Abstract
Ubiquitination is a dynamic and tightly regulated post-translational modification essential for modulating protein stability, trafficking, and function to preserve cellular homeostasis. This process is orchestrated through a hierarchical enzymatic cascade involving three key enzymes: the E1 ubiquitin-activating enzyme, the E2 ubiquitin-conjugating enzyme, and the E3 ubiquitin ligase. The final step of ubiquitination is catalyzed by the E3 ubiquitin ligase, which facilitates the transfer of ubiquitin from the E2 enzyme to the substrate, thereby dictating which proteins undergo ubiquitination. Emerging evidence underscores the critical roles of ubiquitin ligases in neurodevelopment, regulating fundamental processes such as neuronal polarization, axonal outgrowth, synaptogenesis, and synaptic function. Mutations in genes encoding ubiquitin ligases and the consequent dysregulation of these pathways have been increasingly implicated in a spectrum of neurodevelopmental disorders, including autism spectrum disorder, intellectual disability, and attention-deficit/hyperactivity disorder. This review synthesizes current knowledge on the molecular mechanisms underlying neurodevelopment regulated by Cullin-RING ubiquitin ligases—the largest subclass of ubiquitin ligases—and their involvement in the pathophysiology of neurodevelopmental disorders. A deeper understanding of these mechanisms holds significant promise for informing novel therapeutic strategies, ultimately advancing clinical outcomes for individuals affected by neurodevelopmental disorders.
1. Introduction
Ubiquitination is a dynamic post-translational modification that plays critical roles in maintaining cellular function and homeostasis by modulating protein stability, activity, and localization [1,2]. Ubiquitination refers to the covalent attachment of ubiquitin molecules to a substrate protein. This process is carried out through a sequential enzymatic cascade involving three key enzymes. First, the E1 ubiquitin-activating enzyme activates ubiquitin in an ATP-dependent manner, forming a high-energy thioester bond. Next, the activated ubiquitin is transferred to the E2 ubiquitin-conjugating enzyme. Finally, the E3 ubiquitin ligase facilitates the transfer of ubiquitin from the E2 enzyme to the target protein, determining the substrate specificity [3]. The transfer of ubiquitin to the substrate is orchestrated by either a RING (really interesting new gene) finger domain, a HECT (homologous to the E6-AP carboxyl terminus) domain, or an RBR (RING-between-RING) domain, leading to the classification of ubiquitin ligases into RING-type, HECT-type or RBR-type based on the domain they possess [4,5]. Ubiquitin ligases function as either single-protein entities which contain both E2- and substrate-binding domains, or multi-subunit complexes which harbor a protein responsible for binding E2, and a substrate receptor protein.
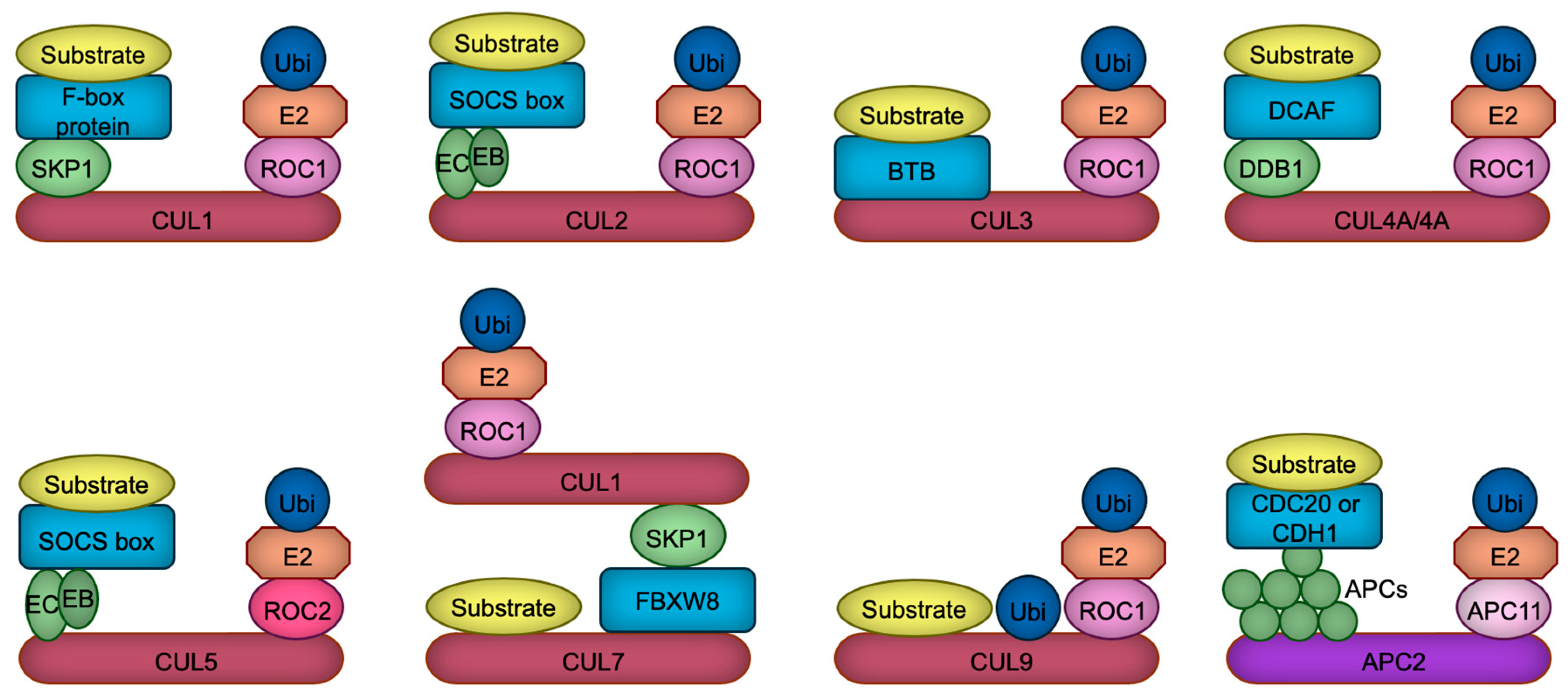
The human and rodent genomes encode more than 600 ubiquitin ligases, which play pivotal roles in various cellular processes, including proliferation, differentiation, and apoptosis [6]. These ligases are implicated in numerous pathophysiological conditions, such as cancer, immune dysfunctions, aging, and developmental disorders [7]. The largest subclass of ubiquitin ligases is the Cullin-RING ubiquitin ligase (CRL), in which Cullin protein serves as a scaffold to bind both the RING domain protein ROC1/2 (also called RBX1/2), and the substrate receptor protein directly or indirectly through an adaptor protein [8,9]. Mammalian cells contain eight (CUL1, 2, 3, 4A, 4B, 5, 7, 9) Cullin proteins, each of which builds their specific ubiquitin ligase complexes [8,9]. For example, CUL1-based ubiquitin ligase uses F-box proteins as a substrate receptor which is linked to CUL1 via a SKP1 adaptor protein [10], while CUL4A or CUL4B employs DDB1 adaptor protein which recruits DCAF substrate receptor proteins [11,12] (Figure 1). In contrast to CUL1, CUL2, CUL3, CUL4A, CUL4B, and CUL5, recent structural investigations have demonstrated that CUL7 and CUL9 directly interact with their substrates, with the ubiquitylation module being supplied by CUL1 in the case of CUL7 [13]. CUL9, on the other hand, employs its intrinsic RBR domain to help catalyze ubiquitylation [14]. All Cullin proteins are thought to use ROC1 as a RING domain protein, except for CUL5 which uses ROC2 [15] (Figure 1). In addition to these Cullin-based complexes, APC/C (anaphase promoting complex/cyclosome) ubiquitin ligase constitutes a similar structure in which APC2 serves as a main scaffold, APC11 as a RING domain protein, and CDC20 or CDH1 as a substrate receptor [16] (Figure 1). Due to this structural similarity and its significant functions in neurodevelopment [17], we include and discuss APC/C ubiquitin ligase in this review.
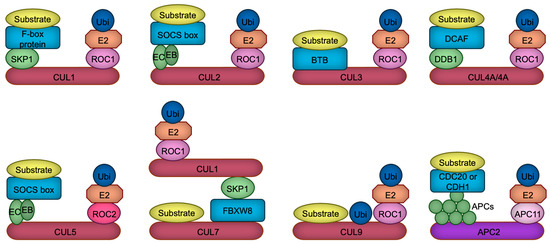
Figure 1.
The composition of CRL ubiquitin ligases. Cullin proteins (red and purple) serve to link the substrate binding module with the ubiquitylation module. CUL1, CUL2, CUL4A, CUL4B, CUL5, and APC2 interact with substrate receptors (blue) through adaptor proteins (green), whereas CUL3 directly associates with substrate receptors. In contrast, CUL7 and CUL9 appear to bypass substrate receptors, binding directly to their substrates. CUL7 recruits CRL1 as a ubiquitylation module, whereas CUL9 uniquely harbors an RBR domain, which facilitates the catalysis of ubiquitylation through a ROC1-based ubiquitylation module. Ubiquitin, the E2 enzyme, the linker connecting the E2 enzyme to Cullin, and the substrate are represented in dark blue, orange, light pink, and yellow, respectively. EB, Elongin B; EC, Elongin C; APCs, APC proteins.
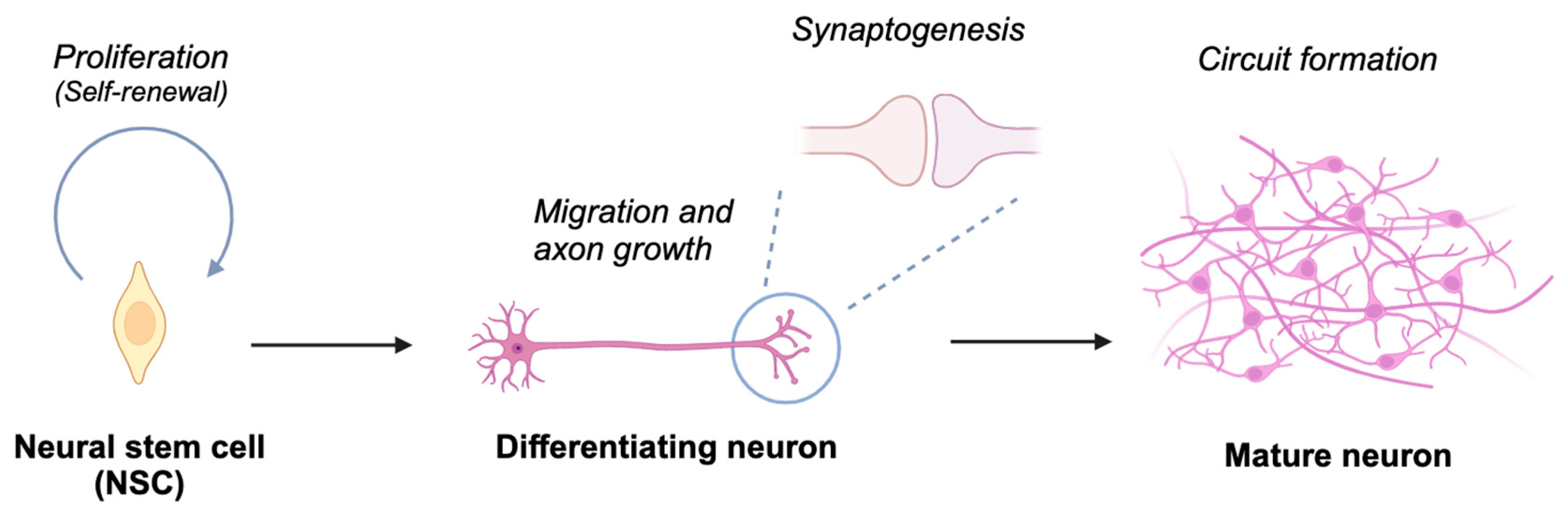
Ubiquitination has been demonstrated to play a significant role in neurodevelopmental processes such as neural stem cell proliferation/differentiation, axonal/dendritic growth, migration, synaptogenesis, and synaptic function (Figure 2). Thus, dysregulation of these processes has been linked to several neurodevelopmental disorders (NDDs), including autism spectrum disorder (ASD), intellectual disability (ID), and attention-deficit/hyperactivity disorder (ADHD). Genomic studies have identified both CRLs and other E3 ligases, including HECT-type E6-AP (also known as UBE3A), SMURF1, and RBR-type ARIH2, as being linked to NDDs [18,19,20,21,22], underscoring the need for comparative analysis and potential interaction between CRLs and other ubiquitin ligases in neurodevelopment. This review aims to explore the role of CRLs-catalyzed ubiquitination in neurodevelopment and its implications for NDDs, highlighting their potential as therapeutic targets to bridge the gap between fundamental research and clinical applications. Readers are referred to excellent reviews on ubiquitin ligases and neurodevelopment for a more comprehensive understanding of ubiquitination in neurodevelopment, focusing on areas such as immune dysregulation [23], axon development [24]. and spatiotemporal regulation of HECT-type ubiquitin ligases [25].
