Identification of E3 Ubiquitin Ligase Substrates Using Biotin Ligase-Based Proximity Labeling Approaches
Abstract
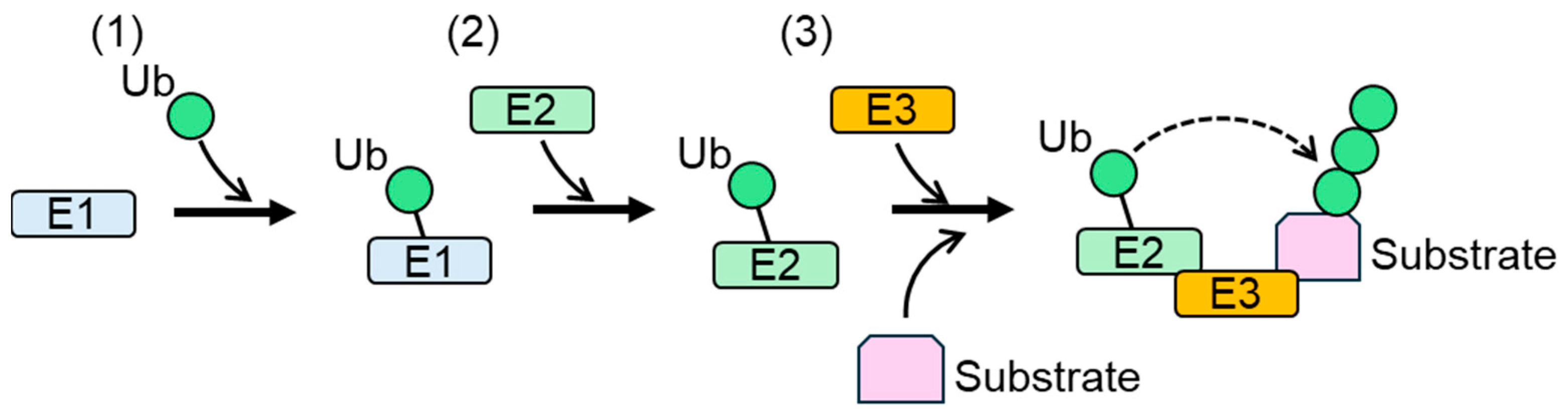
1. Background of Protein Ubiquitylation
2. Pathological Roles of Protein Ubiquitylation
2.1. Ubiquitylation and Neurodegenerative Diseases
2.2. Ubiquitylation and Neurodevelopmental Disorders
2.3. Ubiquitylation and Cancer
3. Ubiquitylation as a Therapeutic Target
4. Biotin-Ligase-Based Protein–Protein Interactor Screening System
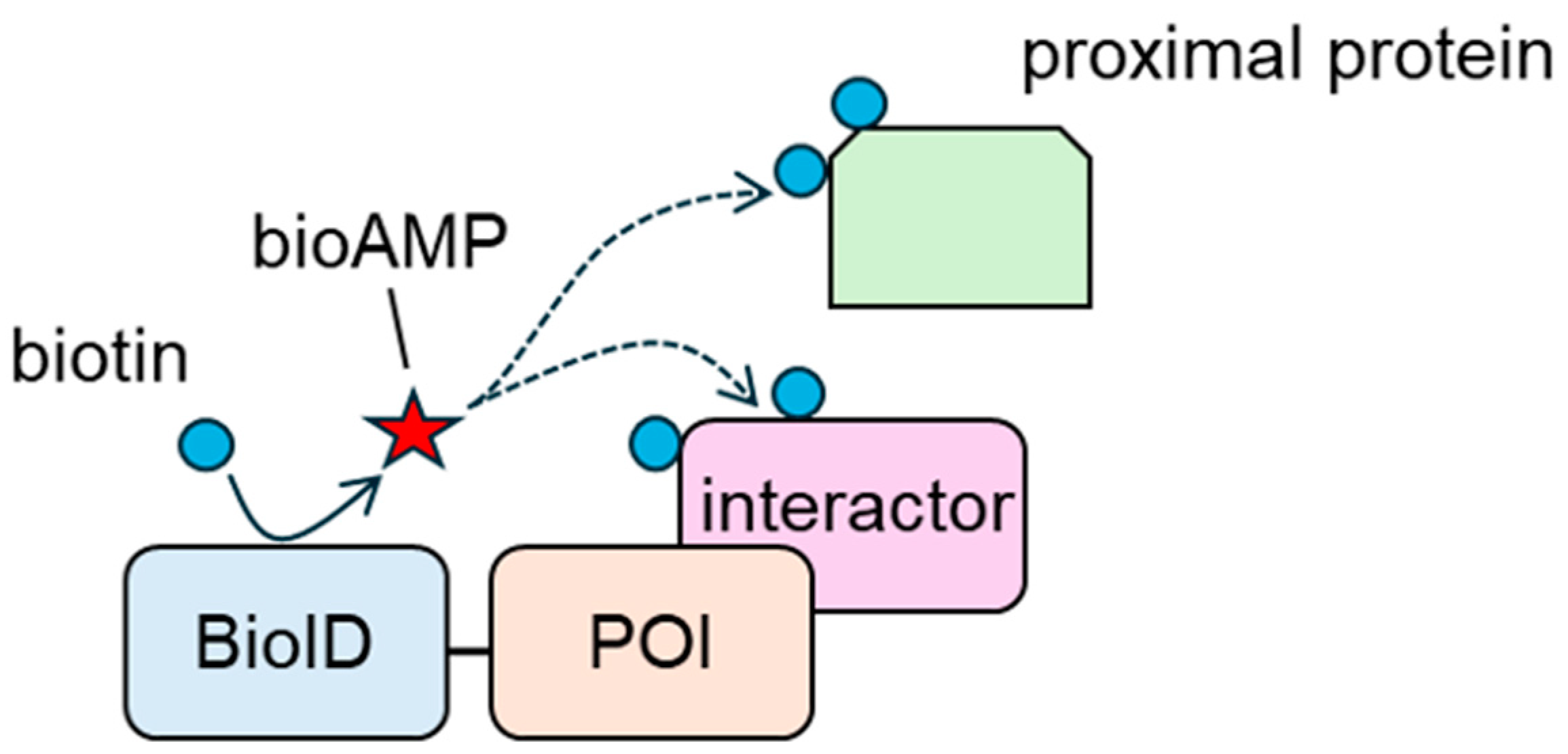
4.1. Basic Information About BioID and BioID-Based Interactor Screening Systems
4.2. Improved BioID and Other Biotin Ligases for Proximity Ligation
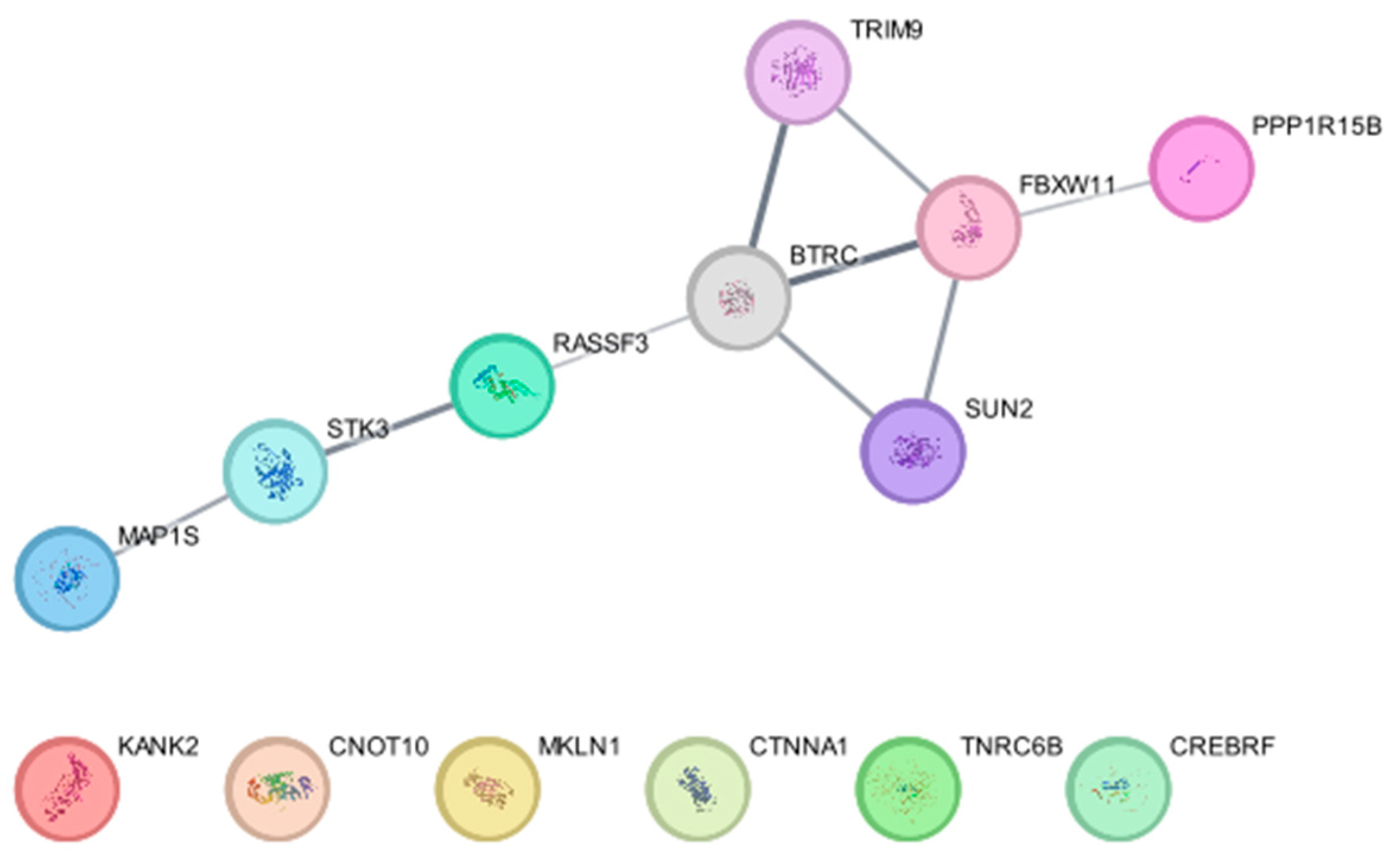
5. Identification of Ub E3 Ligase Substrates Using Biotin-Ligase-Based Proximity Labeling Approaches
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PTM | post-translational modification |
| Ub | ubiquitin |
| ERAD | endoplasmic reticulum-associated degradation |
| MVB | multivesicular body |
| EGFR | epidermal growth factor receptor |
| MHC-I | class I Major Histocompatibility complex |
| ESCRT | endosomal sorting complex required for transport |
| PINK1 | phosphatase and tensin homolog-induced kinase 1 |
| DDR | DNA damage response |
| 53BP1 | TP53-binding protein 1 |
| BRCA | breast cancer type 1 susceptibility protein |
| PLK1 | polo-like kinase 1 |
| CHIP | carboxyl terminus of Hsp70-interacting protein |
| APP | amyloid-β precursor protein |
| BACE1 | β-site app-cleaving enzyme 1 |
| DSM-5 | the New Diagnostic and Statistical Manual of Mental Disorders, 5th Edition |
| TRAF4 | tumor necrosis factor receptor-associated factor 4 |
| RB | the retinoblastoma protein |
| MDM2 | mouse double minute 2 homolog |
| TRIM | Tripartite motif-containing |
| FBXW | F-Box and WD repeat domain containing |
| SKP | S-phase kinase associated protein |
| β-TrCP | β-transducin repeat-containing protein |
| bioAMP | biotinoyl-5′-AMP |
| NE | nuclear envelope |
| LAP | lamina-associated polypeptide |
| SUN | SAD1/UNC84 domain protein |
| eIF | eukaryotic initiation factor |
| RNF | ring finger protein |
| IMiD | immunomodulatory drug |
| CRBN | cereblon |
| IKZF | Ikaros family zinc finger protein |
| ZMYM | zinc finger MYM-type containing |
| STAM | signal transducing adapter molecule |
| HGS | hepatocyte growth factor-regulated tyrosine kinase substrate |
| PROTAC | proteolysis-targeting chimera |
| POI | protein of interest |
| ER | endoplasmic reticulum |
| PPI | protein–protein interaction |
| BASU | modified biotin ligase derived from Bacillus subtilis |
| VCL | vinculin |
| ETF | eukaryotic translation termination factor |
| HDAC | histone deacetylase |
| KI | knock-in |
References
- Samarasinghe, K.T.G.; Crews, C.M. Targeted protein degradation: A promise for undruggable proteins. Cell Chem. Biol. 2021, 28, 934–951. [Google Scholar] [CrossRef]
- Scheffner, M.; Nuber, U.; Huibregtse, J.M. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature 1995, 373, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Sumara, I.; Pangou, E. Non-proteolytic ubiquitylation in cellular signaling and human disease. Commun. Biol. 2022, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Coscoy, L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 2005, 309, 127–130. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.J.; Laugesen, S.H.; Ellgaard, L. Cellular functions and molecular mechanisms of non-lysine ubiquitination. Open Biol. 2019, 9, 190147. [Google Scholar] [CrossRef]
- Mulder, M.P.C.; Witting, K.F.; Ovaa, H. Cracking the Ubiquitin Code: The Ubiquitin Toolbox. Curr. Issues Mol. Biol. 2020, 37, 1–20. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.; Li, K.; Li, H.; Zhu, Y.; Zhai, Y.; Lu, B.; Fan, Y.; Liu, Z.; Chen, X.; et al. Ubiquitination and deubiquitination in cancer: From mechanisms to novel therapeutic approaches. Mol. Cancer 2024, 23, 148. [Google Scholar] [CrossRef]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, X. Current methodologies in protein ubiquitination characterization: From ubiquitinated protein to ubiquitin chain architecture. Cell Biosci. 2022, 12, 126. [Google Scholar] [CrossRef]
