Abstract
Background/Objectives: Optimal activation of the quadriceps femoris, particularly the vastus medialis, while minimizing excessive activation of the vastus lateralis, is crucial for treating knee injuries like ACL ruptures and patellofemoral pain syndrome. Restoring proper muscle balance may enhance rehabilitation outcomes, but effective strategies for modulating muscle activity remain unclear. High-velocity low-amplitude hip manipulation has shown potential to influence neuromuscular function, yet its impact on quadriceps activation during knee extension has not been well studied. Therefore, the main aim of this study is to examine the effects of a single session of high-velocity low-amplitude hip manipulation on quadriceps femoris muscle activation and maximum voluntary contraction during knee extension. Methods: This study utilizes a randomized controlled design. Thirty physically active men and women (mean age: 21.9 ± 1.7 years) were randomly assigned to either an experimental group (n = 15; receiving hip joint manipulation) or a control group (n = 15; undergoing a sham intervention). Participants in the intervention group received a treatment involving hip manipulation and short-duration traction. Muscle activity of the rectus femoris, vastus lateralis, and vastus medialis was assessed using surface electromyography before and after the intervention, while muscle performance was measured by evaluating isometric knee extension strength in the lower limb. The isometric strength test was conducted in a seated position with the knee flexed at 60 degrees in Biodex System 4. Results: This study finds that the experimental group had significantly higher vastus lateralis mean amplitude (p = 0.020; effect size = 0.186) and vastus medialis mean amplitude (p < 0.001; effect size = 0.577) of electromyography root mean square electromyography compared to the control group. The experimental group also showed greater vastus medialis max amplitude (p < 0.001; effect size = 0.435). No significant differences were noted for rectus femoris mean amplitude (p = 0.078; effect size = 0.110), vastus lateralis max amplitude (p = 0.363; effect size = 0.031), rectus femoris max amplitude (p = 0.069; effect size = 0.117), or median frequency of the raw electromyography signal across muscle groups. Conclusions: In conclusion, high-velocity low-amplitude hip manipulation significantly enhances vastus medialis activation, highlighting its potential to improve quadriceps balance. These findings support the incorporation of hip manipulation into rehabilitation protocols.
1. Introduction
Knee injuries, affecting both young individuals and adults, are a prevalent issue in musculoskeletal health [1,2]. For example, reduced quadriceps strength was associated with an increased risk of further cartilage deterioration in the lateral patellofemoral joint in women. This implies that improving quadriceps strength could potentially help prevent the progression of structural damage in the patellofemoral joint in women [3]. One of the key factors driving this degeneration is the weakening of the quadriceps femoris (QF) muscle, a critical muscle group that plays a central role in maintaining knee stability and proper biomechanics [4].
The quadriceps muscle is essential for stabilizing the knee joint during movement, and any deficit in its strength or neuromuscular control can significantly alter knee biomechanics, leading to instability [5,6]. Neuromuscular impairments, such as the inhibition or abnormal activation of the quadriceps, can result in clinical symptoms that disrupt normal joint function, increasing the risk of further injury [7]. Proper neuromuscular control, therefore, is essential for achieving active joint stability, which supports safe and efficient movement in daily activities or during training [8].
There is growing evidence suggesting that addressing hip joint biomechanics, particularly in relation to hip abduction, external rotation, and neuromuscular coordination, is crucial for mitigating knee dysfunction [9]. A specific focus on the role of the hip external rotators, such as the gluteus maximus, piriformis, and deep hip rotators, may help in improving lower limb alignment and function [10]. Dysfunction or weakness in these external rotators can contribute to poor control of the femur during dynamic movements, increasing the risk of knee valgus and altering lower limb kinematics [11]. For example, knee valgus has been linked to decreased strength in the hip extensor muscles [12]. Additionally, an increased Q-angle (the angle formed between the quadriceps muscle and the patellar tendon) has been implicated in altered patellar tracking, which may compound issues related to knee valgus [13]. This altered Q-angle, often resulting from dysfunctional hip mechanics, may place additional strain on the knee joint, contributing to conditions such as patellofemoral pain syndrome and anterior cruciate ligament injuries [14,15,16,17].
Physiotherapists have increasingly focused on strengthening the QF muscle to restore knee function [18]. Open and closed kinetic chain exercises have been widely used in rehabilitation programs to enhance quadriceps strength, while also targeting the hip adductors and external rotators to improve overall lower limb function and alignment [19,20,21]. Clinical research has shown that quadriceps training can significantly reduce knee pain, with a systematic review providing strong evidence for its efficacy [22]. Additionally, studies such as the one by Fukuda et al. [23] suggest that combining quadriceps strengthening with exercises targeting the hip abductors and external rotators yields better results in terms of pain relief and knee function compared to quadriceps training alone.
Recent research has highlighted the potential of manual therapy to enhance neuromuscular function [24]. Among various techniques, the high-velocity low-amplitude (HVLA) technique is one of the oldest and most commonly used in chiropractic care. It encompasses different forms, including the diversified technique, Gonstead adjustment, and Thompson Terminal Point technique [25]. Manipulation, typically characterized by high-velocity low-amplitude thrusts, is commonly used in cases of joint pain or restricted range of motion [26]. Its effects extend beyond biomechanical changes, also engaging neurophysiological and supraspinal mechanisms that impact muscle function and neuromuscular control [27]. For example, an HVLA hip distraction has been shown to enhance gluteus maximus strength in individuals with knee injuries and weakness in the lower extremities [28]. In another study [29], manipulations involving high-velocity low-amplitude in the ankle area may help boost hip abductor strength in people with a history of ankle sprains and unilateral weakness, as assessed during a tensor fascia latae muscle test.