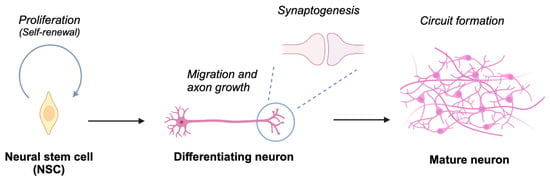
Figure 2.
Schematic depiction of neuronal differentiation processes. Neural stem cells undergo proliferation and self-renewal. Upon initiating differentiation, these cells develop axons and establish synaptic connections with other neurons, culminating in the assembly of neural circuits essential for processing information in response to both environmental and endogenous stimuli. Created in BioRender. Nakagawa, T. (2025) https://BioRender.com/t69e513 (accessed on 5 March 2025).
2. Human Genetics of CRL in NDD Patients
Exome sequencing and chromosomal microarray testing of NDD patients identified de novo heterozygous loss-of-function mutations in CUL3 on chromosome 2 [26,27], homozygous or compound heterozygous loss-of-function mutations in CUL7 on chromosome 6, and hemizygous loss-of-function mutations in CUL4B on X chromosome [28,29] in NDD patients. Furthermore, variants of genes encoding CRL1 substrate receptors have been implicated in NDD patients. These include FBXO10 [30], FBXO11 [31,32], FBXO28 [32,33], FBXO31 [34], FBXO47 [35], FBXL3 [36], FBXL4 [37,38], FBXL10 (also known as KDM2B) [39], β-TrCP1 (also known as FBXW1) [40,41,42], β-TrCP2 (also known as FBXW11) [41], and FBXW7 [43,44,45]. Additionally, variants in CRL3 substrate receptors, such as KLHL15 [46], KLHL17 (also known as actinfilin) [21], KLHL20 [47], KCTD7 [48], and KCTD13 [49] are also relevant. Likewise, variants in CRL4 substrate receptors, including DCAF1 (also known as VprBP) [50], DCAF14 (also known as PHIP, BRWD2 and RepID) [51,52], COP1 [53], and CRBN [54,55] have been implicated in these patients. Lastly, mutations in gene encoding APC/C substrate receptor CDH1 [56] have also been associated with NDD. These findings suggest the involvement of these genes in NDDs, thereby prompting further research to elucidate how these mutations contribute to the pathogenesis of NDDs.
3. Behavioral Phenotypes of Mice with CRL Mutations
Mouse models have been developed to investigate the mechanisms underlying the pathogenesis of NDDs [57,58,59]. To assess the suitability of these models for NDD research, various behavioral evaluations have been designed and conducted [60,61]. For instance, social interaction deficits are quantified through open field and three-chamber social interaction tests, while impairments in learning and memory are assessed using a range of maze and contextual fear conditioning paradigms [60,61]. Additionally, increased distance traveled serves as an indicator of hyperactivity, and anxiety is primarily evaluated through open field and elevated plus-maze tests [60,61]. Several CRL mutant mouse models have been shown to exhibit these NDD-associated behaviors, as summarized in Table 1. These findings suggest that mutations in these genes are most likely causal, rather than merely correlative, in the etiology of NDDs.

Table 1.
NDD-related behavioral abnormalities exhibited by CRL-related gene mutations in mice. A check mark denotes the presence, while a minus sign in parentheses indicates the absence of the behavioral abnormalities. *, these mice exhibit hypoactivity. N.T., not tested; DA, dopaminergic; Glu, glutamatergic.
4. CRLs in Neural Stem Cell Proliferation and Differentiation
In the following sections, we will provide an overview of the current understanding of the molecular mechanisms underlying neurodevelopment and NDDs, as derived from studies on CRLs.
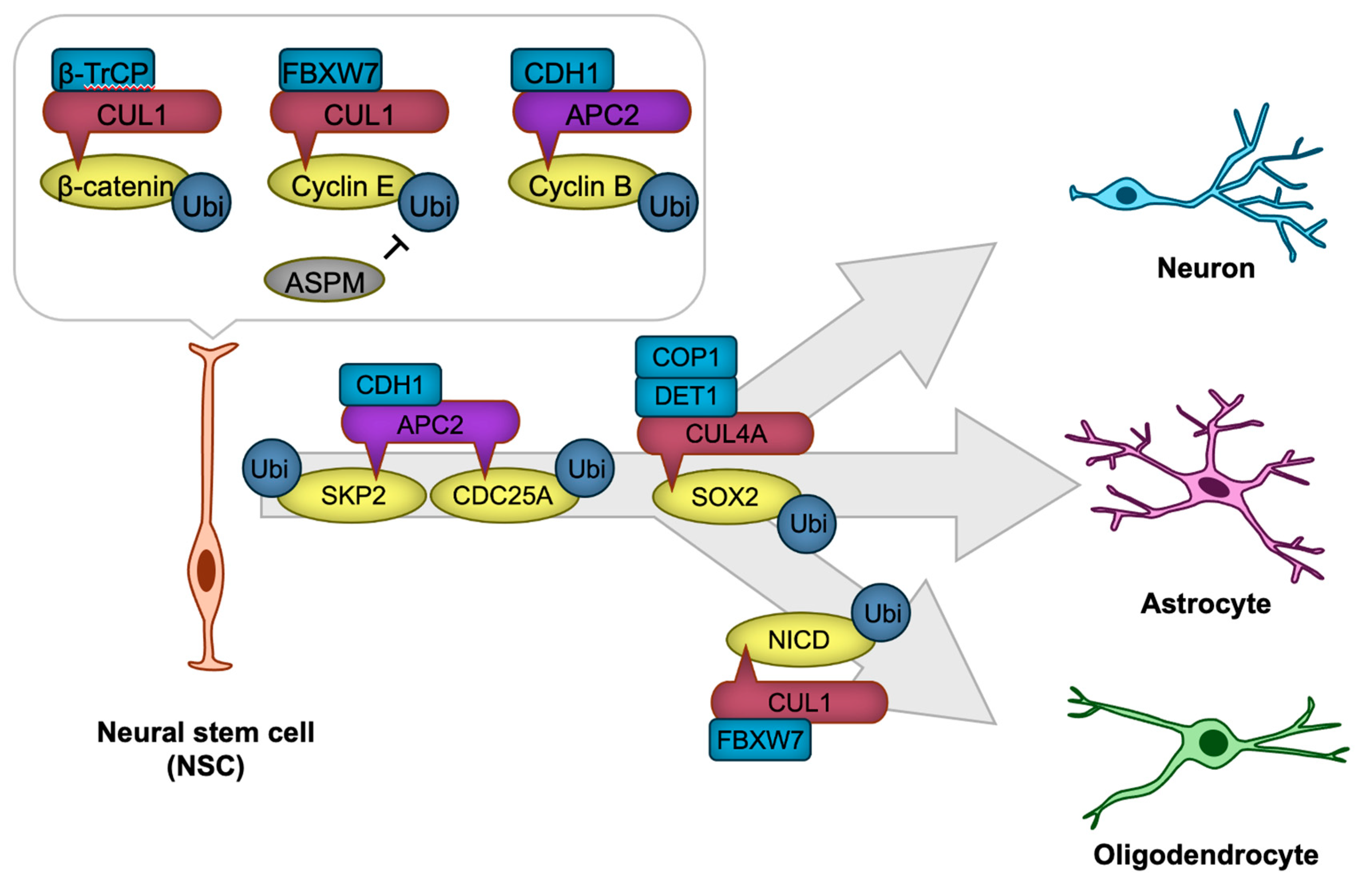
Neural stem cells (NSCs) are multipotent progenitor cells that give rise to neurons, astrocytes, and oligodendrocytes during brain development [72,73]. NSCs first give rise to radial glial cells (RGCs), which serve dual roles as neural progenitors and as scaffolds that guide migrating neurons. RGCs divide asymmetrically, producing either neurons directly or intermediate progenitors (IPs) as an intermediary step in neurogenesis. IPs undergo a limited number of divisions, amplifying the production of neurons to meet developmental demands. Mature neurons are generated from the differentiation of IPs or directly from RGCs in a highly regulated process. This sequential progression from NSCs to RGCs, then to IPs, and finally to mature neurons ensures the proper formation and organization of the nervous system, which is essential for the precise development of neural structures and circuits [74,75,76]. The tightly regulated processes of NSC proliferation and differentiation rely heavily on the dynamic control of protein expression and degradation. CRLs play a pivotal role in these processes by regulating key signaling pathways and transcription factors (Figure 3).
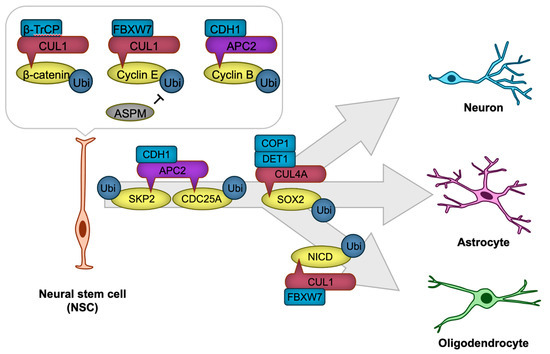
Figure 3.
CRLs in neural stem cell (NSC) proliferation and differentiation. The transcription factor β-catenin and cyclins are targeted for degradation by CRL1 or APC/C to regulate NSC proliferation. NSC differentiation is precisely regulated through CRL1, CRL4A, or APC/C-mediated degradation of key cell cycle regulators, including SKP2 and CDC25A, as well as transcription factors such as SOX2 and NICD, thereby ensuring the timely generation of neurons or glial cells. The ‘-|’ symbol represents an inhibitory interaction.
NSC proliferation is governed by the precise regulation of cell cycle progression, which is controlled by cyclins, cyclin-dependent kinases (CDKs), and their inhibitors. Ubiquitination ensures the timely degradation of these cell cycle regulators, thus maintaining proper cell cycle transitions. For example, APC/C ubiquitin ligase recognizes mitotic regulators such as cyclin B through the CDH1 substrate receptor (APC/CCDH1), and targets them for ubiquitination, promoting the progression of mitosis [77,78]. In Cdh1-deficient neural stem cells, aberrantly elevated cyclin B-associated CDK activity induces DNA damage-mediated apoptotic cell death [78], potentially by disrupting the precise regulation of S phase timing, thereby eliciting DNA replication stress [77]. Another key regulator is the CUL1-based ubiquitin ligase complex (CRL1, also known as SKP1-CUL1-F-box SCF ubiquitin ligase), which ubiquitinates cyclin E for degradation with the use of the FBXW7 substrate receptor (CRL1FBXW7). ASPM suppresses this SCFFBXW7-mediated ubiquitination, thereby stabilizing cyclin E. This stabilization facilitates the shortening of the G1 phase in the cell cycle, promoting NSC proliferation [79].
NSC differentiation requires the fine-tuned suppression of self-renewal programs and the activation of lineage-specific transcriptional networks. Ubiquitination modulates this process by targeting signaling pathway components and transcription factors that drive differentiation. For instance, the Notch signaling pathway, which maintains NSC and RGC self-renewal, is also regulated by the ubiquitin ligase CRL1FBXW7, which ubiquitinates and targets Notch intracellular domain (NICD) for proteasomal degradation [80]. This degradation facilitates the transition from NSC maintenance to astrocytic differentiation [80]. APC/C also plays a critical role in NSC differentiation. APC/CCDH1 ubiquitinates and facilitates the degradation of the CDK activator CDC25A and SKP2, a substrate receptor of SCF ubiquitin ligase that targets CDK inhibitors for degradation [78]. This process prevents cell cycle re-entry, thereby promoting neuronal differentiation [78]. Similarly, the Wnt/β-catenin signaling pathway, a critical regulator of NSC maintenance, is modulated by ubiquitination. The E3 ligase CRL1β-TrCP ubiquitinates β-catenin, leading to its degradation and attenuation of Wnt signaling [81,82].