- Hurley, J.H.; Lee, S.; Prag, G. Ubiquitin-binding domains. Biochem. J. 2006, 399, 361–372. [Google Scholar] [CrossRef]
- Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009, 78, 477–513. [Google Scholar] [CrossRef] [PubMed]
- van Nocker, S.; Sadis, S.; Rubin, D.M.; Glickman, M.; Fu, H.; Coux, O.; Wefes, I.; Finley, D.; Vierstra, R.D. The multiubiquitin-chain-binding protein Mcb1 is a component of the 26S proteasome in Saccharomyces cerevisiae and plays a nonessential, substrate-specific role in protein turnover. Mol. Cell Biol. 1996, 16, 6020–6028. [Google Scholar] [CrossRef]
- Deveraux, Q.; Ustrell, V.; Pickart, C.; Rechsteiner, M. A 26 S protease subunit that binds ubiquitin conjugates. J. Biol. Chem. 1994, 269, 7059–7061. [Google Scholar] [CrossRef] [PubMed]
- Szlanka, T.; Haracska, L.; Kiss, I.; Deák, P.; Kurucz, E.; Andó, I.; Virágh, E.; Udvardy, A. Deletion of proteasomal subunit S5a/Rpn10/p54 causes lethality, multiple mitotic defects and overexpression of proteasomal genes in Drosophila melanogaster. J. Cell Sci. 2003, 116, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, M.; Sasaki, T.; Nishimoto, T.; Kobayashi, H. Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc. Natl. Acad. Sci. USA 2002, 99, 745–750. [Google Scholar] [CrossRef]
- Collins, G.A.; Sha, Z.; Kuo, C.L.; Erbil, B.; Goldberg, A.L. Mammalian Ddi2 is a shuttling factor containing a retroviral protease domain that influences binding of ubiquitylated proteins and proteasomal degradation. J. Biol. Chem. 2022, 298, 101875. [Google Scholar] [CrossRef]
- Lipinszki, Z.; Kiss, P.; Pál, M.; Deák, P.; Szabó, A.; Hunyadi-Gulyas, E.; Klement, E.; Medzihradszky, K.F.; Udvardy, A. Developmental-stage-specific regulation of the polyubiquitin receptors in Drosophila melanogaster. J. Cell Sci. 2009, 122, 3083–3092. [Google Scholar] [CrossRef]
- Zou, T.; Lin, Z. The Involvement of Ubiquitination Machinery in Cell Cycle Regulation and Cancer Progression. Int. J. Mol. Sci. 2021, 22, 5754. [Google Scholar] [CrossRef]
- Tsai, B.; Ye, Y.; Rapoport, T.A. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 2002, 3, 246–255. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.J. Expanding role of ubiquitination in NF-κB signaling. Cell Res. 2011, 21, 6–21. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 2007, 315, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Lehner, P.J. Endosomal transport via ubiquitination. Trends Cell Biol. 2011, 21, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Trempe, J.F.; Sauvé, V.; Grenier, K.; Seirafi, M.; Tang, M.Y.; Ménade, M.; Al-Abdul-Wahid, S.; Krett, J.; Wong, K.; Kozlov, G.; et al. Structure of parkin reveals mechanisms for ubiquitin ligase activation. Science 2013, 340, 1451–1455. [Google Scholar] [CrossRef]
- Harper, J.W.; Ordureau, A.; Heo, J.M. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 93–108. [Google Scholar] [CrossRef]
- Gatti, M.; Pinato, S.; Maiolica, A.; Rocchio, F.; Prato, M.G.; Aebersold, R.; Penengo, L. RNF168 promotes noncanonical K27 ubiquitination to signal DNA damage. Cell Rep. 2015, 10, 226–238. [Google Scholar] [CrossRef]
- Peters, J.M. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006, 7, 644–656. [Google Scholar] [CrossRef]
- Jerabkova, K.; Sumara, I. Cullin 3, a cellular scripter of the non-proteolytic ubiquitin code. Semin. Cell Dev. Biol. 2019, 93, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Le Guerroué, F.; Youle, R.J. Ubiquitin signaling in neurodegenerative diseases: An autophagy and proteasome perspective. Cell Death Differ. 2021, 28, 439–454. [Google Scholar] [CrossRef]
- Liu, N.; Lin, M.M.; Wang, Y. The Emerging Roles of E3 Ligases and DUBs in Neurodegenerative Diseases. Mol. Neurobiol. 2023, 60, 247–263. [Google Scholar] [CrossRef]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Calì, F. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- Ebstein, F.; Küry, S.; Papendorf, J.J.; Krüger, E. Neurodevelopmental Disorders (NDD) Caused by Genomic Alterations of the Ubiquitin-Proteasome System (UPS): The Possible Contribution of Immune Dysregulation to Disease Pathogenesis. Front. Mol. Neurosci. 2021, 14, 733012. [Google Scholar] [CrossRef]