Despite the growing body of research on manipulation [30,31], the immediate effect of lateral hip manipulation on quadriceps muscle function has not been thoroughly explored. Given the interconnectedness of hip and knee biomechanics [32], investigating this relationship could provide valuable insights into novel rehabilitation strategies for individuals with quadriceps weakness, patellofemoral pain, or knee degeneration. This study aimed to assess the immediate effects of a single lateral hip manipulation on quadriceps muscle activation, measured using electromyography (EMG), and neuromuscular function, measured using isometric knee extension strength.
2. Materials and Methods
This research adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for documenting randomized experimental studies [33].
2.1. Trial Design
To assess the impact of hip manipulation on quadriceps femoris neuromuscular performance, a prospective, randomized double-blind design was implemented. Participants were randomly assigned to two equal groups: one group underwent the intervention (manipulation), while the other group received a sham treatment. The allocation ratio for both groups was 1:1. A 1:1 allocation ratio means that each participant has an equal probability of being assigned to either the intervention or sham treatment group. This ensures that the groups are balanced in size, reducing selection bias and improving the validity of comparisons between them.
Approval for the study was granted by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk (Resolution No. NKBBN/866/2022-2023). Participants received a comprehensive explanation of the study protocol in clear, accessible language, and written informed consent was secured from each volunteer. This consent explicitly indicated that participants could withdraw from the study at any point without facing any penalties. The research adhered to the ethical principles for studies involving human subjects as outlined in the Declaration of Helsinki.
2.2. Participants
This research was carried out following the specific inclusion criteria established beforehand: (i) participants must be healthy individuals (ii) aged between 19 and 26 years and (iii) able to attend all intervention and assessment sessions. The exclusion criteria included (i) any history of surgery involving the lower limbs or lumbar spine, (ii) recent lower limb injuries occurring within the past 6 months, (iii) experiencing pain in the ankle, knee, or hip, (iv) ankle joint hypermobility, (v) presence of rheumatic diseases, (vi) neurological disorders, (vii) cancer-related conditions, (viii) connective tissue disorders, (ix) symptoms indicative of spinal root compression, sciatica, or spinal canal stenosis, and (x) prior treatment involving manipulation.
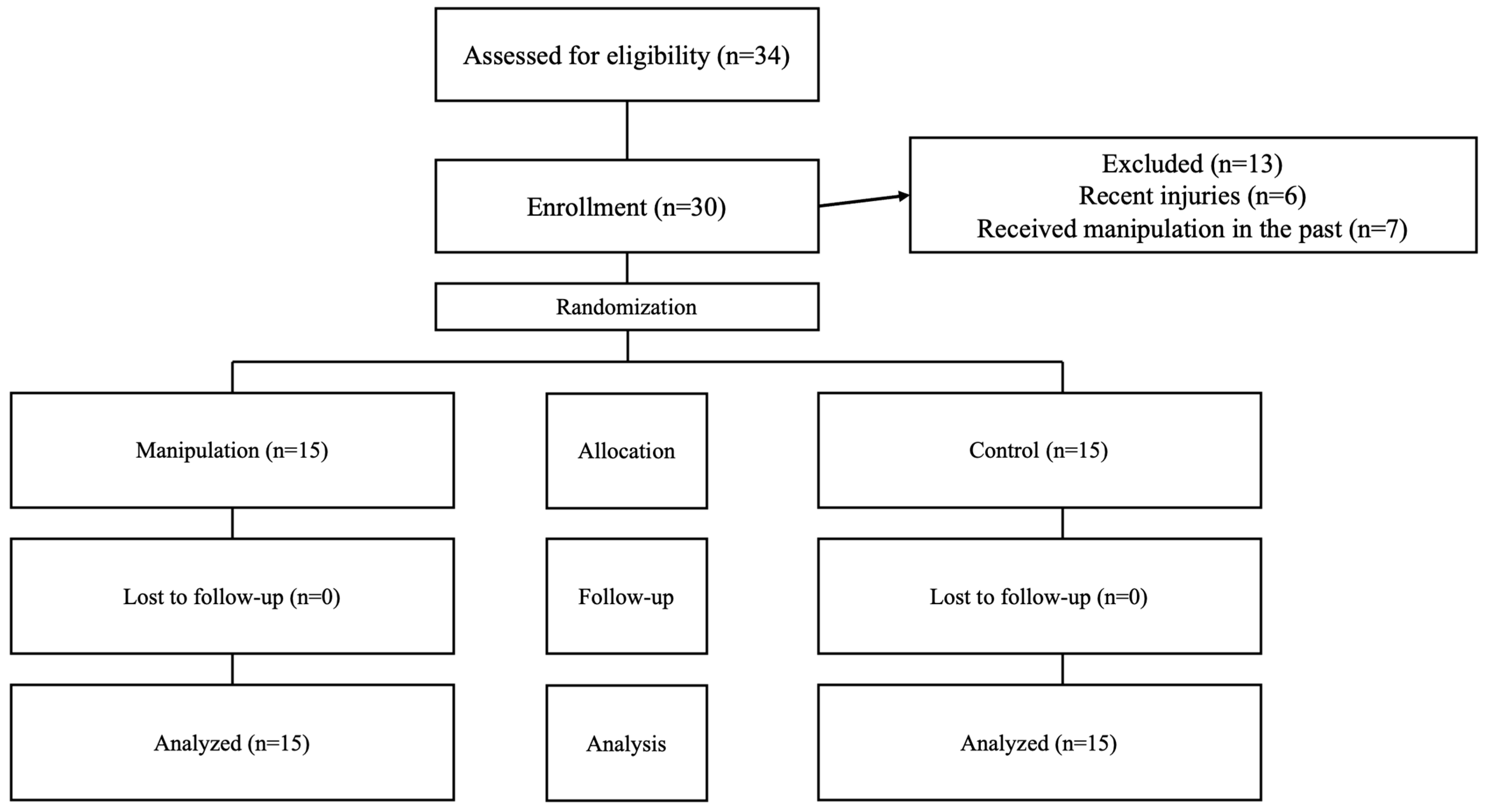
Participants were recruited through announcements in the primary intervention area, social media outreach, and direct contact. This straightforward sampling approach initially attracted 43 volunteers. After screening, 13 were excluded, resulting in a final group of 30 eligible individuals, both men and women, who expressed a desire to participate. Among these, 22 were men and 8 were women, all of whom met the established criteria for group assignment (see Figure 1).