In addition to cell cycle and signaling pathways, ubiquitination directly regulates transcription factors critical for NSC identity and differentiation. SOX2, a core transcription factor that maintains NSC pluripotency, is subject to ubiquitination. In human pluripotent stem cell-derived NSC, CUL4A-based Cullin-RING ubiquitin ligase with DET1-COP1 heterodimer as a substrate receptor (CRL4ADET1-COP1) ubiquitinates SOX2 for degradation, leading to neural differentiation [83].
The dysregulation of ubiquitination in NSC proliferation and differentiation has profound implications for NDDs. Mutations in genes encoding CRL-related proteins (please refer to Section 2) and FBXW7 regulator ASPM [84,85] are identified in NDD patients and these could be caused by aberrant NSC proliferation of differentiation.
5. CRLs in Neuronal Polarization
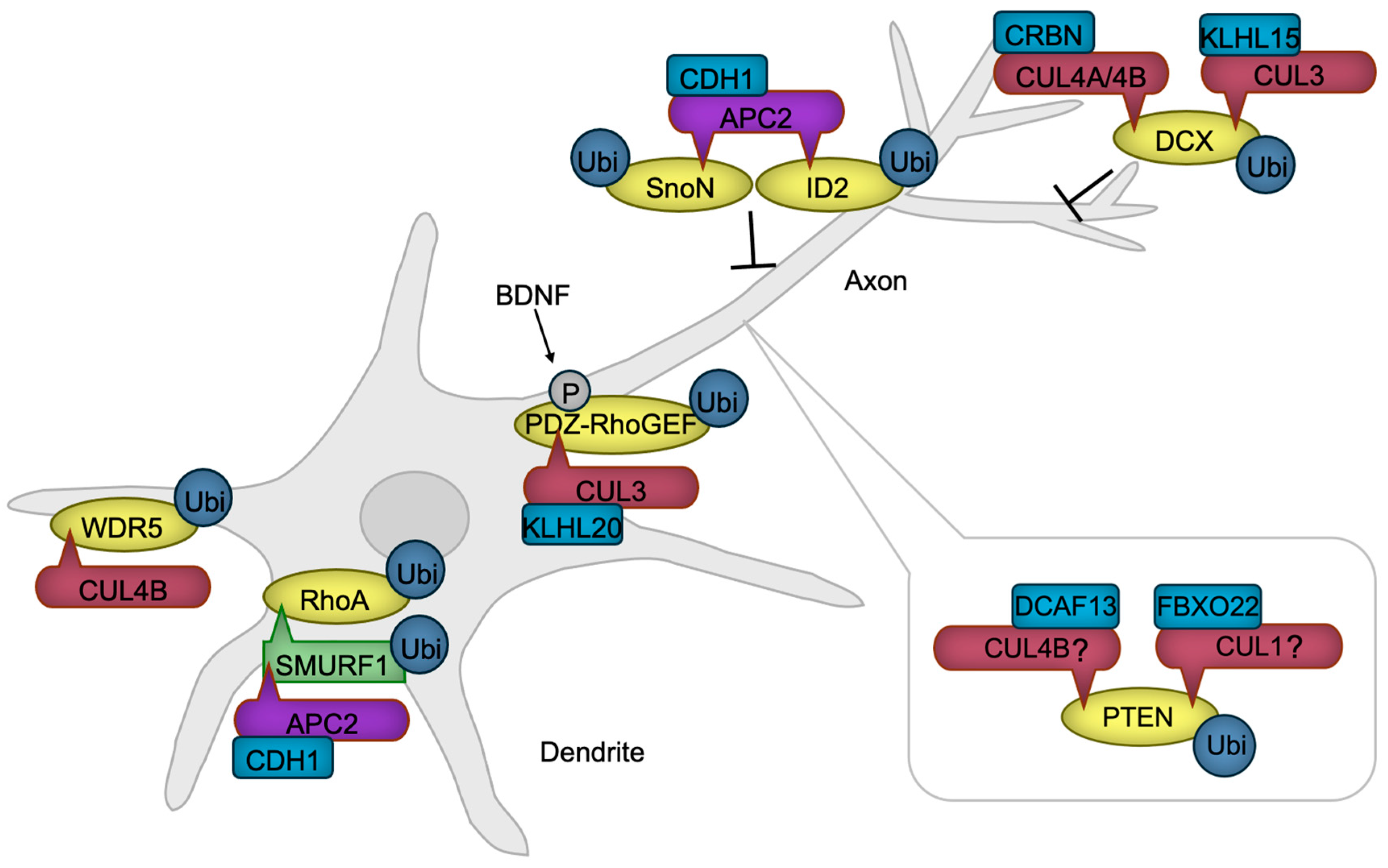
Immature neurons extend processes known as neurites, which subsequently differentiate into axon, the intracellular signal-transmitting structure, or dendrites, the primary signal-receiving components of neurons. The developmental process that defines this distinction is known as neuronal polarization, signifying the establishment of distinct subcellular compartments to enable precise signal transmission and reception [86]. Neuronal polarization is initiated by extracellular symmetry-breaking cues, followed by cytoskeletal rearrangements [87], with actin filaments (F-actin) guiding axon/dendrite extension and microtubules playing an essential role in axon stabilization [88,89]. By modulating protein functions, CRLs ensure the spatial and temporal coordination of neuronal polarization (Figure 4).
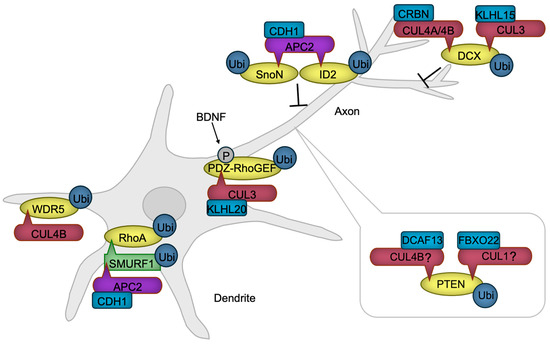
Figure 4.
CRLs in neuronal polarization. APC/C promotes dendrite formation by targeting SMURF1, a negative regulator of RhoA, along with transcription factors SnoN and ID2, positive regulators of axon formation, for ubiquitylation and subsequent degradation. RhoA, essential for dendrite formation, is also regulated through the degradation of its activator, PDZ-RhoGEF, by CRL3. BDNF enhances this degradation, thereby accelerating axon formation. CUL4B-mediated degradation of WDR5 further promotes neurite growth. PTEN, a negative regulator of axonogenesis, is maintained at low levels in axons through ubiquitylation-mediated degradation. While the specific ubiquitin ligases remain unidentified, CRL4B and CRL1 are known to ubiquitylate PTEN in non-neuronal cancer cells, suggesting that these ligases may also regulate PTEN in developing neurons. DCX, which facilitates axonal and dendritic complexity, is negatively regulated by CRL3 and CRL4A/B. BDNF, Brain-derived neurotrophic factor. The ‘-|’ symbol represents an inhibitory interaction.
The protein kinase AKT facilitates axon formation by phosphorylating GSK-3β. This phosphorylation negates the GSK-3β’s function, leading to the activation of microtubule-binding proteins and subsequent stabilization of microtubules [90]. PTEN is a negative regulator of AKT activity [91], necessitating PTEN inactivation within the axon. Notably, the HECT-type ubiquitin ligase NEDD4 is reported to facilitate PTEN degradation through ubiquitination, thereby promoting neurite outgrowth [92] and axon branching [93]. However, the decrease in ubiquitination and accumulation of PTEN were not detected in NEDD4-deficient neurons [94], indicating that the E3 ubiquitin ligase for PTEN in developing axon awaits to be revealed. Although not in neuronal cells, CRL4BDCAF13 [95,96] and SCFFBXO22 [97] are reported to ubiquitinate PTEN for degradation, providing the possibility that Cullin-RING ubiquitin ligases are involved in axon formation through PTEN regulation.
The activity of a small G protein RhoA is critical for dendrite formation through promoting actin arc formation, which prevents microtubule protrusion and axon growth [98]. RhoA is stabilized in dendrites by the ubiquitin ligase APC/CCDH1 that ubiquitinates the RhoA destabilizing HECT-type ubiquitin ligase SMURF1 for degradation [99]. In the axon, the activity of RhoA is downregulated by a mechanism in which RhoA activator PDZ-RhoGEF is targeted for degradation by the ubiquitin ligase CUL3 with KLHL20 serving as a substrate receptor (CRL3KLHL20) [100]. A symmetry-breaking signaling by BDNF induces the phosphorylation of PDZ-RhoGEF, potentiating its ubiquitination and subsequent degradation [100], facilitating neuronal polarization.
The interplay between F-actin and microtubules, mediated by coupling proteins, is also implicated in axon and dendrite formation [101]. One such coupling protein, DCX, is subject to negative regulation by CRL3KLHL15 [102], CRL4ACRBN [103], and CRL4BCRBN [103]. The ubiquitination and subsequent proteasomal degradation of DCX by these CRLs have been shown to attenuate axonal and dendritic complexity and length [102,103].
An additional regulatory layer in the process of neuronal polarization is provided by transcriptional mechanisms, whose activities are also modulated by ubiquitination. For instance, APC/CCDH1 facilitates the ubiquitination of SnoN [104], a transcription factor that promotes the expression of positive regulators of axon growth, and ID2 [105], an inhibitor of axon growth repressors, thereby suppressing axon extension. We have also demonstrated that the ubiquitin ligase CUL4B regulates NGF-induced neurite extension via transcriptional modulation [106,107]. CUL4B mediates the ubiquitination and subsequent degradation of WDR5 [106], a key component of the histone H3 lysine 4 methyltransferase transcriptional complex [108], in rat neuroblastoma PC12 cells, downregulating the neuronal gene expression [109]. These findings underscore the critical role of transcriptional regulation in neuronal process formation.
Dysregulation of ubiquitination in neuronal polarization also has profound implications for NDDs. Mutations in genes encoding CRL-related proteins (please refer to Section 2), as well as DCX [110,111], SMURF1 [21,22], and PTEN [112,113] are identified in NDD patients and these could be caused by aberrant neuronal polarization.
6. CRLs in Neuronal Migration
NSCs are generated, and remain, adjacent to the brain ventricle, while differentiating neurons migrate towards the cortical surface, with later-differentiated neurons passing by earlier-generated neurons during corticogenesis [76,114]. This inside-out layering of the cortex is guided by various mechanisms, some of which are regulated by CRLs (Figure 5).
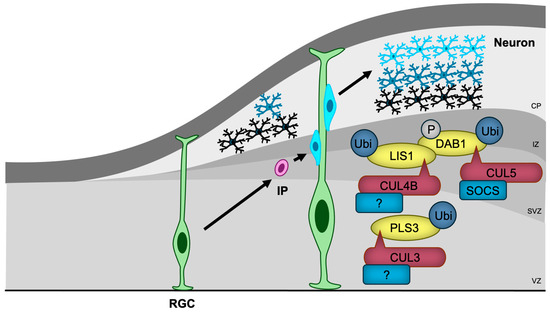
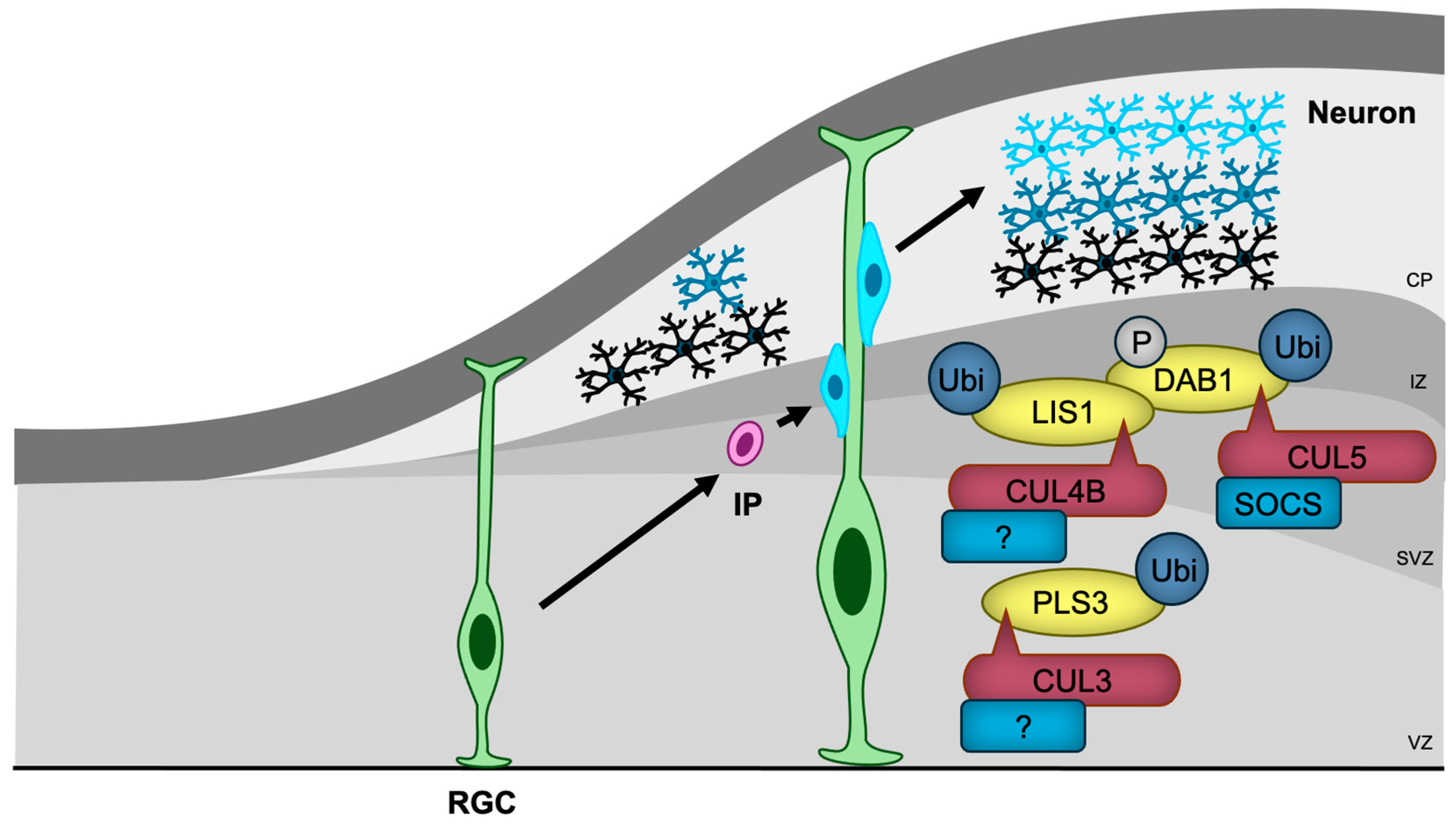
Figure 5.