- Haouari, S.; Vourc’h, P.; Jeanne, M.; Marouillat, S.; Veyrat-Durebex, C.; Lanznaster, D.; Laumonnier, F.; Corcia, P.; Blasco, H.; Andres, C.R. The Roles of NEDD4 Subfamily of HECT E3 Ubiquitin Ligases in Neurodevelopment and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 3882. [Google Scholar] [CrossRef] [PubMed]
- Cruz Walma, D.A.; Chen, Z.; Bullock, A.N.; Yamada, K.M. Ubiquitin ligases: Guardians of mammalian development. Nat. Rev. Mol. Cell Biol. 2022, 23, 350–367. [Google Scholar] [CrossRef]
- van Huizen, M.; Kikkert, M. The Role of Atypical Ubiquitin Chains in the Regulation of the Antiviral Innate Immune Response. Front. Cell Dev. Biol. 2019, 7, 392. [Google Scholar] [CrossRef]
- Loix, M.; Zelcer, N.; Bogie, J.F.J.; Hendriks, J.J.A. The ubiquitous role of ubiquitination in lipid metabolism. Trends Cell Biol. 2024, 34, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Lee, S.J.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef]
- Pratt, W.B.; Gestwicki, J.E.; Osawa, Y.; Lieberman, A.P. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 353–371. [Google Scholar] [CrossRef]
- Tardiff, D.F.; Jui, N.T.; Khurana, V.; Tambe, M.A.; Thompson, M.L.; Chung, C.Y.; Kamadurai, H.B.; Kim, H.T.; Lancaster, A.K.; Caldwell, K.A.; et al. Yeast reveal a “druggable” Rsp5/Nedd4 network that ameliorates α-synuclein toxicity in neurons. Science 2013, 342, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, Z.W.; Mao, C.Y.; Shi, C.H.; Xu, Y.M. CHIP as a therapeutic target for neurological diseases. Cell Death Dis. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Hong, Y.; Yin, P.; Li, S.; Li, X.J. Differential HspBP1 expression accounts for the greater vulnerability of neurons than astrocytes to misfolded proteins. Proc. Natl. Acad. Sci. USA 2017, 114, E7803–E7811. [Google Scholar] [CrossRef]
- Zhuang, L.; Peng, F.; Huang, Y.; Li, W.; Huang, J.; Chu, Y.; Ren, P.; Sun, Y.; Zhang, Y.; Xue, E.; et al. CHIP modulates APP-induced autophagy-dependent pathological symptoms in Drosophila. Aging Cell 2020, 19, e13070. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Noda, S.; Hattori, N. Pathogenic insights to Parkin-linked model mice. Neurosci. Res. 2020, 159, 47–51. [Google Scholar] [CrossRef]
- Goudarzi, S.; Hosseini, A.; Abdollahi, M.; Haghi-Aminjan, H. Insights Into Parkin-Mediated Mitophagy in Alzheimer’s Disease: A Systematic Review. Front. Aging Neurosci. 2021, 13, 674071. [Google Scholar] [CrossRef]
- Cummins, N.; Tweedie, A.; Zuryn, S.; Bertran-Gonzalez, J.; Götz, J. Disease-associated tau impairs mitophagy by inhibiting Parkin translocation to mitochondria. EMBO J. 2019, 38, e99360. [Google Scholar] [CrossRef]
- Sliter, D.A.; Martinez, J.; Hao, L.; Chen, X.; Sun, N.; Fischer, T.D.; Burman, J.L.; Li, Y.; Zhang, Z.; Narendra, D.P.; et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 2018, 561, 258–262. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Meng, X.; Wu, X.; Tong, H.; Zhang, X.; Qu, S. Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model. Cell Death Dis. 2017, 8, e2574. [Google Scholar] [CrossRef]
- Lai, Y.J.; Zhu, B.L.; Sun, F.; Luo, D.; Ma, Y.L.; Luo, B.; Tang, J.; Xiong, M.J.; Liu, L.; Long, Y.; et al. Estrogen receptor α promotes Cav1.2 ubiquitination and degradation in neuronal cells and in APP/PS1 mice. Aging Cell 2019, 18, e12961. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Modi, P.K.; Sharma, P. Aberrant activation of neuronal cell cycle caused by dysregulation of ubiquitin ligase Itch results in neurodegeneration. Cell Death Dis. 2020, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zenge, C.; Ordureau, A. Ubiquitin system mutations in neurological diseases. Trends Biochem. Sci. 2024, 49, 875–887. [Google Scholar] [CrossRef]
- Kishino, T.; Lalande, M.; Wagstaff, J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 1997, 15, 70–73. [Google Scholar] [CrossRef]
- Matsuura, T.; Sutcliffe, J.S.; Fang, P.; Galjaard, R.J.; Jiang, Y.H.; Benton, C.S.; Rommens, J.M.; Beaudet, A.L. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat. Genet. 1997, 15, 74–77. [Google Scholar] [CrossRef]