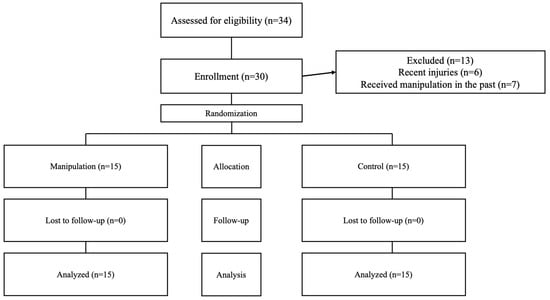
Figure 1.
Flowchart showing participant progression throughout the study steps.
2.3. Interventions
Participants in the intervention group received a treatment HVLA manipulation and short-duration traction [34]. The procedure was conducted as follows: Participants were first informed about the intervention’s nature. They were positioned supine with a 30° flexion at the hip joint and their upper limbs resting alongside their trunk. The therapist, an expert with over 25 years of experience, positioned themselves laterally to the participant, carefully wrapping both hands around the proximal thigh, as close to the pelvis as possible. Then, the therapist performed a thigh movement, moving it laterally, parallel to the ground, until the maximum felt resistance of the tissue tension in the hip joint was reached. From this point, a distance of 1 cm was measured between the outer side of the patient’s thigh and the therapist’s body to standardize the repeatability of subsequent interventions. At this point, the therapist made sure that the participant did not feel any discomfort and then performed a 1 s lateral manipulation. This technique was performed once for each participant.
Participants in the control group received a sham intervention. This procedure closely mirrored the HVLA manipulation, but during the lateral movement of the thigh, no actual tissue tension was applied. When the thigh reached the lateral position, the therapist used their torso to prevent any further lateral movement, thereby inhibiting any increase in tissue tension at the hip joint. The therapist confirmed that the participant did not experience discomfort, and then executed a movement that mimicked the manipulation without applying any additional tissue tension [16]. The temperature of the room was kept at 22 °C, with a relative humidity level of 50%.
2.4. Outcomes
Located at the Academy of Physical Education in Gdańsk, the Physical Exercise Laboratory of the Department of Biomechanics and Sports Engineering served as the study venue. To enhance the reliability of the therapeutic assessment, each volunteer was tested twice by the same physiotherapists. The measurement team consisted of three physiotherapists, each holding a PhD degree and with a minimum of 5 years of experience in assessing muscular activity using EMG and in measuring muscular strength with the Biodex system. Measurements were taken both before and immediately after the manipulation or placebo of the hip joint.
To ensure repeatability and objectivity, the Biodex System 4 dynamometer (Biodex Medical Systems, Inc., Shirley, NY, USA) chair settings were utilized for better patient stabilization, following the manufacturer’s guidelines. During the initial assessment, the settings for the movable components (Positioning Chair) were recorded and replicated for the second assessment. Participants were secured using leather straps. In this position, each participant performed three maximum voluntary isometric contractions (MVIC) lasting 5 s each. A 30 s rest was provided between each repetition, and participants received verbal encouragement to maximize their effort during the measurements.
2.4.1. Surface Electromyography
Surface electromyography (SEMG) data were recorded from the quadriceps femoris (QF) during the MVIC of the knee extension. Specifically, the signals from the rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM) muscles were collected. Electrode placement and skin preparation—including shaving, abrading, and cleansing with alcohol—were conducted in accordance with the SENIAM guidelines.
During each 5 s MVIC, the middle 3 s of data were analyzed. The SEMG signals were differentially amplified with a gain of 500 using the TeleMyo DTS system (Noraxon, Scottsdale, AZ, USA) and Ag/AgCl 1-cm2 surface electrodes (Sorimex, Toruń, Poland). The signals underwent band-pass filtering between 15 and 500 Hz and were sampled at 1500 Hz with 16-bit resolution via an analog-to-digital converter. Subsequently, the SEMG data were archived and processed using MyoResearch 2.8 software (Noraxon, Scottsdale, AZ, USA) [30].
Signal processing included full rectification and smoothing via the root mean square (RMS) method, utilizing a 100 ms moving time window. Average values from all three MVIC trials were calculated, including the mean and maximum amplitude of electromyography root mean square (EMGRMS, measured in microvolts) and the median frequency of the raw SEMG signal power spectrum (EMGMED, in Hz). Additionally, percentage changes from pre-manipulation to post-manipulation measurements were computed for analysis. Finally, the VL-VM ratio, calculated by dividing the EMG values of VL by those of VM, was also determined pre- and post-intervention.
2.4.2. Isometric Knee Extension Strength
Using the Biodex System 4 (Biodex Medical Systems Inc., Shirley, NY, USA), the muscle strength of the knee extensors was evaluated. According to the manufacturer’s guidelines, participants were seated in the device with their back supported, their tested lower limb fully extended at the knee joint (neutral in both sagittal and frontal planes), and the hip positioned at 90° flexion. The lower limb was secured to the device’s arm with leather straps, ensuring that the device’s rotation shaft aligned with the knee joint’s anatomical axis for movement in the sagittal plane. Each participant then performed three MVIC, each lasting 5 s, with a 30 s rest between repetitions [35]. Participants received verbal encouragement to maximize their effort during each contraction. For further analysis, the highest peak torque (Nm) from the three attempts was chosen, and peak torque was normalized to each participant’s body mass (Nm × kg−1).
