y.; Heaton, N.; Portmann, B. Possible involvement of CCL1-CCR8 interaction in lymphocytic recruitment in IgG4-related sclerosing cholangitis. J. Hepatol. 2013, 59, 1059–1064. [Google Scholar] [CrossRef]
- Grote, D.; Souabni, A.; Busslinger, M.; Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 2006, 133, 53–61. [Google Scholar] [CrossRef]
- Sakai, R.; Ito, M.; Komai, K.; Iizuka-Koga, M.; Matsuo, K.; Nakayama, T.; Yoshie, O.; Amano, K.; Nishimasu, H.; Nureki, O.; et al. Kidney GATA3(+) regulatory T cells play roles in the convalescence stage after antibody-mediated renal injury. Cell Mol. Immunol. 2021, 18, 1249–1261. [Google Scholar] [CrossRef]
- Heinz, S.; Romanoski, C.E.; Benner, C.; Glass, C.K. The selection and function of cell type-specific enhancers. Nat. Rev. Mol. Cell Biol. 2015, 16, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Krendl, C.; Shaposhnikov, D.; Rishko, V.; Ori, C.; Ziegenhain, C.; Sass, S.; Simon, L.; Müller, N.S.; Straub, T.; Brooks, K.E.; et al. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc. Natl. Acad. Sci. USA 2017, 114, E9579–E9588. [Google Scholar] [CrossRef] [PubMed]
- Vale, W.; Rivier, J.; Vaughan, J.; McClintock, R.; Corrigan, A.; Woo, W.; Karr, D.; Spiess, J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 1986, 321, 776–779. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, X.; Zhao, C.; Geng, R.; Wu, K.; Yuan, M.; Ai, N.; Ge, W. Rescue of bmp15 deficiency in zebrafish by mutation of inha reveals mechanisms of BMP15 regulation of folliculogenesis. PLoS Genet. 2023, 19, e1010954. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Singh, S.K.; Rajput, S.; Allawadhi, P.; Khurana, A.; Weiskirchen, R.; Navik, U. Therapeutic potential of stem cells in regeneration of liver in chronic liver diseases: Current perspectives and future challenges. Pharmacol. Ther. 2024, 253, 108563. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Wolf, S.D.; Ehlting, C.; Müller-Dott, S.; Poschmann, G.; Petzsch, P.; Lautwein, T.; Wang, S.; Helm, B.; Schilling, M.; Saez-Rodriguez, J.; et al. Hepatocytes reprogram liver macrophages involving control of TGF-β activation, influencing liver regeneration and injury. Hepatol. Commun. 2023, 7, e0208. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Honda, C.K.; Kurozumi, S.; Fujii, T.; Pourquier, D.; Khellaf, L.; Boissiere, F.; Horiguchi, J.; Oyama, T.; Shirabe, K.; Colinge, J.; et al. Cancer-associated fibroblast spatial heterogeneity and EMILIN1 expression in the tumor microenvironment modulate TGF-β activity and CD8(+) T-cell infiltration in breast cancer. Theranostics 2024, 14, 1873–1885. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, C.; Song, X.; Yan, J.; Xu, J.; Zhou, Y.; Sun, Z.; Zheng, Q.; Zhang, Y.; Chen, R.; Jin, X.; et al. Tauroursodeoxycholic Acid Induces Liver Regeneration and Alleviates Fibrosis Through GATA3 Activation. Biomedicines 2025, 13, 910. https://doi.org/10.3390/biomedicines13040910
Bai C, Song X, Yan J, Xu J, Zhou Y, Sun Z, Zheng Q, Zhang Y, Chen R, Jin X, et al. Tauroursodeoxycholic Acid Induces Liver Regeneration and Alleviates Fibrosis Through GATA3 Activation. Biomedicines. 2025; 13(4):910. https://doi.org/10.3390/biomedicines13040910
Chicago/Turabian StyleBai, Chongyang, Xiaojing Song, Jiexi Yan, Jun Xu, Yongqiang Zhou, Zongbin Sun, Qiuxia Zheng, Yue Zhang, Ruixi Chen, Xiaoyi Jin, and et al. 2025. "Tauroursodeoxycholic Acid Induces Liver Regeneration and Alleviates Fibrosis Through GATA3 Activation" Biomedicines 13, no. 4: 910. https://doi.org/10.3390/biomedicines13040910
APA StyleBai, C., Song, X., Yan, J., Xu, J., Zhou, Y., Sun, Z., Zheng, Q., Zhang, Y., Chen, R., Jin, X., Shao, Y., Xie, Y., Yang, L., Zhong, F., Zhang, Y., Li, J., Li, R., Yan, S., & Li, X. (2025). Tauroursodeoxycholic Acid Induces Liver Regeneration and Alleviates Fibrosis Through GATA3 Activation. Biomedicines, 13(4), 910. https://doi.org/10.3390/biomedicines13040910