- Sutcliffe, J.S.; Jiang, Y.H.; Galijaard, R.J.; Matsuura, T.; Fang, P.; Kubota, T.; Christian, S.L.; Bressler, J.; Cattanach, B.; Ledbetter, D.H.; et al. The E6-Ap ubiquitin-protein ligase (UBE3A) gene is localized within a narrowed Angelman syndrome critical region. Genome Res. 1997, 7, 368–377. [Google Scholar] [CrossRef]
- Bossuyt, S.N.V.; Punt, A.M.; de Graaf, I.J.; van den Burg, J.; Williams, M.G.; Heussler, H.; Elgersma, Y.; Distel, B. Loss of nuclear UBE3A activity is the predominant cause of Angelman syndrome in individuals carrying UBE3A missense mutations. Hum. Mol. Genet. 2021, 30, 430–442. [Google Scholar] [CrossRef]
- Greer, P.L.; Hanayama, R.; Bloodgood, B.L.; Mardinly, A.R.; Lipton, D.M.; Flavell, S.W.; Kim, T.K.; Griffith, E.C.; Waldon, Z.; Maehr, R.; et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 2010, 140, 704–716. [Google Scholar] [CrossRef]
- Chowdhury, S.; Shepherd, J.D.; Okuno, H.; Lyford, G.; Petralia, R.S.; Plath, N.; Kuhl, D.; Huganir, R.L.; Worley, P.F. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 2006, 52, 445–459. [Google Scholar] [CrossRef]
- Rial Verde, E.M.; Lee-Osbourne, J.; Worley, P.F.; Malinow, R.; Cline, H.T. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 2006, 52, 461–474. [Google Scholar] [CrossRef]
- Shepherd, J.D.; Rumbaugh, G.; Wu, J.; Chowdhury, S.; Plath, N.; Kuhl, D.; Huganir, R.L.; Worley, P.F. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 2006, 52, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Zhou, J.; Luo, S.; Huang, R.; Zhao, C.; Diao, A. Nedd4 E3 ubiquitin ligase promotes cell proliferation and autophagy. Cell Prolif. 2015, 48, 338–347. [Google Scholar] [CrossRef]
- Zhu, H.; Kavsak, P.; Abdollah, S.; Wrana, J.L.; Thomsen, G.H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999, 400, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, T.; Fukuchi, M.; Murakami, G.; Chiba, T.; Tanaka, K.; Imamura, T.; Miyazono, K. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 2001, 276, 12477–12480. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, M.; Bose, R.; Pye, M.; Zhang, L.; Miller, B.; Ching, P.; Sakuma, R.; Luga, V.; Roncari, L.; Attisano, L.; et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 2009, 137, 295–307. [Google Scholar] [CrossRef]
- Choi, B.H.; Che, X.; Chen, C.; Lu, L.; Dai, W. WWP2 is required for normal cell cycle progression. Genes. Cancer 2015, 6, 371–377. [Google Scholar] [CrossRef]
- Di Marcotullio, L.; Greco, A.; Mazzà, D.; Canettieri, G.; Pietrosanti, L.; Infante, P.; Coni, S.; Moretti, M.; De Smaele, E.; Ferretti, E.; et al. Numb activates the E3 ligase Itch to control Gli1 function through a novel degradation signal. Oncogene 2011, 30, 65–76. [Google Scholar] [CrossRef]
- Kawabe, H.; Neeb, A.; Dimova, K.; Young, S.M., Jr.; Takeda, M.; Katsurabayashi, S.; Mitkovski, M.; Malakhova, O.A.; Zhang, D.E.; Umikawa, M.; et al. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron 2010, 65, 358–372. [Google Scholar] [CrossRef]
- Hsia, H.E.; Kumar, R.; Luca, R.; Takeda, M.; Courchet, J.; Nakashima, J.; Wu, S.; Goebbels, S.; An, W.; Eickholt, B.J.; et al. Ubiquitin E3 ligase Nedd4-1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth. Proc. Natl. Acad. Sci. USA 2014, 111, 13205–13210. [Google Scholar] [CrossRef]
- Stouffs, K.; Verloo, P.; Brock, S.; Régal, L.; Beysen, D.; Ceulemans, B.; Jansen, A.C.; Meuwissen, M.E.C. Recurrent NEDD4L Variant in Periventricular Nodular Heterotopia, Polymicrogyria and Syndactyly. Front. Genet. 2020, 11, 26. [Google Scholar] [CrossRef]
- Bryan, B.; Cai, Y.; Wrighton, K.; Wu, G.; Feng, X.H.; Liu, M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005, 579, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.L.; Lu, H.; Shelly, M.; Gao, H.; Poo, M.M. Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development. Neuron 2011, 69, 231–243. [Google Scholar] [CrossRef]
- Ambrozkiewicz, M.C.; Schwark, M.; Kishimoto-Suga, M.; Borisova, E.; Hori, K.; Salazar-Lázaro, A.; Rusanova, A.; Altas, B.; Piepkorn, L.; Bessa, P.; et al. Polarity Acquisition in Cortical Neurons Is Driven by Synergistic Action of Sox9-Regulated Wwp1 and Wwp2 E3 Ubiquitin Ligases and Intronic miR-140. Neuron 2018, 100, 1097–1115.e1015. [Google Scholar] [CrossRef]