2.5. Sample Size
Using G*Power software (version 3.1.9.6, Universität Düsseldorf, Düsseldorf, Germany), the sample size for the study was calculated. The analysis was based on an ANCOVA model, which assumed a moderate effect size of 0.6 and a power of 0.85 for two groups [34]. This approach resulted in a suggested sample size of 28 participants.
2.6. Randomization
Participants were assigned identification numbers in a 1:1 ratio through a simple randomization process using Research Randomizer software (Social Psychology Network, Wesleyan University, Middletown, CT, USA). To maintain allocation concealment, participants were randomized and assigned prior to the initial evaluation. There were no alterations to group assignments for any of the participants.
2.7. Blinding
Participants were kept unaware of the intervention, preventing them from observing others, while a control therapy was administered to the control group to ensure uniform conditions across all groups. Additionally, the evaluators conducting the assessments were blinded to the group assignments of the participants.
2.8. Statistical Methods
Prior to conducting any inferential analyses, the normality of the sample was assessed using the Shapiro–Wilk test. Although some variables did not show normality (p < 0.05), the sample size of 30 is sufficient for the Central Limit Theorem to apply [36], which allows us to assume that the sampling distribution of the mean is approximately normal. As such, these variables are considered normal for the purposes of parametric analysis. Additionally, the homogeneity of variance assumption was examined using Levene’s test, with results indicating no significant violation (p > 0.05). Based on these findings, no violations of the assumptions for parametric tests were observed, supporting the use of parametric inferential methods for the analysis.
An ANCOVA was conducted to compare the intervention and control groups, using baseline levels as a covariate. This analysis aimed to evaluate the differences in post-intervention outcomes between the two groups while controlling for initial variations. The effect size was calculated using the partial eta squared (), and the magnitude of the differences was interpreted as follows [37]: 0.00–0.01, trivial; 0.01–0.06, small; 0.06–0.14, medium; >0.14, large.
The within-group differences were assessed using Cohen’s d, with the following thresholds for effect sizes [38]: 0.0–0.2 indicating a trivial effect, 0.2–0.6 representing a small effect, 0.6–1.2 denoting a moderate effect, and values greater than 1.2 signifying a large effect. Additionally, a repeated measures ANOVA was conducted to compare the percentage difference between post- and pre-intervention scores, calculated using the formula (post − pre)/pre × 100. Using IBM SPSS Statistics software (Version 29.0.2.0, Armonk, NY, USA: IBM Corp), statistical analyses were performed with a significance level set at p < 0.05.
3. Results
Of the 43 volunteers initially recruited, 6 were excluded due to lower limb injuries sustained in the previous six months, and 7 were excluded due to having received prior manipulation. After these exclusions, the remaining 30 participants were assigned to both groups and completed the intervention (Figure 1). All 30 participants were analyzed and included in the statistical evaluation.
Among the participants, 22 were men and 8 were women. The overall mean age was 22.0 ± 1.7 years, with an average height of 179.9 ± 9.5 cm and body mass of 75.5 ± 13.9 kg. A detailed breakdown of the main anthropometric and demographic data for each group is provided in Table 1.

Table 1.
Demographic and anthropometric information.
Table 2 presents the descriptive statistics and inferential statistics comparing within-group differences (post–pre) for the main outcomes. The ANCOVA, using the baseline as covariable, revealed that experimental group presented significantly greater VL mean amplitude of EMGRMS (F = 6.155; p = 0.020; = 0.186) and VM mean amplitude of EMGRMS (F = 36.784; p < 0.001; = 0.577) than control, while no significant differences between groups were found on the RF mean amplitude of EMGRMS (F = 3.349; p = 0.078; = 0.110).

Table 2.
Descriptive statistics (mean ± standard deviation) and inferential statistics comparing within-group differences (post–pre) for the main outcomes.
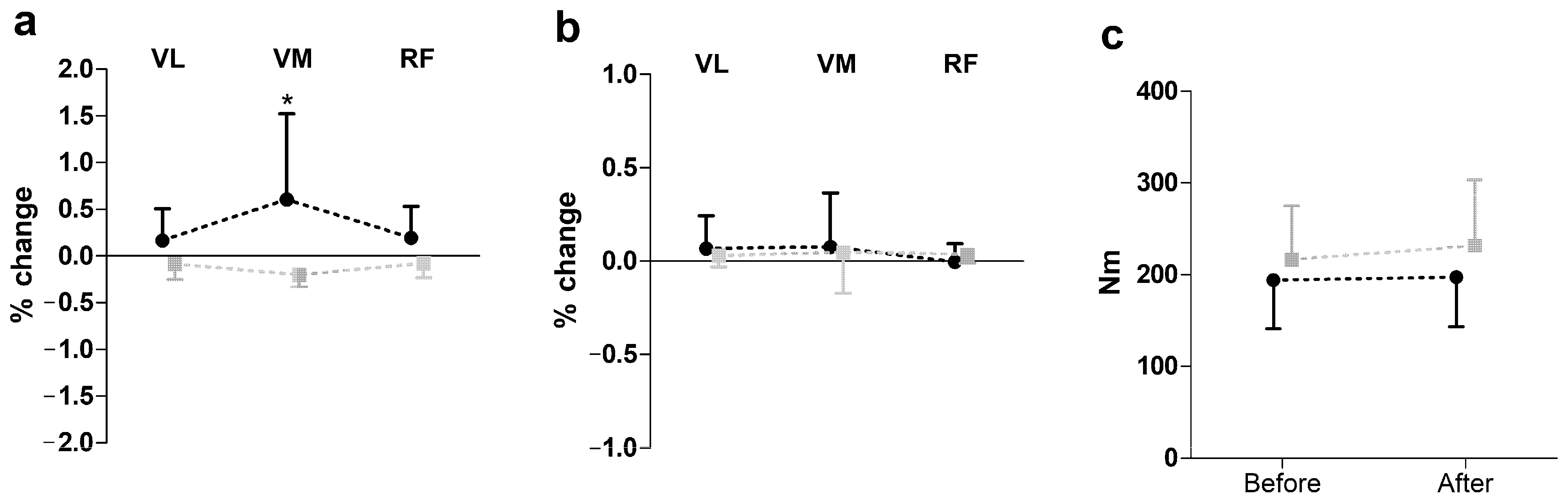
Figure 2 presents the results of SEMG and peak torque changes (post-pre) following hip manipulation during knee extension. A two-way repeated measures ANOVA revealed a significant group effect on SEMG amplitude (F = 16.68, p ≤ 0.01, η2 = 0.37), with the hip manipulation group showing an increase in amplitude, while the control group showed a decrease. However, no significant effects were observed for peak torque during knee extension.