CRLs in neuronal migration. RGCs, the first differentiated cells derived from neural stem cells, generate IPs that migrate towards the cortical surface, with later-differentiated neurons passing by earlier-generated neurons. CRL3, CRL4B, and CRL5 regulate neuronal migration by ubiquitylating key migration regulatory proteins, including PLS3, LIS1, and DAB1, respectively. The substrate receptors of CUL3 and CUL4B for PLS3 and LIS1, respectively, have yet to be identified. RGC, radial glial cell; IP, intermediate progenitor; VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; CP, cortical plate.
The most well-characterized regulator of neuronal migration is Reelin/DAB1 signaling [115]. Reelin binds to its receptors and activates tyrosine kinases, leading to the phosphorylation of DAB1 [116]. Phosphorylated DAB1 then interacts with several proteins, including LIS1, a regulator of microtubule-based dynein-dynactin motor proteins, which facilitates cytoskeletal reorganization essential for migration [117]. CUL5 with SOCS proteins as substrate receptors (CRL5SOCS) targets phosphorylated DAB1 for ubiquitination and degradation, thus preventing DAB1 hyperactivation [118]. CUL5 knockdown-induced accumulation of phosphorylated DAB1 results in excessive migration and abnormal superficial positioning [118]. Furthermore, CUL4B was shown to bind to LIS1 [119], suggesting that CRL4B may also play a role in regulating neuronal migration through Reelin signaling.
In contrast, the heterozygous deletion of Cul3, a condition that mirrors NDD in humans, leads to impaired migration of embryonic neurons and cortical lamination abnormalities in mice [63]. Proteomic analysis has identified PLS3, an actin-bundling protein, as a critical substrate of CUL3, and the accumulation of PLS3 caused by Cul3 haploinsufficiency disrupts actin filament organization and results in abnormal adhesion with impaired migration [63]. The substrate receptor responsible for PLS3 recognition remains unidentified.
The generation of the cerebellum and hippocampus also depends on proper neuronal migration [120,121]. FBXO41, a neuron-specific substrate receptor of CRL1 ubiquitin ligase, has been shown to promote neuronal migration in the cerebellum (from the external to the internal granule layer) [122], and hippocampus (from the hilus to the granule cell layer) [123]. It localizes to the centrosome and disassembles primary cilia, an antenna-like structure that receives several signaling ligands [124]. However, the involvement of primary cilia and the substrates of SCFFBXO41 in neuronal migration remain unclear.
In addition to CUL3 and CUL4B, mutations in genes encoding Reelin [125,126], DAB1 [127], and LIS1 [128] have been identified in patients with NDDs, indicating that defects in neuronal migration may, at least in part, contribute to the pathogenesis of these disorders.
7. CRLs in Synaptogenesis and Synaptic Function
Elongating axons extend towards the dendrites of target neurons, where synapses are established [129,130]. At the presynaptic terminal, neurons secrete neurotransmitters such as glutamate, dopamine, acetylcholine (ACh), and gamma-aminobutyric acid (GABA), which bind to their corresponding receptors in the specialized postsynaptic density (PSD) region of the postsynaptic neurons [131], thereby enabling communication and the transmission of information. CLRs play a significant role in synaptogenesis and synaptic function (Figure 6).
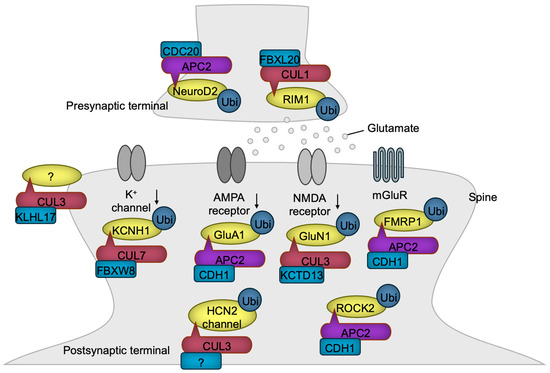
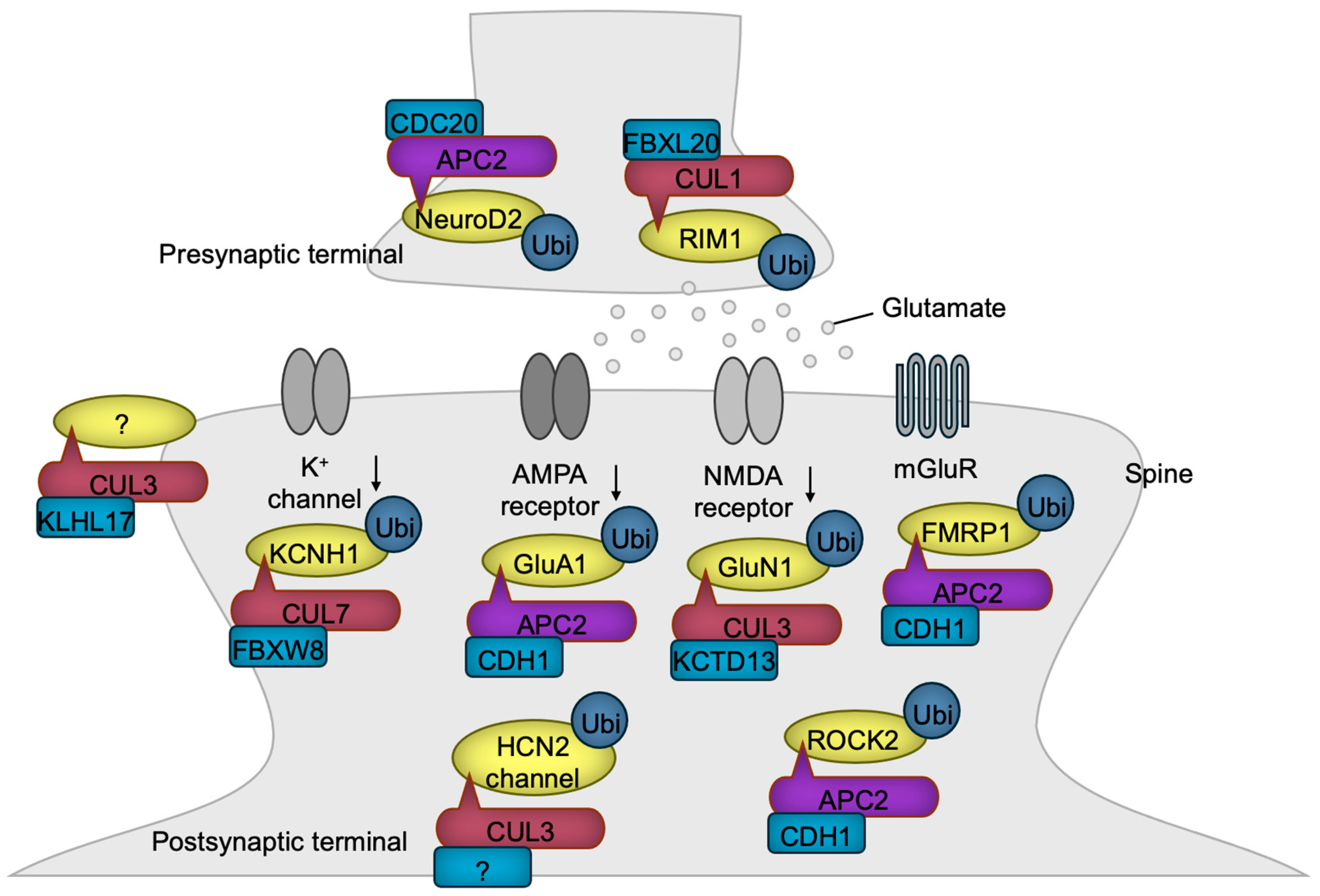
Figure 6.
CRLs in synaptogenesis and synaptic function. CRL1 and APC/C regulate presynaptic terminal functions, while CRL3, CRL7, and APC/C control postsynaptic functions through the ubiquitylation of the indicated substrates. Notably, KLHL17-loaded CRL3 has been shown to promote spine enlargement and enhance synaptic activity, although the specific substrate responsible for this function remains unidentified. The substrate receptor of CUL3 for HCN2 channel has yet to be identified. The black downward arrows denote inactivation.
Presynaptic development is transcriptionally regulated by APC/CCDC20. In this process, the ubiquitination and degradation of the transcription factor NeuroD2 lead to the downregulation of Complexin II expression. Complexin II acts as a negative regulator of presynaptic differentiation, and its downregulation promotes the formation of presynaptic structures [132]. Both the presynaptic active zones and PSDs are supported by scaffolding proteins, the abundance of which is frequently regulated by ubiquitin ligases [133]. For example, FBXL20 (also known as SCRAPPER) localizes at the presynaptic terminal, where it targets RIM1, a Ca2+-sensing synaptic vesicle regulator, for ubiquitination and degradation, ensuring the proper release of glutamate [134].
Glutamate stimulates postsynaptic neurons by activating four types of ion channels—AMPA, kainate, NMDA, and GluD receptors [135]—and the G-protein-coupled metabotropic receptor (mGluR) [136]. Chronic synaptic activity triggers the degradation of the AMPA receptor, a mechanism critical for preventing the toxic hyperactivation of postsynaptic neurons, known as excitotoxicity. APC/CCDH1 plays a pivotal role in the downregulation of the AMPA receptor by targeting the GluA1 (also known as GluR1) subunit for ubiquitination and degradation in response to synaptic stimuli [137]. Periodic synaptic activation induces long-term potentiation (LTP) or long-term depression (LTD) mediated through glutamate receptors, which are the foundations of synaptic plasticity essential for learning, memory, and other processes frequently dysregulated in NDD patients [138]. APC/CCDH1 is involved in mGluR-mediated LTD by synaptic stimulus-dependent ubiquitination of FMRP1, a negative regulator of LTD, leading to its degradation [139]. Accordingly, the loss of CDH1 in excitatory neurons of the mouse brain impairs LTD, resulting in sustained cell surface expression of the AMPA receptor and defective degradation of FMRP1 [139]. The levels of the NMDA receptor are also regulated by CRL3KCTD13 which targets GluN1 for ubiquitination and degradation [140]. In patients with epileptic seizures, the expression of KCTD13 is diminished, likely leading to the heightened activation of excitatory synaptic transmission and an increased susceptibility to epilepsy [140].