- Miyazaki, K.; Ozaki, T.; Kato, C.; Hanamoto, T.; Fujita, T.; Irino, S.; Watanabe, K.; Nakagawa, T.; Nakagawara, A. A novel HECT-type E3 ubiquitin ligase, NEDL2, stabilizes p73 and enhances its transcriptional activity. Biochem. Biophys. Res. Commun. 2003, 308, 106–113. [Google Scholar] [CrossRef]
- Killick, R.; Niklison-Chirou, M.; Tomasini, R.; Bano, D.; Rufini, A.; Grespi, F.; Velletri, T.; Tucci, P.; Sayan, B.S.; Conforti, F.; et al. p73: A multifunctional protein in neurobiology. Mol. Neurobiol. 2011, 43, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Halvardson, J.; Zhao, J.J.; Zaghlool, A.; Wentzel, C.; Georgii-Hemming, P.; Månsson, E.; Ederth Sävmarker, H.; Brandberg, G.; Soussi Zander, C.; Thuresson, A.C.; et al. Mutations in HECW2 are associated with intellectual disability and epilepsy. J. Med. Genet. 2016, 53, 697–704. [Google Scholar] [CrossRef]
- Berko, E.R.; Cho, M.T.; Eng, C.; Shao, Y.; Sweetser, D.A.; Waxler, J.; Robin, N.H.; Brewer, F.; Donkervoort, S.; Mohassel, P.; et al. De novo missense variants in HECW2 are associated with neurodevelopmental delay and hypotonia. J. Med. Genet. 2017, 54, 84–86. [Google Scholar] [CrossRef]
- Ullman, N.L.; Smith-Hicks, C.L.; Desai, S.; Stafstrom, C.E. De Novo HECW2 Mutation Associated With Epilepsy, Developmental Decline, and Intellectual Disability: Case Report and Review of Literature. Pediatr. Neurol. 2018, 85, 76–78. [Google Scholar] [CrossRef]
- Acharya, A.; Kavus, H.; Dunn, P.; Nasir, A.; Folk, L.; Withrow, K.; Wentzensen, I.M.; Ruzhnikov, M.R.Z.; Fallot, C.; Smol, T.; et al. Delineating the genotypic and phenotypic spectrum of HECW2-related neurodevelopmental disorders. J. Med. Genet. 2022, 59, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Drinjakovic, J.; Jung, H.; Campbell, D.S.; Strochlic, L.; Dwivedy, A.; Holt, C.E. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 2010, 65, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Hou, Q.; Jarzylo, L.; Amato, S.; Gilbert, J.; Shang, F.; Man, H.Y. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J. Neurochem. 2011, 119, 27–39. [Google Scholar] [CrossRef]
- Han, C.; Cui, K.; Bi, X.; Wang, L.; Sun, M.; Yang, L.; Liu, L. Association between polymorphism of the NEDD4 gene and cognitive dysfunction of schizophrenia patients in Chinese Han population. BMC Psychiatry 2019, 19, 405. [Google Scholar] [CrossRef]
- Ekberg, J.A.; Boase, N.A.; Rychkov, G.; Manning, J.; Poronnik, P.; Kumar, S. Nedd4-2 (NEDD4L) controls intracellular Na+-mediated activity of voltage-gated sodium channels in primary cortical neurons. Biochem. J. 2014, 457, 27–31. [Google Scholar] [CrossRef]
- Dibbens, L.M.; Ekberg, J.; Taylor, I.; Hodgson, B.L.; Conroy, S.J.; Lensink, I.L.; Kumar, S.; Zielinski, M.A.; Harkin, L.A.; Sutherland, G.R.; et al. NEDD4-2 as a potential candidate susceptibility gene for epileptic photosensitivity. Genes. Brain Behav. 2007, 6, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Karri, D.; Shen, H.; Shao, J.; Dasgupta, S.; Huang, S.; Edwards, D.P.; Ittmann, M.M.; O’Malley, B.W.; Yi, P. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J. Clin. Investig. 2018, 128, 3129–3143. [Google Scholar] [CrossRef]
- Singh, R.; Meng, H.; Shen, T.; Lumahan, L.E.V.; Nguyen, S.; Shen, H.; Dasgupta, S.; Qin, L.; Karri, D.; Zhu, B.; et al. TRAF4-mediated nonproteolytic ubiquitination of androgen receptor promotes castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2218229120. [Google Scholar] [CrossRef] [PubMed]
- Harakandi, C.; Nininahazwe, L.; Xu, H.; Liu, B.; He, C.; Zheng, Y.C.; Zhang, H. Recent advances on the intervention sites targeting USP7-MDM2-p53 in cancer therapy. Bioorg Chem. 2021, 116, 105273. [Google Scholar] [CrossRef]
- Jin, J.O.; Lee, G.D.; Nam, S.H.; Lee, T.H.; Kang, D.H.; Yun, J.K.; Lee, P.C. Sequential ubiquitination of p53 by TRIM28, RLIM, and MDM2 in lung tumorigenesis. Cell Death Differ. 2021, 28, 1790–1803. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Long, Y.; Maimaitijiang, A.; Su, Z.; Li, W.; Li, J. Unraveling the Guardian: p53’s Multifaceted Role in the DNA Damage Response and Tumor Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 12928. [Google Scholar] [CrossRef]