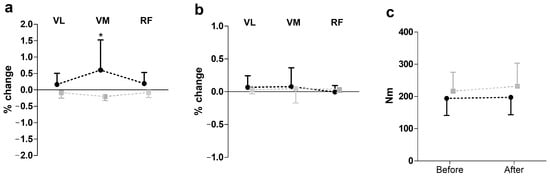
Figure 2.
Impact of hip manipulation on surface electromyography (SEMG) and performance during knee extension. Black circles: hip manipulated group; gray squares: sham controlled group; VL: vastus lateralis; VM: vastus medialis; RF: rectus femoris. Percentage changes in surface electromyography amplitude (a), median frequency (b), and peak torque (c) * Significant difference with all other muscle in both groups except RF in hip-manipulated group at p ≤ 0.05.
Table 3 presents the VL-VM ratio data. A significantly greater VL-VM ratio for the mean amplitude of EMGRMS (F = 4.537; p = 0.042; = 0.144) was observed in the control group after the intervention. However, no significant differences between groups were found for the VL-VM ratio in the median frequency of the raw SEMG signal power spectrum (F = 0.023; p = 0.882; = 0.001) or for the maximum amplitude of EMGRMS (F = 0.797; p = 0.380; = 0.029).

Table 3.
Descriptive statistics (mean ± standard deviation) and inferential statistics comparing within-group differences (post–pre) and between-group (experimental vs. control) for the vastus lateralis (VL)–vastus medialis (VM) ratio.
4. Discussion
Our study found that high-velocity low-amplitude hip manipulation resulted in significantly greater muscle activation in the VL mean amplitude of EMGRMS, as well as in the VM mean amplitude and maximum amplitude of EMGRMS, compared to the control group that only received a sham intervention. However, the intervention did not have a significant effect on the RF when compared to the sham intervention.
The VM benefited the most from the high-velocity low-amplitude hip intervention, showing significantly greater mean and maximum amplitudes of EMGRMS. Additionally, there were significant within-group enhancements in both variables, in addition to the previously mentioned between-group differences. Previous research [39] has indicated that similar manipulative techniques can effectively alter motor unit recruitment patterns in other muscle groups. For instance, studies on spinal manipulation have shown an increase in the recruitment of lower-threshold lower-twitch torque motor units, highlighting the broader implications of these interventions on muscular function. Additionally, a previous study [30] utilizing manipulation found it to be significantly effective in enhancing muscle activation in the infraspinatus compared to the control group. Finally, a study on hip manipulation [34] found a significant increase (p > 0.05) in EMGRMS amplitude in the gluteus medius muscle.
High-velocity manipulations may stimulate the muscle’s neuromuscular pathways effectively, enhancing the recruitment of motor units and improving the firing rate [40]. The low-amplitude component could promote greater muscle activation while minimizing fatigue, allowing for sustained engagement of the VM. Moreover, such manipulations might facilitate increased blood flow and metabolic processes in the muscle, further contributing to enhanced muscle function [41]. The significant within-group enhancements suggest that the intervention not only produced immediate benefits, but also fostered ongoing improvements in muscle coordination and activation over time.
The observed enhancements in mean and maximum EMGRMS in the VL and VM following hip manipulation may be explained by the activation and recruitment of motor units specific to these muscles. Both the VL and VM play essential roles in knee extension and stabilization, and the hip manipulation likely created an optimal biomechanical environment that facilitated improved neuromuscular communication and muscle engagement during the intervention [41]. Increased EMGRMS values suggest that the manipulation enhanced the muscle’s ability to generate force effectively, possibly through increased motor unit synchronization and recruitment, which are known to influence muscle performance. Conversely, the lack of significant changes in the median frequency of the raw SEMG signal power spectrum may indicate that, while the overall muscle activation intensity increased, there were no substantial shifts in the muscle fiber recruitment pattern or the spectral properties of the electrical activity. This could be attributed to the fact that the manipulation primarily affected the magnitude of activation without altering the frequency, which may require more intensive or prolonged interventions to influence [42].