Dysfunction of the dopamine system is implicated in the phenotypes associated with NDDs [141,142]. Consistently, mice with a heterozygous Cul3 deletion in DA neurons exhibit hyperactivity of DA neurons accompanied by the behavioral abnormalities such as increased locomotion, impaired working memory, and deficits in sensorimotor gating (please refer to Table 1), all of which were reversed by the forced inactivation of the DA neuron [65]. The hyperexcitability of DA neurons was shown to be caused by the accumulation of HCN2 channels, a substrate of CUL3 [65]. These findings confirm the critical role of elevated DA activity in NDDs.
Potassium channels are essential for maintaining neuronal activity by regulating membrane potential [143]. The potassium channel Kv10.1 (also known as EAG1 or KCNH1), whose gene is mutated in NDDs [144,145], undergoes ubiquitination-mediated degradation, a process driven by CRL7FBXW8 [146]. Consequently, the overexpression of CUL7 diminishes potassium currents, whereas reduced CUL7 expression leads to enhanced potassium currents in non-neuronal cells that exogenously express Kv10.1 [146]. The extent to which these findings can be extrapolated to neuronal function requires further investigation.
The postsynaptic sites in dendrites often form specialized protruded structures known as spines [147]. KLHL17 has been shown to enlarge spines and facilitate synaptic activity. Since this function requires the BTB domain, which is essential for association with CUL3, CRL3KLHL17 likely contributes to this process, although the specific substrate remains to be identified [67]. Dendrites are dynamic structures that must be maintained to ensure the proper functioning of the brain. One destabilizing factor of dendrites is ROCK2, whose levels are regulated by APC/CCDH1 [71]. Therefore, the loss of CDH1 leads to the accumulation of ROCK2, resulting in dendritic spine disruption and learning deficits, which can be rescued by a ROCK inhibitor [71].
8. Therapeutic Implications and Future Directions
The critical roles of Cullin-RING ubiquitin ligases (CRLs) in neurodevelopment and their involvement in NDDs, as summarized in Table 2, highlight the potential for therapeutic targeting. Given that mutations in genes encoding CRL-related proteins have been identified in NDD patients (as discussed in Section 2), modulating ubiquitination pathways represents a promising approach to mitigate disease phenotypes. One potential therapeutic strategy involves the use of small-molecule inhibitors to regulate CRL activity. For instance, MLN4924 (also known as pevonedistat) is a NEDD8-activating enzyme (NAE) inhibitor and disrupts all CRL functions, leading to the stabilization of CRL substrates [148]. MLN4924 has been demonstrated to be brain-permeable [149], and to alleviate ischemic brain injury [150]. Consistently, MLN4924 can prevent neuronal cell death induced by oxidative stress in hippocampal neurons and SH-SY5Y neural cells [151]. Furthermore, MLN4924 enhances the proliferative capacity and inhibits differentiation in corneal stem cells, thereby accelerating corneal epithelial wound healing [152]. These findings suggest the potential of MLN4924 as a therapeutic agent for NDDs associated with impaired neural stem cell proliferation or excessive cell death. MLN4924 has already entered clinical trials for cancer therapy [153], and the results from these trials will provide valuable insights into its adverse effects and clinical tolerability. In addition to a global CRL inhibitor, the targeted inhibition of specific CRLs may offer enhanced therapeutic potential for NDDs, necessitating the development of highly selective inhibitors aimed at specific CRL complexes or substrate receptors.

Table 2.
CRL scaffolds, substrate receptors, their substrates, and the functions of ubiquitination discussed in this article. *, CUL4B does not require substrate receptor to bind to WDR5. NSC, neural stem cell; LTD, long-term depression.
Not all of the CRL-related proteins discussed in this review have been identified as mutated, but the increasing sample sizes may reveal mutations in these genes. In relation, patient-specific genetic and molecular profiling can guide therapeutic interventions for NDDs. Whole-genome, transcriptome, and proteomic analyses could aid in identifying individual CRL-related mutations, enabling the selection of personalized therapeutic strategies. In addition, patient-derived induced pluripotent stem cells (iPSCs) offer an invaluable platform for modeling disease mechanisms and testing novel therapeutics in a personalized context [154].
Despite significant progress in understanding the role of CRLs in neurodevelopment, several key questions remain unanswered. Future research should focus on: (1) identifying substrate specificity; many CRL substrate receptors remain uncharacterized. Understanding their target specificity is essential for developing selective therapeutic interventions: (2) developing selective CRL modulators; current pharmacological tools lack specificity for individual CRL complexes, though CUL-level inhibitors are emerging, as exemplified by DI-1548 and DI-1859 for CUL3 [155], and 33-11 and KH-4-43 for CUL4 [156]. Advances in structure-based drug design may enable the development of highly selective CRL inhibitors or activators [157]: (3) reconciling the conflicting findings; for instance, as shown in Table 1, mice with a whole-body heterozygous knockout of the Cul3 gene do not exhibit an anxiety-like phenotype [62,63], while those with a heterozygous knockout of Cul3 in neural progenitor cells do exhibit an anxiety-like phenotype [64]. Furthermore, the reduction in Cul4b shortens neurite extension in PC12 cells [106], whereas Cul4b knockout does not affect dendritic length in hippocampal neurons [66]. Interestingly, the knockdown of Cul4b in cultured cerebral neurons increases the length of total neurites, axons, and dendrites [103]. Clarifying the factors contributing to these inconsistencies could shed light on the default functions of CRLs and the underlying causes of phenotypic heterogeneity often observed in NDD patients [158,159]. (4) Exploring non-degradative ubiquitination; while ubiquitination is often associated with protein degradation, non-degradative ubiquitin modifications also play critical roles in signaling pathways [160]. Investigating these roles could reveal novel therapeutic targets. (5) Investigating the role of CRLs in human tissues; a major limitation of current neurodevelopmental models is their reliance on rodent models. Human and rodent brains exhibit notable differences, such as fewer outer RGCs [161], and a reduced variety of interneuron cell types [162] in rodents. Human neurons differentiated from patient-derived iPSCs are being utilized to study neuronal functions [163], but these neurons lack the context of the brain’s native environment. Although studying the human brain raises ethical concerns, the development of human brain organoid techniques provides researchers with access to human brain tissue in vitro [164]. Pioneering studies are already revealing key functions of NDD-associated genes in differentiating human neural cells using brain organoids [165,166,167]. The functions of CRLs can similarly be investigated in the context of human brain development, which is crucial for obtaining more disease-relevant insights into CRL functions.
9. Concluding Remarks
The ubiquitination pathway, particularly through the action of CRLs, plays a fundamental role in neurodevelopment and the pathophysiology of NDDs. As our understanding of ubiquitin-mediated neurodevelopmental regulation continues to expand, new opportunities for treating NDDs are likely to emerge, bringing us closer to precision medicine-based interventions for these complex disorders.
Author Contributions
Conceptualization, T.N. and M.N.; investigation, H.A., T.N. and M.N.; writing—original draft preparation, H.A. and T.N.; writing—review and editing, M.N. and T.H.; supervision, T.N.; project administration, T.N.; funding acquisition, T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by KAKENHI (grant number: 23K06367).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors acknowledge Keiichi I. Nakayama, Keiko Nakayama, and Yue Xiong for guiding and supporting for our research on CRLs. We would like to also thank lab members for the productive discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Sun, L.J. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 2009, 33, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.B.; Hristova, V.A.; Weissman, A.M. HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 2012, 125 Pt 3, 531–537. [Google Scholar] [CrossRef]
- Uchida, C.; Kitagawa, M. RING-, HECT-, and RBR-type E3 Ubiquitin Ligases: Involvement in Human Cancer. Curr. Cancer Drug Targets 2016, 16, 157–174. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Damgaard, R.B. The ubiquitin system: From cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef]
- Petroski, M.D.; Deshaies, R.J. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2005, 6, 9–20. [Google Scholar] [CrossRef]
- Harper, J.W.; Schulman, B.A. Cullin-RING Ubiquitin Ligase Regulatory Circuits: A Quarter Century Beyond the F-Box Hypothesis. Annu. Rev. Biochem. 2021, 90, 403–429. [Google Scholar] [CrossRef]
- Skaar, J.R.; Pagan, J.K.; Pagano, M. Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 369–381. [Google Scholar] [CrossRef]
- Jackson, S.; Xiong, Y. CRL4s: The CUL4-RING E3 ubiquitin ligases. Trends Biochem. Sci. 2009, 34, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakagawa, T. CUL4-Based Ubiquitin Ligases in Chromatin Regulation: An Evolutionary Perspective. Cells 2025, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Hopf, L.V.M.; Baek, K.; Klügel, M.; von Gronau, S.; Xiong, Y.; Schulman, B.A. Structure of CRL7(FBXW8) reveals coupling with CUL1-RBX1/ROC1 for multi-cullin-RING E3-catalyzed ubiquitin ligation. Nat. Struct. Mol. Biol. 2022, 29, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ghetko, D.; Hopf, L.V.M.; Tripathi-Giesgen, I.; Du, J.; Kostrhon, S.; Vu, D.T.; Beier, V.; Steigenberger, B.; Prabu, J.R.; Stier, L.; et al. Noncanonical assembly, neddylation and chimeric cullin-RING/RBR ubiquitylation by the 1.8 MDa CUL9 E3 ligase complex. Nat. Struct. Mol. Biol. 2024, 31, 1083–1094. [Google Scholar] [CrossRef]
- Kamura, T.; Maenaka, K.; Kotoshiba, S.; Matsumoto, M.; Kohda, D.; Conaway, R.C.; Conaway, J.W.; Nakayama, K.I. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes. Dev. 2004, 18, 3055–3065. [Google Scholar] [CrossRef]
- Yamano, H. APC/C: Current understanding and future perspectives. F1000Research 2019, 8, 725. [Google Scholar] [CrossRef]
- Fuchsberger, T.; Lloret, A.; Viña, J. New Functions of APC/C Ubiquitin Ligase in the Nervous System and Its Role in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1057. [Google Scholar] [CrossRef]
- Banerjee-Basu, S.; Packer, A. SFARI Gene: An evolving database for the autism research community. Dis. Model Mech. 2010, 3, 133–135. [Google Scholar]