- Yang, F.; Xu, J.; Li, H.; Tan, M.; Xiong, X.; Sun, Y. FBXW2 suppresses migration and invasion of lung cancer cells via promoting β-catenin ubiquitylation and degradation. Nat. Commun. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, W.; Yang, F.; Chen, G.; Li, H.; Zhao, Y.; Liu, P.; Tan, M.; Xiong, X.; Sun, Y. The β-TrCP-FBXW2-SKP2 axis regulates lung cancer cell growth with FBXW2 acting as a tumour suppressor. Nat. Commun. 2017, 8, 14002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, H.; Jin, J. Applications of protein ubiquitylation and deubiquitylation in drug discovery. J. Biol. Chem. 2024, 300, 107264. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G., Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.A.; An, J.; Nowak, R.P.; Yuan, J.C.; Fink, E.C.; Berry, B.C.; Ebert, B.L.; Fischer, E.S. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. eLife 2018, 7, e38430. [Google Scholar] [CrossRef]
- Krönke, J.; Fink, E.C.; Hollenbach, P.W.; MacBeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 2015, 523, 183–188. [Google Scholar] [CrossRef]
- Matyskiela, M.E.; Couto, S.; Zheng, X.; Lu, G.; Hui, J.; Stamp, K.; Drew, C.; Ren, Y.; Wang, M.; Carpenter, A.; et al. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol. 2018, 14, 981–987. [Google Scholar] [CrossRef]
- Yamanaka, S.; Murai, H.; Saito, D.; Abe, G.; Tokunaga, E.; Iwasaki, T.; Takahashi, H.; Takeda, H.; Suzuki, T.; Shibata, N.; et al. Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF. EMBO J. 2021, 40, e105375. [Google Scholar] [CrossRef]
- Matyskiela, M.E.; Lu, G.; Ito, T.; Pagarigan, B.; Lu, C.C.; Miller, K.; Fang, W.; Wang, N.Y.; Nguyen, D.; Houston, J.; et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 2016, 535, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, K.; Usuda, J.; Iwamoto, Y.; Fukumoto, H.; Nakamura, T.; Yoneda, T.; Narita, N.; Saijo, N.; Nishio, K. Mechanisms of action of the novel sulfonamide anticancer agent E7070 on cell cycle progression in human non-small cell lung cancer cells. Investig. New Drugs 2001, 19, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Goralski, M.; Gaskill, N.; Capota, E.; Kim, J.; Ting, T.C.; Xie, Y.; Williams, N.S.; Nijhawan, D. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 2017, 356, eaal3755. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Gough, S.M.; Flanagan, J.J.; Teh, J.; Andreoli, M.; Rousseau, E.; Pannone, M.; Bookbinder, M.; Willard, R.; Davenport, K.; Bortolon, E.; et al. Oral Estrogen Receptor PROTAC Vepdegestrant (ARV-471) Is Highly Efficacious as Monotherapy and in Combination with CDK4/6 or PI3K/mTOR Pathway Inhibitors in Preclinical ER+ Breast Cancer Models. Clin. Cancer Res. 2024, 30, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.P.; Ma, C.; De Laurentiis, M.; Iwata, H.; Hurvitz, S.A.; Wander, S.A.; Danso, M.; Lu, D.R.; Perkins Smith, J.; Liu, Y.; et al. VERITAC-2: A Phase III study of vepdegestrant, a PROTAC ER degrader, versus fulvestrant in ER+/HER2- advanced breast cancer. Future Oncol. 2024, 20, 2447–2455. [Google Scholar] [CrossRef]
- Cronan, J.E. Biotin protein ligase as you like it: Either extraordinarily specific or promiscuous protein biotinylation. Proteins 2024, 92, 435–448. [Google Scholar] [CrossRef]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef]
- Chapman-Smith, A.; Cronan, J.E., Jr. Molecular biology of biotin attachment to proteins. J. Nutr. 1999, 129, 477s–484s. [Google Scholar] [CrossRef]
- Beckett, D.; Kovaleva, E.; Schatz, P.J. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 1999, 8, 921–929. [Google Scholar] [CrossRef]
- Lane, M.D.; Rominger, K.L.; Young, D.L.; Lynen, F. The enzymatic synthesis of holotranscarboxylase from apotranscarboxylase and (+)-biotin. II. investigation of the reaction mechanism. J. Biol. Chem. 1964, 239, 2865–2871. [Google Scholar] [PubMed]
- Kwon, K.; Beckett, D. Function of a conserved sequence motif in biotin holoenzyme synthetases. Protein Sci. 2000, 9, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Sears, R.M.; May, D.G.; Roux, K.J. BioID as a Tool for Protein-Proximity Labeling in Living Cells. Methods Mol. Biol. 2019, 2012, 299–313. [Google Scholar] [CrossRef]