The pronounced effect of manipulation on the VM and VL, as opposed to the RF, can be attributed to the critical role within the quadriceps muscle group. For instance, VM is essential for stabilizing the patella and facilitating knee extension, particularly during activities that require dynamic knee stabilization [43]. By manipulating the hip, a more favorable mechanical environment may be established, enhancing the VM’s activation and function. In contrast, the RF did not exhibit significant differences between the intervention and control groups. This disparity could be due to the RF’s more complex role, as it also acts as a hip flexor in addition to its knee extension function [44]. The manipulation may not have sufficiently targeted the RF’s activation patterns, limiting its responsiveness to the intervention. This highlights the importance of understanding the specific biomechanical roles of each muscle in the quadriceps group when evaluating the effects of physiotherapeutic interventions.
Despite the findings of our study, there are several limitations that should be acknowledged. The sample size did not include sports athletes, which may limit the generalizability of the results to athletic populations. Additionally, the age group targeted in this study restricts the applicability of the findings to older populations, suggesting that future research should aim for a more diverse participant pool. This study’s focus on acute outcomes also leaves the long-term effects of HVLA hip manipulation on muscle activation uncertain; therefore, future studies should explore the chronic effects of such interventions over extended periods. Moreover, investigating variations in manipulation techniques and their specific impacts on different muscle groups—especially those, like the RF, that did not show significant changes—could offer deeper insights into neuromuscular dynamics. The use of advanced imaging or biomechanical assessments could also help elucidate the underlying mechanisms of action, particularly regarding motor unit recruitment patterns and the influence of muscle architecture on activation responses. A further limitation is the possibility that participants were able to distinguish between the real and sham interventions, despite efforts to carefully design the sham procedure to mimic the HVLA manipulation without applying tissue tension. The therapist’s skill and adherence to the protocol aimed to minimize biases; however, future studies could incorporate additional measures, such as blinding participants to the type of treatment, to reduce this risk further.
Despite the limitations, the findings of our study have implications for practitioners in the fields of physical therapy. The effectiveness of high-velocity low-amplitude hip manipulation in enhancing muscle activation, particularly in the VM and VL, suggests that incorporating this technique into rehabilitation protocols or warm-up could improve outcomes for participants recovering from knee injuries or those seeking to enhance athletic performance. Practitioners should consider the specific roles of these muscles in knee stabilization and extension when designing individualized intervention plans.
5. Conclusions
In conclusion, our study demonstrates that a single session of high-velocity low-amplitude hip manipulation enhances the activation of both the vastus lateralis (VL) and vastus medialis (VM) during knee extension, while having no significant effect on the rectus femoris (RF) when compared to a sham intervention. These findings suggest that high-velocity low-amplitude hip manipulation can effectively modulate quadriceps muscle activation, potentially optimizing neuromuscular function and offering a beneficial approach in the rehabilitation of knee injuries such as ACL ruptures and patellofemoral pain syndrome. Future research should focus on investigating the long-term effects and explore various manipulation techniques to further clarify their impacts on muscle activation and rehabilitation outcomes.
Author Contributions
R.S.: Conceptualization, data curation, investigation, methodology, project administration, writing—original draft, and writing—review and editing; K.S.: data curation, investigation, and methodology; M.S.: data curation, investigation, and methodology; B.N.; project administration, writing—original draft, and writing—review and editing; P.A.: methodology, data curation, writing—review and editing, R.L.: formal analysis, writing—original draft, and writing—review and editing, P.Ł.: formal analysis, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk (Resolution No. NKBBN/866/2022-2023), accessed date 17 December 2022. Participants received a detailed explanation of this study, including a simplified description of the protocol. Before participating, they provided written informed consent, confirming their voluntary participation and understanding that they could withdraw from this study at any time without penalty. This study adhered to the ethical principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data can be provided upon a reasonable request to the corresponding author.
Acknowledgments
The materials obtained during the study are secured and are in the possession of the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Snoeker, B.; Turkiewicz, A.; Magnusson, K.; Frobell, R.; Yu, D.; Peat, G.; Englund, M. Risk of Knee Osteoarthritis after Different Types of Knee Injuries in Young Adults: A Population-Based Cohort Study. Br. J. Sports Med. 2020, 54, 725–730. [Google Scholar] [CrossRef]