- Gentile, J.K.; Tan, W.H.; Horowitz, L.T.; Bacino, C.A.; Skinner, S.A.; Barbieri-Welge, R.; Bauer-Carlin, A.; Beaudet, A.L.; Bichell, T.J.; Lee, H.S.; et al. A neurodevelopmental survey of Angelman syndrome with genotype-phenotype correlations. J. Dev. Behav. Pediatr. 2010, 31, 592–601. [Google Scholar] [CrossRef]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Calì, F. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraj, Y.; Taha, R.Z.; Al-Dous, E.; Ahram, D.; Abbasi, S.; Abuazab, E.; Shaath, H.; Habbab, W.; Errafii, K.; Bejaoui, Y.; et al. The genetic landscape of autism spectrum disorder in the Middle Eastern population. Front. Genet. 2024, 15, 1363849. [Google Scholar] [CrossRef] [PubMed]
- Ebstein, F.; Küry, S.; Papendorf, J.J.; Krüger, E. Neurodevelopmental Disorders (NDD) Caused by Genomic Alterations of the Ubiquitin-Proteasome System (UPS): The Possible Contribution of Immune Dysregulation to Disease Pathogenesis. Front. Mol. Neurosci. 2021, 14, 733012. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.J.; Tomé, D.; Almeida, R.D. The Ubiquitinated Axon: Local Control of Axon Development and Function by Ubiquitin. J. Neurosci. 2021, 41, 2796–2813. [Google Scholar] [CrossRef]
- Ambrozkiewicz, M.C.; Lorenz, S. Understanding ubiquitination in neurodevelopment by integrating insights across space and time. Nat. Struct. Mol. Biol. 2025, 32, 14–22. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Vives, L.; Fu, W.; Egertson, J.D.; Stanaway, I.B.; Phelps, I.G.; Carvill, G.; Kumar, A.; Lee, C.; Ankenman, K.; et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 2012, 338, 1619–1622. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Raymond, F.L.; O’Meara, S.; Edkins, S.; Teague, J.; Butler, A.; Dicks, E.; Stevens, C.; Tofts, C.; Avis, T.; et al. Mutations in CUL4B, which encodes a ubiquitin E3 ligase subunit, cause an X-linked mental retardation syndrome associated with aggressive outbursts, seizures, relative macrocephaly, central obesity, hypogonadism, pes cavus, and tremor. Am. J. Hum. Genet. 2007, 80, 345–352. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Chen, B.; Zhang, X.; Guo, C.; Zhou, H.; Li, J.; Gao, G.; Guo, Y.; Yan, C.; et al. Mutation in CUL4B, which encodes a member of cullin-RING ubiquitin ligase complex, causes X-linked mental retardation. Am. J. Hum. Genet. 2007, 80, 561–566. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Vives, L.; Girirajan, S.; Karakoc, E.; Krumm, N.; Coe, B.P.; Levy, R.; Ko, A.; Lee, C.; Smith, J.D.; et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012, 485, 246–250. [Google Scholar] [CrossRef]
- Gregor, A.; Sadleir, L.G.; Asadollahi, R.; Azzarello-Burri, S.; Battaglia, A.; Ousager, L.B.; Boonsawat, P.; Bruel, A.L.; Buchert, R.; Calpena, E.; et al. De Novo Variants in the F-Box Protein FBXO11 in 20 Individuals with a Variable Neurodevelopmental Disorder. Am. J. Hum. Genet. 2018, 103, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Meerbrei, T.; Gerstner, T.; Toutain, A.; Lynch, S.A.; Stals, K.; Maxton, C.; Lemke, J.R.; Bernat, J.A.; Bombei, H.M.; et al. De novo missense variants in FBXO11 alter its protein expression and subcellular localization. Hum. Mol. Genet. 2022, 31, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.; Myers, C.T.; Muir, A.M.; Calvert, S.; Basinger, A.; Perry, M.S.; Rodan, L.; Helbig, K.L.; Chambers, C.; Gorman, K.M.; et al. FBXO28 causes developmental and epileptic encephalopathy with profound intellectual disability. Epilepsia 2021, 62, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.; Sritharan, K.; Mittal, K.; Vasli, N.; Araujo, C.; Jamil, T.; Rafiq, M.A.; Anwar, Z.; Mikhailov, A.; Rauf, S.; et al. Truncation of the E3 ubiquitin ligase component FBXO31 causes non-syndromic autosomal recessive intellectual disability in a Pakistani family. Hum. Genet. 2014, 133, 975–984. [Google Scholar] [CrossRef]
- Harripaul, R.; Vasli, N.; Mikhailov, A.; Rafiq, M.A.; Mittal, K.; Windpassinger, C.; Sheikh, T.I.; Noor, A.; Mahmood, H.; Downey, S.; et al. Mapping autosomal recessive intellectual disability: Combined microarray and exome sequencing identifies 26 novel candidate genes in 192 consanguineous families. Mol. Psychiatry 2018, 23, 973–984. [Google Scholar] [CrossRef]
- Ansar, M.; Paracha, S.A.; Serretti, A.; Sarwar, M.T.; Khan, J.; Ranza, E.; Falconnet, E.; Iwaszkiewicz, J.; Shah, S.F.; Qaisar, A.A.; et al. Biallelic variants in FBXL3 cause intellectual disability, delayed motor development and short stature. Hum. Mol. Genet. 2019, 28, 972–979. [Google Scholar] [CrossRef]
- Bonnen, P.E.; Yarham, J.W.; Besse, A.; Wu, P.; Faqeih, E.A.; Al-Asmari, A.M.; Saleh, M.A.; Eyaid, W.; Hadeel, A.; He, L.; et al. Mutations in FBXL4 cause mitochondrial encephalopathy and a disorder of mitochondrial DNA maintenance. Am. J. Hum. Genet. 2013, 93, 471–481. [Google Scholar] [CrossRef]
- Gai, X.; Ghezzi, D.; Johnson, M.A.; Biagosch, C.A.; Shamseldin, H.E.; Haack, T.B.; Reyes, A.; Tsukikawa, M.; Sheldon, C.A.; Srinivasan, S.; et al. Mutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathy. Am. J. Hum. Genet. 2013, 93, 482–495. [Google Scholar] [CrossRef]
- Charng, W.L.; Karaca, E.; Coban Akdemir, Z.; Gambin, T.; Atik, M.M.; Gu, S.; Posey, J.E.; Jhangiani, S.N.; Muzny, D.M.; Doddapaneni, H.; et al. Exome sequencing in mostly consanguineous Arab families with neurologic disease provides a high potential molecular diagnosis rate. BMC Med. Genomics 2016, 9, 42. [Google Scholar] [CrossRef]
- Ruzzo, E.K.; Pérez-Cano, L.; Jung, J.Y.; Wang, L.K.; Kashef-Haghighi, D.; Hartl, C.; Singh, C.; Xu, J.; Hoekstra, J.N.; Leventhal, O.; et al. Inherited and De Novo Genetic Risk for Autism Impacts Shared Networks. Cell 2019, 178, 850–866.e26. [Google Scholar] [CrossRef]
- Holt, R.J.; Young, R.M.; Crespo, B.; Ceroni, F.; Curry, C.J.; Bellacchio, E.; Bax, D.A.; Ciolfi, A.; Simon, M.; Fagerberg, C.R.; et al. De Novo Missense Variants in FBXW11 Cause Diverse Developmental Phenotypes Including Brain, Eye, and Digit Anomalies. Am. J. Hum. Genet. 2019, 105, 640–657. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.K.; Zhang, P.; Urresti, J.; Amar, M.; Pramod, A.B.; Chen, J.; Thomas, A.; Corominas, R.; Lin, G.N.; Iakoucheva, L.M. Full-length isoform transcriptome of the developing human brain provides further insights into autism. Cell Rep. 2021, 36, 109631. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.E.M.; Costain, G.; Blok, L.E.R.; Silk, M.A.; Nguyen, T.B.; Dong, X.; Alhuzaimi, D.E.; Dowling, J.J.; Walker, S.; Amburgey, K.; et al. Germline variants in tumor suppressor FBXW7 lead to impaired ubiquitination and a neurodevelopmental syndrome. Am. J. Hum. Genet. 2022, 109, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Meier-Abt, F.; Kraemer, D.; Braun, N.; Reinehr, M.; Stutz-Grunder, E.; Steindl, K.; Rauch, A. Further evidence that the neurodevelopmental gene FBXW7 predisposes to Wilms tumor. Am. J. Med. Genet. A 2024, 194, e63528. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Li, H.; Dai, Y.; Wang, X.; Liu, L. Case report: A novel FBXW7 gene variant causes global developmental delay. Front. Genet. 2024, 15, 1436462. [Google Scholar] [CrossRef]
- Mignon-Ravix, C.; Cacciagli, P.; Choucair, N.; Popovici, C.; Missirian, C.; Milh, M.; Mégarbané, A.; Busa, T.; Julia, S.; Girard, N.; et al. Intragenic rearrangements in X-linked intellectual deficiency: Results of a-CGH in a series of 54 patients and identification of TRPC5 and KLHL15 as potential XLID genes. Am. J. Med. Genet. A 2014, 164A, 1991–1997. [Google Scholar] [CrossRef]
- Sleyp, Y.; Valenzuela, I.; Accogli, A.; Ballon, K.; Ben-Zeev, B.; Berkovic, S.F.; Broly, M.; Callaerts, P.; Caylor, R.C.; Charles, P.; et al. De novo missense variants in the E3 ubiquitin ligase adaptor KLHL20 cause a developmental disorder with intellectual disability, epilepsy, and autism spectrum disorder. Genet. Med. 2022, 24, 2464–2474. [Google Scholar] [CrossRef]
- Mastrangelo, M.; Sartori, S.; Simonati, A.; Brinciotti, M.; Moro, F.; Nosadini, M.; Pezzini, F.; Doccini, S.; Santorelli, F.M.; Leuzzi, V. Progressive myoclonus epilepsy and ceroidolipofuscinosis 14: The multifaceted phenotypic spectrum of KCTD7-related disorders. Eur. J. Med. Genet. 2019, 62, 103591. [Google Scholar] [CrossRef]
- Golzio, C.; Willer, J.; Talkowski, M.E.; Oh, E.C.; Taniguchi, Y.; Jacquemont, S.; Reymond, A.; Sun, M.; Sawa, A.; Gusella, J.F.; et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 2012, 485, 363–367. [Google Scholar] [CrossRef]
- Clothier, J.L.; Grooms, A.N.; Porter-Gill, P.A.; Gill, P.S.; Schaefer, G.B. Identification of DCAF1 by Clinical Exome Sequencing and Methylation Analysis as a Candidate Gene for Autism and Intellectual Disability: A Case Report. J. Pers. Med. 2022, 12, 886. [Google Scholar] [CrossRef]
- Webster, E.; Cho, M.T.; Alexander, N.; Desai, S.; Naidu, S.; Bekheirnia, M.R.; Lewis, A.; Retterer, K.; Juusola, J.; Chung, W.K. De novo PHIP-predicted deleterious variants are associated with developmental delay, intellectual disability, obesity, and dysmorphic features. Cold Spring Harb. Mol. Case Stud. 2016, 2, a001172. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Hoischen, A.; Coe, B.P.; Carvill, G.L.; Van Esch, H.; Bosch, D.G.M.; Andersen, U.A.; Baker, C.; Bauters, M.; Bernier, R.A.; et al. A genotype-first approach identifies an intellectual disability-overweight syndrome caused by PHIP haploinsufficiency. Eur. J. Hum. Genet. 2018, 26, 54–63. [Google Scholar] [CrossRef]
- Glessner, J.T.; Wang, K.; Cai, G.; Korvatska, O.; Kim, C.E.; Wood, S.; Zhang, H.; Estes, A.; Brune, C.W.; Bradfield, J.P.; et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009, 459, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.J.; Pucilowska, J.; Lombardi, R.Q.; Rooney, J.P. A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology 2004, 63, 1927–1931. [Google Scholar] [CrossRef]
- Sheereen, A.; Alaamery, M.; Bawazeer, S.; Al Yafee, Y.; Massadeh, S.; Eyaid, W. A missense mutation in the CRBN gene that segregates with intellectual disability and self-mutilating behaviour in a consanguineous Saudi family. J. Med. Genet. 2017, 54, 236–240. [Google Scholar] [CrossRef]
- Rodríguez, C.; Sánchez-Morán, I.; Álvarez, S.; Tirado, P.; Fernández-Mayoralas, D.M.; Calleja-Pérez, B.; Almeida, Á.; Fernández-Jaén, A. A novel human Cdh1 mutation impairs anaphase promoting complex/cyclosome activity resulting in microcephaly, psychomotor retardation, and epilepsy. J. Neurochem. 2019, 151, 103–115. [Google Scholar] [CrossRef]
- Gonzalez-Sulser, A. Rodent genetic models of neurodevelopmental disorders and epilepsy. Eur. J. Paediatr. Neurol. 2020, 24, 66–69. [Google Scholar] [CrossRef]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering animal models used to study autism spectrum disorder: Current state and optimizing future. Genes. Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef]
- Damianidou, E.; Mouratidou, L.; Kyrousi, C. Research models of neurodevelopmental disorders: The right model in the right place. Front. Neurosci. 2022, 16, 1031075. [Google Scholar] [CrossRef]