- Kim, D.I.; Jensen, S.C.; Noble, K.A.; Kc, B.; Roux, K.H.; Motamedchaboki, K.; Roux, K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 2016, 27, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–887. [Google Scholar] [CrossRef]
- Kido, K.; Yamanaka, S.; Nakano, S.; Motani, K.; Shinohara, S.; Nozawa, A.; Kosako, H.; Ito, S.; Sawasaki, T. AirID, a novel proximity biotinylation enzyme, for analysis of protein-protein interactions. eLife 2020, 9, e54983. [Google Scholar] [CrossRef]
- Ramanathan, M.; Majzoub, K.; Rao, D.S.; Neela, P.H.; Zarnegar, B.J.; Mondal, S.; Roth, J.G.; Gai, H.; Kovalski, J.R.; Siprashvili, Z.; et al. RNA-protein interaction detection in living cells. Nat. Methods 2018, 15, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.S.; Chafin, L.; Farkas, D.; Adair, J.; Elhance, A.; Farkas, L.; Bednash, J.S.; Londino, J.D. MicroID2: A Novel Biotin Ligase Enables Rapid Proximity-Dependent Proteomics. Mol. Cell. Proteom. 2022, 21, 100256. [Google Scholar] [CrossRef]
- Kubitz, L.; Bitsch, S.; Zhao, X.; Schmitt, K.; Deweid, L.; Roehrig, A.; Barazzone, E.C.; Valerius, O.; Kolmar, H.; Béthune, J. Engineering of ultraID, a compact and hyperactive enzyme for proximity-dependent biotinylation in living cells. Commun. Biol. 2022, 5, 657. [Google Scholar] [CrossRef]
- De Munter, S.; Görnemann, J.; Derua, R.; Lesage, B.; Qian, J.; Heroes, E.; Waelkens, E.; Van Eynde, A.; Beullens, M.; Bollen, M. Split-BioID: A proximity biotinylation assay for dimerization-dependent protein interactions. FEBS Lett. 2017, 591, 415–424. [Google Scholar] [CrossRef]
- Schopp, I.M.; Amaya Ramirez, C.C.; Debeljak, J.; Kreibich, E.; Skribbe, M.; Wild, K.; Béthune, J. Split-BioID a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat. Commun. 2017, 8, 15690. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.F.; Branon, T.C.; Rajeev, S.; Svinkina, T.; Udeshi, N.D.; Thoudam, T.; Kwak, C.; Rhee, H.W.; Lee, I.K.; Carr, S.A.; et al. Split-TurboID enables contact-dependent proximity labeling in cells. Proc. Natl. Acad. Sci. USA 2020, 117, 12143–12154. [Google Scholar] [CrossRef] [PubMed]
- Schaack, G.A.; Sullivan, O.M.; Mehle, A. Identifying Protein-Protein Interactions by Proximity Biotinylation with AirID and splitAirID. Curr. Protoc. 2023, 3, e702. [Google Scholar] [CrossRef]
- Antonin, W.; Ungricht, R.; Kutay, U. Traversing the NPC along the pore membrane: Targeting of membrane proteins to the INM. Nucleus 2011, 2, 87–91. [Google Scholar] [CrossRef]
- Ungricht, R.; Klann, M.; Horvath, P.; Kutay, U. Diffusion and retention are major determinants of protein targeting to the inner nuclear membrane. J. Cell Biol. 2015, 209, 687–703. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Loïodice, I.; Alves, A.; Rabut, G.; Van Overbeek, M.; Ellenberg, J.; Sibarita, J.B.; Doye, V. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol. Biol. Cell 2004, 15, 3333–3344. [Google Scholar] [CrossRef]
- Willems, A.R.; Schwab, M.; Tyers, M. A hitchhiker’s guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 2004, 1695, 133–170. [Google Scholar] [CrossRef]
- Coyaud, E.; Mis, M.; Laurent, E.M.; Dunham, W.H.; Couzens, A.L.; Robitaille, M.; Gingras, A.C.; Angers, S.; Raught, B. BioID-based Identification of Skp Cullin F-box (SCF)β-TrCP1/2 E3 Ligase Substrates. Mol. Cell. Proteom. 2015, 14, 1781–1795. [Google Scholar] [CrossRef]
- Okamoto, T.; Wu, Y.; Matsuhisa, K.; Saito, A.; Sakaue, F.; Imaizumi, K.; Kaneko, M. Hypertonicity-responsive ubiquitin ligase RNF183 promotes Na, K-ATPase lysosomal degradation through ubiquitination of its β1 subunit. Biochem. Biophys. Res. Commun. 2020, 521, 1030–1035. [Google Scholar] [CrossRef]
- Yamanaka, S.; Horiuchi, Y.; Matsuoka, S.; Kido, K.; Nishino, K.; Maeno, M.; Shibata, N.; Kosako, H.; Sawasaki, T. A proximity biotinylation-based approach to identify protein-E3 ligase interactions induced by PROTACs and molecular glues. Nat. Commun. 2022, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, M.; Sandow, J.J.; Maher, B.; Susanto, O.; Vervoort, S.J.; Devlin, J.R.; Garama, D.; Condina, M.R.; Steele, J.R.; Kahrood, H.V.; et al. Mapping the IMiD-dependent cereblon interactome using BioID-proximity labelling. FEBS J. 2024, 291, 4892–4912. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Gomila, O.; Merino-Cacho, L.; Muratore, V.; Perez, C.; Taibi, V.; Maspero, E.; Azkargorta, M.; Iloro, I.; Trulsson, F.; Vertegaal, A.C.O.; et al. BioE3 identifies specific substrates of ubiquitin E3 ligases. Nat. Commun. 2023, 14, 7656. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Lumpkin, R.J.; Tsai, R.W.; Su, S.; Zhao, X.; Xiong, Y.; Chen, J.; Mageed, N.; Donovan, K.A.; Fischer, E.S.; et al. Ubiquitin-specific proximity labeling for the identification of E3 ligase substrates. Nat. Chem. Biol. 2024, 20, 1227–1236. [Google Scholar] [CrossRef]