- Gage, B.E.; McIlvain, N.M.; Collins, C.L.; Fields, S.K.; Dawn Comstock, R. Epidemiology of 6.6 Million Knee Injuries Presenting to United States Emergency Departments From 1999 Through 2008. Acad. Emerg. Med. 2012, 19, 378–385. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Segal, N.A.; Guermazi, A.; Roemer, F.; Felson, D.T.; Nevitt, M.C.; Lewis, C.E.; Stefanik, J.J. Sex-Specific Influence of Quadriceps Weakness on Worsening Patellofemoral and Tibiofemoral Cartilage Damage: A Prospective Cohort Study. Arthritis Care Res. 2019, 71, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Øiestad, B.E.; Juhl, C.B.; Eitzen, I.; Thorlund, J.B. Knee Extensor Muscle Weakness Is a Risk Factor for Development of Knee Osteoarthritis. A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2015, 23, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lepley, A.S.; Gribble, P.A.; Thomas, A.C.; Tevald, M.A.; Sohn, D.H.; Pietrosimone, B.G. Quadriceps Neural Alterations in Anterior Cruciate Ligament Reconstructed Patients: A 6-month Longitudinal Investigation. Scand. J. Med. Sci. Sports 2015, 25, 828–839. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Roos, E.M. A Pragmatic Approach to Prevent Post-Traumatic Osteoarthritis after Sport or Exercise-Related Joint Injury. Best Pract. Res. Clin. Rheumatol. 2019, 33, 158–171. [Google Scholar] [CrossRef]
- Pietrosimone, B.G.; McLeod, M.M.; Lepley, A.S. A Theoretical Framework for Understanding Neuromuscular Response to Lower Extremity Joint Injury. Sports Health A Multidiscip. Approach 2012, 4, 31–35. [Google Scholar] [CrossRef]
- Blasimann, A.; Koenig, I.; Baert, I.; Baur, H.; Vissers, D. Which Assessments Are Used to Analyze Neuromuscular Control by Electromyography after an Anterior Cruciate Ligament Injury to Determine Readiness to Return to Sports? A Systematic Review. BMC Sports Sci. Med. Rehabil. 2021, 13, 142. [Google Scholar] [CrossRef]
- Nagelli, C.; Di Stasi, S.; Tatarski, R.; Chen, A.; Wordeman, S.; Hoffman, J.; Hewett, T.E. Neuromuscular Training Improves Self-Reported Function and Single-Leg Landing Hip Biomechanics in Athletes After Anterior Cruciate Ligament Reconstruction. Orthop. J. Sports Med. 2020, 8, 2325967120959347. [Google Scholar] [CrossRef]
- Malloy, P.; Wichman, D.M.; Garcia, F.; Espinoza-Orías, A.; Chahla, J.; Nho, S.J. Impaired Lower Extremity Biomechanics, Hip External Rotation Muscle Weakness, and Proximal Femoral Morphology Predict Impaired Single-Leg Squat Performance in People With FAI Syndrome. Am. J. Sports Med. 2021, 49, 2984–2993. [Google Scholar] [CrossRef]
- Cashman, G.E. The Effect of Weak Hip Abductors or External Rotators on Knee Valgus Kinematics in Healthy Subjects: A Systematic Review. J. Sport Rehabil. 2012, 21, 273–284. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Alzhrani, M.; Alshahrani, S.N.; Alghamdi, W.; Alqahtani, M.; Alzahrani, H. Is Hip Muscle Strength Associated with Dynamic Knee Valgus in a Healthy Adult Population? A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7669. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.-D.; Boling, M.C.; Levine, B.; Shultz, S.J. Relationships Between Lower Extremity Alignment and the Quadriceps Angle. Clin. J. Sport Med. 2009, 19, 201–206. [Google Scholar] [CrossRef]
- Ford, K.; Nguyen, A.-D.; Dischiavi, S.; Hegedus, E.; Zuk, E.; Taylor, J. An Evidence-Based Review of Hip-Focused Neuromuscular Exercise Interventions to Address Dynamic Lower Extremity Valgus. Open Access J. Sports Med. 2015, 6, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-D.; Huang, W.-S.; Lai, P.-T. Muscle Activation of Vastus Medialis Oblique and Vastus Lateralis in Sling-Based Exercises in Patients with Patellofemoral Pain Syndrome: A Cross-Over Study. Evid.-Based Complement. Altern. Med. 2015, 2015, 740315. [Google Scholar] [CrossRef]
- Reiman, M.P.; Bolgla, L.A.; Loudon, J.K. A Literature Review of Studies Evaluating Gluteus Maximus and Gluteus Medius Activation during Rehabilitation Exercises. Physiother. Theory Pract. 2012, 28, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, A.M.; Pasanen, K.; Krosshaug, T.; Vasankari, T.; Kannus, P.; Heinonen, A.; Kujala, U.M.; Avela, J.; Perttunen, J.; Parkkari, J. Association between Frontal Plane Knee Control and Lower Extremity Injuries: A Prospective Study on Young Team Sport Athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000311. [Google Scholar] [CrossRef]
- Vahtrik, D.; Gapeyeva, H.; Aibast, H.; Ereline, J.; Kums, T.; Haviko, T.; Märtson, A.; Schneider, G.; Pääsuke, M. Quadriceps Femoris Muscle Function Prior and after Total Knee Arthroplasty in Women with Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2017–2025. [Google Scholar] [CrossRef]
- Khayambashi, K.; Mohammadkhani, Z.; Ghaznavi, K.; Lyle, M.A.; Powers, C.M. The Effects of Isolated Hip Abductor and External Rotator Muscle Strengthening on Pain, Health Status, and Hip Strength in Females With Patellofemoral Pain: A Randomized Controlled Trial. J. Orthop. Sports Phys. Ther. 2012, 42, 22–29. [Google Scholar] [CrossRef]
- Chang, W.-D.; Huang, W.-S.; Lee, C.-L.; Lin, H.-Y.; Lai, P.-T. Effects of Open and Closed Kinetic Chains of Sling Exercise Therapy on the Muscle Activity of the Vastus Medialis Oblique and Vastus Lateralis. J. Phys. Ther. Sci. 2014, 26, 1363–1366. [Google Scholar] [CrossRef]