- Takao, K.; Yamasaki, N.; Miyakawa, T. Impact of brain-behavior phenotypying of genetically-engineered mice on research of neuropsychiatric disorders. Neurosci. Res. 2007, 58, 124–132. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, D.; Sun, H.; Qu, Y.; Su, X. Behavioral tests for evaluating the characteristics of brain diseases in rodent models: Optimal choices for improved outcomes (Review). Mol. Med. Rep. 2022, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Amar, M.; Pramod, A.B.; Yu, N.K.; Herrera, V.M.; Qiu, L.R.; Moran-Losada, P.; Zhang, P.; Trujillo, C.A.; Ellegood, J.; Urresti, J.; et al. Autism-linked Cullin3 germline haploinsufficiency impacts cytoskeletal dynamics and cortical neurogenesis through RhoA signaling. Mol. Psychiatry 2021, 26, 3586–3613. [Google Scholar] [CrossRef]
- Morandell, J.; Schwarz, L.A.; Basilico, B.; Tasciyan, S.; Dimchev, G.; Nicolas, A.; Sommer, C.; Kreuzinger, C.; Dotter, C.P.; Knaus, L.S.; et al. Cul3 regulates cytoskeleton protein homeostasis and cell migration during a critical window of brain development. Nat. Commun. 2021, 12, 3058. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Chen, C.; Wang, H.; Cui, W.; Tan, Z.; Robinson, H.; Gao, N.; Luo, B.; Zhang, L.; et al. CUL3 Deficiency Causes Social Deficits and Anxiety-like Behaviors by Impairing Excitation-Inhibition Balance through the Promotion of Cap-Dependent Translation. Neuron 2020, 105, 475–490.e6. [Google Scholar] [CrossRef]
- Gao, N.; Liu, Z.; Wang, H.; Shen, C.; Dong, Z.; Cui, W.; Xiong, W.C.; Mei, L. Deficiency of Cullin 3, a Protein Encoded by a Schizophrenia and Autism Risk Gene, Impairs Behaviors by Enhancing the Excitability of Ventral Tegmental Area (VTA) DA Neurons. J. Neurosci. 2023, 43, 6249–6267. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsai, M.S.; Lin, C.Y.; Yu, I.S.; Chen, Y.T.; Lin, S.R.; Juan, L.W.; Hsu, H.M.; Lee, L.J.; Lin, S.W. Rescue of the genetically engineered Cul4b mutant mouse as a potential model for human X-linked mental retardation. Hum. Mol. Genet. 2012, 21, 4270–4285. [Google Scholar] [CrossRef]
- Hu, H.T.; Huang, T.N.; Hsueh, Y.P. KLHL17/Actinfilin, a brain-specific gene associated with infantile spasms and autism, regulates dendritic spine enlargement. J. Biomed. Sci. 2020, 27, 103. [Google Scholar] [CrossRef]
- Arbogast, T.; Razaz, P.; Ellegood, J.; McKinstry, S.U.; Erdin, S.; Currall, B.; Aneichyk, T.; Lerch, J.P.; Qiu, L.R.; Rodriguiz, R.M.; et al. Kctd13-deficient mice display short-term memory impairment and sex-dependent genetic interactions. Hum. Mol. Genet. 2019, 28, 1474–1486. [Google Scholar] [CrossRef]
- Rajadhyaksha, A.M.; Ra, S.; Kishinevsky, S.; Lee, A.S.; Romanienko, P.; DuBoff, M.; Yang, C.; Zupan, B.; Byrne, M.; Daruwalla, Z.R.; et al. Behavioral characterization of cereblon forebrain-specific conditional null mice: A model for human non-syndromic intellectual disability. Behav. Brain Res. 2012, 226, 428–434. [Google Scholar] [CrossRef]
- Li, M.; Shin, Y.H.; Hou, L.; Huang, X.; Wei, Z.; Klann, E.; Zhang, P. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat. Cell Biol. 2008, 10, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Bobo-Jiménez, V.; Delgado-Esteban, M.; Angibaud, J.; Sánchez-Morán, I.; de la Fuente, A.; Yajeya, J.; Nägerl, U.V.; Castillo, J.; Bolaños, J.P.; Almeida, A. APC/C(Cdh1)-Rock2 pathway controls dendritic integrity and memory. Proc. Natl. Acad. Sci. USA 2017, 114, 4513–4518. [Google Scholar] [CrossRef] [PubMed]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Petit, A.C.; Lledo, P.M. The impact of adult neurogenesis on affective functions: Of mice and men. Mol. Psychiatry 2024, 29, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Florio, M.; Huttner, W.B. Neural progenitors, neurogenesis and the evolution of the neocortex. Development 2014, 141, 2182–2194. [Google Scholar] [CrossRef]
- Taverna, E.; Götz, M.; Huttner, W.B. The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 2014, 30, 465–502. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, H.; Ming, G.L. Genetics of human brain development. Nat. Rev. Genet. 2024, 25, 26–45. [Google Scholar] [CrossRef]
- Pines, J. Cubism and the cell cycle: The many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 2011, 12, 427–438. [Google Scholar] [CrossRef]
- Delgado-Esteban, M.; García-Higuera, I.; Maestre, C.; Moreno, S.; Almeida, A. APC/C-Cdh1 coordinates neurogenesis and cortical size during development. Nat. Commun. 2013, 4, 2879. [Google Scholar] [CrossRef]
- Capecchi, M.R.; Pozner, A. ASPM regulates symmetric stem cell division by tuning Cyclin E ubiquitination. Nat. Commun. 2015, 6, 8763. [Google Scholar] [CrossRef]
- Matsumoto, A.; Onoyama, I.; Sunabori, T.; Kageyama, R.; Okano, H.; Nakayama, K.I. Fbxw7-dependent degradation of Notch is required for control of “stemness” and neuronal-glial differentiation in neural stem cells. J. Biol. Chem. 2011, 286, 13754–13764. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.P.; Zhang, Y.; Wang, C.; Yuan, F.; Li, H.; Yao, Y.; Chen, Y.; Li, C.; Wei, W.; Liu, C.H.; et al. Dynamic ubiquitylation of Sox2 regulates proteostasis and governs neural progenitor cell differentiation. Nat. Commun. 2018, 9, 4648. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Scott, S.; Hampshire, D.J.; Springell, K.; Corry, P.; Abramowicz, M.J.; Mochida, G.H.; Hennekam, R.C.; Maher, E.R.; Fryns, J.P.; et al. Protein-truncating mutations in ASPM cause variable reduction in brain size. Am. J. Hum. Genet. 2003, 73, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Létard, P.; Drunat, S.; Vial, Y.; Duerinckx, S.; Ernault, A.; Amram, D.; Arpin, S.; Bertoli, M.; Busa, T.; Ceulemans, B.; et al. Autosomal recessive primary microcephaly due to ASPM mutations: An update. Hum. Mutat. 2018, 39, 319–332. [Google Scholar] [CrossRef]
- Arimura, N.; Kaibuchi, K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007, 8, 194–205. [Google Scholar] [CrossRef]
- Yogev, S.; Shen, K. Establishing Neuronal Polarity with Environmental and Intrinsic Mechanisms. Neuron 2017, 96, 638–650. [Google Scholar] [CrossRef]
- Jung, M.; Kim, D.; Mun, J.Y. Direct Visualization of Actin Filaments and Actin-Binding Proteins in Neuronal Cells. Front. Cell Dev. Biol. 2020, 8, 588556. [Google Scholar] [CrossRef]
- Meka, D.P.; Kobler, O.; Hong, S.; Friedrich, C.M.; Wuesthoff, S.; Henis, M.; Schwanke, B.; Krisp, C.; Schmuelling, N.; Rueter, R.; et al. Centrosome-dependent microtubule modifications set the conditions for axon formation. Cell Rep. 2022, 39, 110686. [Google Scholar] [CrossRef]
- Yoshimura, T.; Arimura, N.; Kaibuchi, K. Signaling networks in neuronal polarization. J. Neurosci. 2006, 26, 10626–10630. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.J.; Martinez, J.A.; Zochodne, D.W. Disruption of E3 ligase NEDD4 in peripheral neurons interrupts axon outgrowth: Linkage to PTEN. Mol. Cell Neurosci. 2012, 50, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Drinjakovic, J.; Jung, H.; Campbell, D.S.; Strochlic, L.; Dwivedy, A.; Holt, C.E. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 2010, 65, 341–357. [Google Scholar] [CrossRef]
- Hsia, H.E.; Kumar, R.; Luca, R.; Takeda, M.; Courchet, J.; Nakashima, J.; Wu, S.; Goebbels, S.; An, W.; Eickholt, B.J.; et al. Ubiquitin E3 ligase Nedd4-1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth. Proc. Natl. Acad. Sci. USA 2014, 111, 13205–13210. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Jiang, K.; Chen, B.; Wang, K.; Lao, L.; Hou, C.; Wang, F.; Zhang, C.; Shen, H. MicroRNA-300 Regulates the Ubiquitination of PTEN through the CRL4B(DCAF13) E3 Ligase in Osteosarcoma Cells. Mol. Ther. Nucleic Acids 2018, 10, 254–268. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.L.; Zhao, L.W.; Pi, S.B.; Zhang, S.Y.; Tong, C.; Fan, H.Y. The CRL4-DCAF13 ubiquitin E3 ligase supports oocyte meiotic resumption by targeting PTEN degradation. Cell Mol. Life Sci. 2020, 77, 2181–2197. [Google Scholar] [CrossRef]
- Ge, M.K.; Zhang, N.; Xia, L.; Zhang, C.; Dong, S.S.; Li, Z.M.; Ji, Y.; Zheng, M.H.; Sun, J.; Chen, G.Q.; et al. FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat. Commun. 2020, 11, 1720. [Google Scholar] [CrossRef]
- Dupraz, S.; Hilton, B.J.; Husch, A.; Santos, T.E.; Coles, C.H.; Stern, S.; Brakebusch, C.; Bradke, F. RhoA Controls Axon Extension Independent of Specification in the Developing Brain. Curr. Biol. 2019, 29, 3874–3886.e9. [Google Scholar] [CrossRef]
- Kannan, M.; Lee, S.J.; Schwedhelm-Domeyer, N.; Stegmüller, J. The E3 ligase Cdh1-anaphase promoting complex operates upstream of the E3 ligase Smurf1 in the control of axon growth. Development 2012, 139, 3600–3612. [Google Scholar] [CrossRef]
- Lin, M.Y.; Lin, Y.M.; Kao, T.C.; Chuang, H.H.; Chen, R.H. PDZ-RhoGEF ubiquitination by Cullin3-KLHL20 controls neurotrophin-induced neurite outgrowth. J. Cell Biol. 2011, 193, 985–994. [Google Scholar] [CrossRef]
- Dogterom, M.; Koenderink, G.H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Merrill, R.A.; Usachev, A.Y.; Strack, S. The X-linked intellectual disability gene product and E3 ubiquitin ligase KLHL15 degrades doublecortin proteins to constrain neuronal dendritogenesis. J. Biol. Chem. 2021, 296, 100082. [Google Scholar] [CrossRef] [PubMed]
- Shim, T.; Kim, J.Y.; Kim, W.; Lee, Y.I.; Cho, B.; Moon, C. Cullin-RING E3 ubiquitin ligase 4 regulates neurite morphogenesis during neurodevelopment. iScience 2024, 27, 108933. [Google Scholar] [CrossRef] [PubMed]
- Stegmüller, J.; Konishi, Y.; Huynh, M.A.; Yuan, Z.; Dibacco, S.; Bonni, A. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron 2006, 50, 389–400. [Google Scholar] [CrossRef]
- Lasorella, A.; Stegmüller, J.; Guardavaccaro, D.; Liu, G.; Carro, M.S.; Rothschild, G.; de la Torre-Ubieta, L.; Pagano, M.; Bonni, A.; Iavarone, A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 2006, 442, 471–474. [Google Scholar] [CrossRef]
- Nakagawa, T.; Xiong, Y. X-linked mental retardation gene CUL4B targets ubiquitylation of H3K4 methyltransferase component WDR5 and regulates neuronal gene expression. Mol. Cell 2011, 43, 381–391. [Google Scholar] [CrossRef]
- Nakagawa, T.; Xiong, Y. Chromatin regulation by CRL4 E3 ubiquitin ligases: CUL4B targets WDR5 ubiquitylation in the nucleus. Cell Cycle 2011, 10, 4197–4198. [Google Scholar]
- Shilatifard, A. The COMPASS family of histone H3K4 methylases: Mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 2012, 81, 65–95. [Google Scholar] [CrossRef]
- Green, E.M.; Gozani, O. CUL4B: Trash talking at chromatin. Mol. Cell 2011, 43, 321–323. [Google Scholar] [CrossRef][Green Version]
- des Portes, V.; Pinard, J.M.; Billuart, P.; Vinet, M.C.; Koulakoff, A.; Carrié, A.; Gelot, A.; Dupuis, E.; Motte, J.; Berwald-Netter, Y.; et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 1998, 92, 51–61. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Allen, K.M.; Fox, J.W.; Lamperti, E.D.; Berkovic, S.; Scheffer, I.; Cooper, E.C.; Dobyns, W.B.; Minnerath, S.R.; Ross, M.E.; et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 1998, 92, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Dasouki, M.J.; Zhou, X.P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Broix, L.; Nguyen, L. Cerebral cortex development: An outside-in perspective. FEBS Lett. 2017, 591, 3978–3992. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Joly-Amado, A.; Kulkarni, N.; Nash, K.R. Reelin Signaling in Neurodevelopmental Disorders and Neurodegenerative Diseases. Brain Sci. 2023, 13, 1479. [Google Scholar] [CrossRef]