- Mukhopadhyay, U.; Levantovsky, S.; Carusone, T.M.; Gharbi, S.; Stein, F.; Behrends, C.; Bhogaraju, S. A ubiquitin-specific, proximity-based labeling approach for the identification of ubiquitin ligase substrates. Sci. Adv. 2024, 10, eadp3000. [Google Scholar] [CrossRef]
- Uçkun, E.; Wolfstetter, G.; Anthonydhason, V.; Sukumar, S.K.; Umapathy, G.; Molander, L.; Fuchs, J.; Palmer, R.H. In vivo Profiling of the Alk Proximitome in the Developing Drosophila Brain. J. Mol. Biol. 2021, 433, 167282. [Google Scholar] [CrossRef]
- Artan, M.; Barratt, S.; Flynn, S.M.; Begum, F.; Skehel, M.; Nicolas, A.; de Bono, M. Interactome analysis of Caenorhabditis elegans synapses by TurboID-based proximity labeling. J. Biol. Chem. 2021, 297, 101094. [Google Scholar] [CrossRef]
- Uezu, A.; Soderling, S. Identifying Synaptic Proteins by In Vivo BioID from Mouse Brain. Methods Mol. Biol. 2019, 2008, 107–119. [Google Scholar] [CrossRef]
- Howarth, M.; Ting, A.Y. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat. Protoc. 2008, 3, 534–545. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Min, M.; Mayor, U.; Dittmar, G.; Lindon, C. Using in vivo biotinylated ubiquitin to describe a mitotic exit ubiquitome from human cells. Mol. Cell. Proteom. 2014, 13, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Martinez, A.; Lectez, B.; Lee, S.Y.; Franco, M.; Barrio, R.; Dittmar, G.; Mayor, U. Proteomic Analysis of the Ubiquitin Landscape in the Drosophila Embryonic Nervous System and the Adult Photoreceptor Cells. PLoS ONE 2015, 10, e0139083. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Gomila, O.; Muratore, V.; Merino-Cacho, L.; Rodriguez, J.A.; Barrio, R.; Sutherland, J.D. Studying the ubiquitin code through biotin-based labelling methods. Semin. Cell Dev. Biol. 2022, 132, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Merino-Cacho, L.; Barroso-Gomila, O.; Hernández-Sánchez, S.; Ramirez, J.; Mayor, U.; Sutherland, J.D.; Barrio, R. Biotin-Based Strategies to Explore the World of Ubiquitin and Ubiquitin-like Modifiers. ChemBioChem 2024, 25, e202300746. [Google Scholar] [CrossRef]
| Biotin Ligase | Amino Acid Length | Incubation Time for Biotinylation | Reference |
|---|---|---|---|
| BioID | 321 | <16 h | [108] |
| BioID2 | 233 | 16 h | [114] |
| TurboID | 321 | 10 min | [115] |
| Miniturbo | 254 | N.D. | [115] |
| AirID | 317 | 6 h | [116] |
| BASU | 325 | 18 h | [117] |
| MicroID2 | 180 | 3 h | [118] |
| UltraID | 170 | 10 min | [119] |
| Split-BioID | 140 (N), 181 (C) | 16 h | [120] |
| 256 (N), 65 (C) | 24 h | [121] | |
| Split-TurboID | 73 (N) | 4 h | [122] |
| 248 (C) | |||
| Split-AirID | 98 (N) | <24 h | [123] |
| 245 (C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuhisa, K.; Sato, S.; Kaneko, M. Identification of E3 Ubiquitin Ligase Substrates Using Biotin Ligase-Based Proximity Labeling Approaches. Biomedicines 2025, 13, 854. https://doi.org/10.3390/biomedicines13040854
Matsuhisa K, Sato S, Kaneko M. Identification of E3 Ubiquitin Ligase Substrates Using Biotin Ligase-Based Proximity Labeling Approaches. Biomedicines. 2025; 13(4):854. https://doi.org/10.3390/biomedicines13040854
Chicago/Turabian StyleMatsuhisa, Koji, Shinya Sato, and Masayuki Kaneko. 2025. "Identification of E3 Ubiquitin Ligase Substrates Using Biotin Ligase-Based Proximity Labeling Approaches" Biomedicines 13, no. 4: 854. https://doi.org/10.3390/biomedicines13040854
APA StyleMatsuhisa, K., Sato, S., & Kaneko, M. (2025). Identification of E3 Ubiquitin Ligase Substrates Using Biotin Ligase-Based Proximity Labeling Approaches. Biomedicines, 13(4), 854. https://doi.org/10.3390/biomedicines13040854