- Chen, S.; Chang, W.-D.; Wu, J.-Y.; Fong, Y.-C. Electromyographic Analysis of Hip and Knee Muscles during Specific Exercise Movements in Females with Patellofemoral Pain Syndrome. Medicine 2018, 97, e11424. [Google Scholar] [CrossRef] [PubMed]
- Bolgla, L.A.; Boling, M.C. An Update for the Conservative Management of Patellofemoral Pain Syndrome: A Systematic Review of the Literature from 2000 to 2010. Int. J. Sports Phys. Ther. 2011, 6, 112–125. [Google Scholar] [PubMed]
- Fukuda, T.Y.; Rossetto, F.M.; Magalhães, E.; Bryk, F.F.; Garcia Lucareli, P.R.; De Almeida Carvalho, N.A. Short-Term Effects of Hip Abductors and Lateral Rotators Strengthening in Females With Patellofemoral Pain Syndrome: A Randomized Controlled Clinical Trial. J. Orthop. Sports Phys. Ther. 2010, 40, 736–742. [Google Scholar] [CrossRef]
- Studnicki, R.; Tomaszewsk, U.; Hansdorfer-Korzon, R.; Kawczyński, A. Comparing the Acute Effects of Diagonal Mobilization and Nordic Hamstring Curls on Knee Flexion and Extension Strength: A Randomized, Double-Blinded Parallel Study in Young Soccer Players. Appl. Sci. 2024, 14, 8610. [Google Scholar] [CrossRef]
- LaPelusa, A.; Bordoni, B. High-Velocity Low-Amplitude Manipulation Techniques; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Evans, D.W. Mechanisms and Effects of Spinal High-Velocity, Low-Amplitude Thrust Manipulation: Previous Theories. J. Manip. Physiol. Ther. 2002, 25, 251–262. [Google Scholar] [CrossRef]
- Niazi, I.K.; Navid, M.S.; Merkle, C.; Amjad, I.; Kumari, N.; Trager, R.J.; Holt, K.; Haavik, H. A Randomized Controlled Trial Comparing Different Sites of High-Velocity Low Amplitude Thrust on Sensorimotor Integration Parameters. Sci. Rep. 2024, 14, 1159. [Google Scholar] [CrossRef]
- Silva Neto, J.B.; Ismania, C.; de Freitas, D.G.; Cazarini Jr, C.; Martin, R.L.; Fukuda, T.Y. The Effect of a Single High Velocity Low Amplitude Hip Mobilization on Strength in Subjects with Knee Injuries. Musculoskelet. Sci. Pract. 2019, 44, 102051. [Google Scholar] [CrossRef]
- Lawrence, M.A.; Raymond, J.T.; Look, A.E.; Woodard, N.M.; Schicker, C.M.; Swanson, B.T. Effects of Tibiofibular and Ankle Joint Manipulation on Hip Strength and Muscle Activation. J. Manip. Physiol. Ther. 2020, 43, 406–417. [Google Scholar] [CrossRef]
- Studnicki, R.; Naderza, W.; Niespodziński, B.; Hansdorfer-Korzon, R.; Kawczyński, A. Effects of Shoulder Manipulation on Electromyography Measures in Archery Athletes during Recovery from Fatigue: A Comparison between Sexes. Retos 2024, 60, 720–729. [Google Scholar] [CrossRef]
- Studnicki, R.; Szymczyk, P.; Adamczewski, T.; Studzińska, K.; Hansdorfer-Korzon, R.; Silva, A.F.; Kawczyński, A. Manual Traction Is Effective in Alleviating Lumbosacral Spine Pain: Evidence from a Randomized Controlled Trial. Heliyon 2024, 10, e31013. [Google Scholar] [CrossRef]
- Stensgaard Stoltze, J.; Rasmussen, J.; Skipper Andersen, M. On the Biomechanical Relationship between Applied Hip, Knee and Ankle Joint Moments and the Internal Knee Compressive Forces. Int. Biomech. 2018, 5, 63–74. [Google Scholar] [CrossRef]
- Merkow, R.P.; Kaji, A.H.; Itani, K.M.F. The CONSORT Framework. JAMA Surg. 2021, 156, 877–878. [Google Scholar] [CrossRef]
- Studnicki, R.; Skup, K.; Sochaj, M.; Niespodziński, B.; Aschenbrenner, P.; Laskowski, R.; Łuczkiewicz, P. Hip Manipulation Increases Electromyography Amplitude and Hip Joint Performance: A Double-Blind Randomized Controlled Study. Life 2024, 14, 1353. [Google Scholar] [CrossRef]
- Sugiura, Y.; Hatanaka, Y.; Arai, T.; Sakurai, H.; Kanada, Y. Estimations of One Repetition Maximum and Isometric Peak Torque in Knee Extension Based on the Relationship Between Force and Velocity. J. Strength Cond. Res. 2016, 30, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Maroco, J. Análise Estatística Com Utilização Do SPSS [Statistical Analysis with SPSS]; Edições Silabo: Lisbon, Portugal, 2012. [Google Scholar]
- Richardson, J.T.E. Eta Squared and Partial Eta Squared as Measures of Effect Size in Educational Research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Robinault, L.; Holobar, A.; Crémoux, S.; Rashid, U.; Niazi, I.K.; Holt, K.; Lauber, J.; Haavik, H. The Effects of Spinal Manipulation on Motor Unit Behavior. Brain Sci. 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Gyer, G.; Michael, J.; Inklebarger, J.; Tedla, J.S. Spinal Manipulation Therapy: Is It All about the Brain? A Current Review of the Neurophysiological Effects of Manipulation. J. Integr. Med. 2019, 17, 328–337. [Google Scholar] [CrossRef]
- Roberts, A.; Harris, K.; Outen, B.; Bukvic, A.; Smith, B.; Schultz, A.; Bergman, S.; Mondal, D. Osteopathic Manipulative Medicine: A Brief Review of the Hands-On Treatment Approaches and Their Therapeutic Uses. Medicines 2022, 9, 33. [Google Scholar] [CrossRef]
- Pickar, J.G.; Bolton, P.S. Spinal Manipulative Therapy and Somatosensory Activation. J. Electromyogr. Kinesiol. 2012, 22, 785–794. [Google Scholar] [CrossRef]
- Bevilaqua Grossi, D.; Pedro, V.M.; Bérzin, F. Análise Funcional Dos Estabilizadores Patelares. Acta Ortop. Bras. 2004, 12, 99–104. [Google Scholar] [CrossRef]
- Carr, J.C.; Stock, M.S.; Hernandez, J.M.; Ortegon, J.R.; Mota, J.A. Additional Insight into Biarticular Muscle Function: The Influence of Hip Flexor Fatigue on Rectus Femoris Activity at the Knee. J. Electromyogr. Kinesiol. 2018, 42, 36–43. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).