- Gao, Z.; Godbout, R. Reelin-Disabled-1 signaling in neuronal migration: Splicing takes the stage. Cell Mol. Life Sci. 2013, 70, 2319–2329. [Google Scholar] [CrossRef]
- Feng, L.; Allen, N.S.; Simo, S.; Cooper, J.A. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes. Dev. 2007, 21, 2717–2730. [Google Scholar] [CrossRef]
- Stier, A.; Gilberto, S.; Mohamed, W.I.; Royall, L.N.; Helenius, J.; Mikicic, I.; Sajic, T.; Beli, P.; Müller, D.J.; Jessberger, S.; et al. The CUL4B-based E3 ubiquitin ligase regulates mitosis and brain development by recruiting phospho-specific DCAFs. EMBO J. 2023, 42, e112847. [Google Scholar] [CrossRef]
- Butts, T.; Green, M.J.; Wingate, R.J. Development of the cerebellum: Simple steps to make a ‘little brain’. Development 2014, 141, 4031–4041. [Google Scholar] [CrossRef]
- Cossart, R.; Khazipov, R. How development sculpts hippocampal circuits and function. Physiol. Rev. 2022, 102, 343–378. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Holubowska, A.; Schwedhelm-Domeyer, N.; Mitkovski, M.; Lee, S.J.; Kannan, M.; Matz, A.; Vadhvani, M.; Stegmüller, J. Loss of the neuron-specific F-box protein FBXO41 models an ataxia-like phenotype in mice with neuronal migration defects and degeneration in the cerebellum. J. Neurosci. 2015, 35, 8701–8717. [Google Scholar] [CrossRef] [PubMed]
- Quadros, A.; Arazola, R.D.; Álvarez, A.R.; Pires, J.; Meredith, R.M.; Saarloos, I.; Verhage, M.; Toonen, R.F. Neuronal F-Box protein FBXO41 regulates synaptic transmission and hippocampal network maturation. iScience 2022, 25, 104069. [Google Scholar] [CrossRef] [PubMed]
- King, C.R.; AR, A.A.Q.; Chazeau, A.; Saarloos, I.; van der Graaf, A.J.; Verhage, M.; Toonen, R.F. Fbxo41 Promotes Disassembly of Neuronal Primary Cilia. Sci. Rep. 2019, 9, 8179. [Google Scholar] [CrossRef]
- Persico, A.M.; D’Agruma, L.; Maiorano, N.; Totaro, A.; Militerni, R.; Bravaccio, C.; Wassink, T.H.; Schneider, C.; Melmed, R.; Trillo, S.; et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol. Psychiatry 2001, 6, 150–159. [Google Scholar] [CrossRef]
- Skaar, D.A.; Shao, Y.; Haines, J.L.; Stenger, J.E.; Jaworski, J.; Martin, E.R.; DeLong, G.R.; Moore, J.H.; McCauley, J.L.; Sutcliffe, J.S.; et al. Analysis of the RELN gene as a genetic risk factor for autism. Mol. Psychiatry 2005, 10, 563–571. [Google Scholar] [CrossRef]
- Nawa, Y.; Kimura, H.; Mori, D.; Kato, H.; Toyama, M.; Furuta, S.; Yu, Y.; Ishizuka, K.; Kushima, I.; Aleksic, B.; et al. Rare single-nucleotide DAB1 variants and their contribution to Schizophrenia and autism spectrum disorder susceptibility. Hum. Genome Var. 2020, 7, 37. [Google Scholar] [CrossRef]
- Saillour, Y.; Carion, N.; Quelin, C.; Leger, P.L.; Boddaert, N.; Elie, C.; Toutain, A.; Mercier, S.; Barthez, M.A.; Milh, M.; et al. LIS1-related isolated lissencephaly: Spectrum of mutations and relationships with malformation severity. Arch. Neurol. 2009, 66, 1007–1015. [Google Scholar] [CrossRef]
- Südhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Qi, C.; Luo, L.D.; Feng, I.; Ma, S. Molecular mechanisms of synaptogenesis. Front. Synaptic Neurosci. 2022, 14, 939793. [Google Scholar] [CrossRef]
- Kaizuka, T.; Takumi, T. Postsynaptic density proteins and their involvement in neurodevelopmental disorders. J. Biochem. 2018, 163, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, A.H.; Yamada, T.; Wu, B.; Bilimoria, P.M.; Ikeuchi, Y.; de la Iglesia, N.; Shen, J.; Bonni, A. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science 2009, 326, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, H.; Stegmüller, J. The role of E3 ubiquitin ligases in synapse function in the healthy and diseased brain. Mol. Cell Neurosci. 2021, 112, 103602. [Google Scholar] [CrossRef] [PubMed]
- Yao, I.; Takagi, H.; Ageta, H.; Kahyo, T.; Sato, S.; Hatanaka, K.; Fukuda, Y.; Chiba, T.; Morone, N.; Yuasa, S.; et al. SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell 2007, 130, 943–957. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Fu, A.K.; Hung, K.W.; Fu, W.Y.; Shen, C.; Chen, Y.; Xia, J.; Lai, K.O.; Ip, N.Y. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat. Neurosci. 2011, 14, 181–189. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Huang, J.; Ikeuchi, Y.; Malumbres, M.; Bonni, A. A Cdh1-APC/FMRP Ubiquitin Signaling Link Drives mGluR-Dependent Synaptic Plasticity in the Mammalian Brain. Neuron 2015, 86, 726–739. [Google Scholar] [CrossRef]
- Gu, J.; Ke, P.; Guo, H.; Liu, J.; Liu, Y.; Tian, X.; Huang, Z.; Xu, X.; Xu, D.; Ma, Y.; et al. KCTD13-mediated ubiquitination and degradation of GluN1 regulates excitatory synaptic transmission and seizure susceptibility. Cell Death Differ. 2023, 30, 1726–1741. [Google Scholar] [CrossRef]
- Mandic-Maravic, V.; Grujicic, R.; Milutinovic, L.; Munjiza-Jovanovic, A.; Pejovic-Milovancevic, M. Dopamine in Autism Spectrum Disorders-Focus on D2/D3 Partial Agonists and Their Possible Use in Treatment. Front. Psychiatry 2021, 12, 787097. [Google Scholar] [CrossRef] [PubMed]
- Pavăl, D. The dopamine hypothesis of autism spectrum disorder: A comprehensive analysis of the evidence. Int. Rev. Neurobiol. 2023, 173, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Maljevic, S.; Lerche, H. Potassium channels: A review of broadening therapeutic possibilities for neurological diseases. J. Neurol. 2013, 260, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.; Rash, L.D.; Crawford, J.; Ma, L.; Cristofori-Armstrong, B.; Miller, D.; Ru, K.; Baillie, G.J.; Alanay, Y.; Jacquinet, A.; et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat. Genet. 2015, 47, 73–77. [Google Scholar] [CrossRef]
- Kortüm, F.; Caputo, V.; Bauer, C.K.; Stella, L.; Ciolfi, A.; Alawi, M.; Bocchinfuso, G.; Flex, E.; Paolacci, S.; Dentici, M.L.; et al. Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat. Genet. 2015, 47, 661–667. [Google Scholar] [CrossRef]
- Hsu, P.H.; Ma, Y.T.; Fang, Y.C.; Huang, J.J.; Gan, Y.L.; Chang, P.T.; Jow, G.M.; Tang, C.Y.; Jeng, C.J. Cullin 7 mediates proteasomal and lysosomal degradations of rat Eag1 potassium channels. Sci. Rep. 2017, 7, 40825. [Google Scholar] [CrossRef]
- Rochefort, N.L.; Konnerth, A. Dendritic spines: From structure to in vivo function. EMBO Rep. 2012, 13, 699–708. [Google Scholar] [CrossRef]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef]
- Reihe, C.A.; Pekas, N.; Wu, P.; Wang, X. Systemic inhibition of neddylation by 3-day MLN4924 treatment regime does not impair autophagic flux in mouse hearts and brains. Am. J. Cardiovasc. Dis. 2017, 7, 134–150. [Google Scholar]
- Yu, H.; Luo, H.; Chang, L.; Wang, S.; Geng, X.; Kang, L.; Zhong, Y.; Cao, Y.; Wang, R.; Yang, X.; et al. The NEDD8-activating enzyme inhibitor MLN4924 reduces ischemic brain injury in mice. Proc. Natl. Acad. Sci. USA 2022, 119, e2111896119. [Google Scholar] [CrossRef]
- Yu, S.; Xie, L.; Liu, Z.; Li, C.; Liang, Y. MLN4924 Exerts a Neuroprotective Effect against Oxidative Stress via Sirt1 in Spinal Cord Ischemia-Reperfusion Injury. Oxid. Med. Cell Longev. 2019, 2019, 7283639. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, Y.; Wu, S.; Wei, H.; Zou, J.; Xu, S.; Ling, Q.; Kang, M.; Huang, H.; Chen, X.; et al. MLN4924 Promotes Self-Renewal of Limbal Stem Cells and Ocular Surface Restoration. J. Pers. Med. 2023, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.; Peng, Z.; Liu, C.H.; Zhang, L.; Jiang, H. Advances in Cancer Treatment by Targeting the Neddylation Pathway. Front. Cell Dev. Biol. 2021, 9, 653882. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, J.; Chinnaswamy, K.; Stuckey, J.A.; Liu, L.; McEachern, D.; Yang, C.Y.; Bernard, D.; Shen, H.; Rui, L.; et al. Selective inhibition of cullin 3 neddylation through covalent targeting DCN1 protects mice from acetaminophen-induced liver toxicity. Nat. Commun. 2021, 12, 2621. [Google Scholar] [CrossRef]
- Wu, K.; Huynh, K.Q.; Lu, I.; Moustakim, M.; Miao, H.; Yu, C.; Haeusgen, M.J.; Hopkins, B.D.; Huang, L.; Zheng, N.; et al. Inhibitors of cullin-RING E3 ubiquitin ligase 4 with antitumor potential. Proc. Natl. Acad. Sci. USA 2021, 118, e2007328118. [Google Scholar] [CrossRef]
- Tang, Y.; Moretti, R.; Meiler, J. Recent Advances in Automated Structure-Based De Novo Drug Design. J. Chem. Inf. Model. 2024, 64, 1794–1805. [Google Scholar] [CrossRef]
- Kim, S.H.; Macari, S.; Koller, J.; Chawarska, K. Examining the phenotypic heterogeneity of early autism spectrum disorder: Subtypes and short-term outcomes. J. Child. Psychol. Psychiatry 2016, 57, 93–102. [Google Scholar] [CrossRef]
- Warrier, V.; Zhang, X.; Reed, P.; Havdahl, A.; Moore, T.M.; Cliquet, F.; Leblond, C.S.; Rolland, T.; Rosengren, A.; Rowitch, D.H.; et al. Genetic correlates of phenotypic heterogeneity in autism. Nat. Genet. 2022, 54, 1293–1304. [Google Scholar] [CrossRef]
- Liao, Y.; Sumara, I.; Pangou, E. Non-proteolytic ubiquitylation in cellular signaling and human disease. Commun. Biol. 2022, 5, 114. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, J.W.; LaMonica, B.; Kriegstein, A.R. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci. 2011, 14, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Krienen, F.M.; Goldman, M.; Zhang, Q.; Rosario, C.H.D.R.; Florio, M.; Machold, R.; Saunders, A.; Levandowski, K.; Zaniewski, H.; Schuman, B.; et al. Innovations present in the primate interneuron repertoire. Nature 2020, 586, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.J.; Levine, E.S. Uncovering True Cellular Phenotypes: Using Induced Pluripotent Stem Cell-Derived Neurons to Study Early Insults in Neurodevelopmental Disorders. Front. Neurol. 2018, 9, 237. [Google Scholar] [CrossRef]
- Pașca, S.P.; Arlotta, P.; Bateup, H.S.; Camp, J.G.; Cappello, S.; Gage, F.H.; Knoblich, J.A.; Kriegstein, A.R.; Lancaster, M.A.; Ming, G.L.; et al. A framework for neural organoids, assembloids and transplantation studies. Nature 2025, 639, 315–320. [Google Scholar] [CrossRef]
- Fleck, J.S.; Jansen, S.M.J.; Wollny, D.; Zenk, F.; Seimiya, M.; Jain, A.; Okamoto, R.; Santel, M.; He, Z.; Camp, J.G.; et al. Inferring and perturbing cell fate regulomes in human brain organoids. Nature 2023, 621, 365–372. [Google Scholar] [CrossRef]
- Li, C.; Fleck, J.S.; Martins-Costa, C.; Burkard, T.R.; Themann, J.; Stuempflen, M.; Peer, A.M.; Vertesy, Á.; Littleboy, J.B.; Esk, C.; et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature 2023, 621, 373–380. [Google Scholar] [CrossRef]
- Meng, X.; Yao, D.; Imaizumi, K.; Chen, X.; Kelley, K.W.; Reis, N.; Thete, M.V.; Arjun McKinney, A.; Kulkarni, S.; Panagiotakos, G.; et al. Assembloid CRISPR screens reveal impact of disease genes in human neurodevelopment. Nature 2023, 622, 359–366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).