Abstract
Background/Objectives: Reports on oral frailty as a risk factor for chronic constipation are scarce. In this study, we examined the relationship between Oral Frailty Index-8 (OFI-8) and constipation severity. Methods: This cross-sectional analysis involved patients aged ≥65 years (outpatients between November 2020 and November 2021). Patient background (age, sex, body mass index, medical history, lifestyle history, and oral medications), a constipation severity questionnaire (Constipation Scoring System [CSS]), grip strength, walking speed, skeletal muscle mass index (dual-energy X-ray absorptiometry), a frailty questionnaire, an oral frailty questionnaire (OFI-8), an abdominal symptoms quality of life (QOL) questionnaire (Izumo scale), a swallowing evaluation questionnaire (10-item Eating Assessment Tool [EAT-10]), a chronic obstructive pulmonary disease (COPD) evaluation questionnaire (COPD assessment test [CAT]), a simplified QOL evaluation (EuroQol-five dimensions [EQ-5D]), the Dietary Variety Score, a nutritional evaluation (CONtrolling NUTritional Status [CONUT] score), and the 15-item Geriatric Depression Scale (GDS-15) were analyzed. Risk factors for constipation severity (CSS) were examined using multivariate analysis. Patients with advanced gastrointestinal cancer, inflammatory bowel disease, and active gastroduodenal ulcer were excluded. Results: In total, 1029 patients (male/female: 450/579; mean age: 78.3 ± 6.1 years; mean body mass index: 22.9 ± 3) were included. Multivariate analysis demonstrated a significant association between CSS and OFI-8 (β = 0.065), EAT-10 (β = 0.061), sarcopenia (β = 0.050), laxative (β = 0.126), constipation-related QOL score (β = 0.625), diarrhea-related QOL score (β = −0.064), and CAT (β = 0.061). Conclusions: Comprehensive risk factors associated with CSS included a high oral frailty score, impaired swallowing (EAT-10), sarcopenia, laxative use, a high constipation QOL score, a low diarrhea QOL score, and COPD assessment through CAT.
1. Introduction
As the population ages, chronic constipation is becoming a disease that hinders healthy longevity. The prevalence of chronic constipation was 14% (range: 2–35%) in a meta-analysis [1], and the incidence rate increases with age [2,3]. Chronic constipation also reduces quality of life (QOL) [4,5,6]. Furthermore, a link with systemic diseases, including cardiovascular disease, has been suggested [7,8]. The number of patients with chronic constipation will increase in the future, and these patients have been associated with a poorer prognosis [9,10]. Moreover, a greater financial burden on medical systems is anticipated [11], emphasizing the need for appropriate treatment.
In today’s aging society, caring for elderly individuals who are bedridden and require assistance is becoming a challenge. Intervention for frailty and sarcopenia to prevent bedriddenness and the need for nursing care is essential. Frailty places elderly individuals at high risk for adverse health outcomes [12,13]. Recently, the revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria) was published [14]. Within this framework, a new concept called “oral frailty” was developed, focusing on mild decline in oral function. It has become clear that oral frailty appears at a preliminary stage of frailty, and has a significant impact on the progression of frailty [15]. When oral function declines, dietary imbalance occurs, nutritional balance is disrupted, and the risk of falling into a state of low nutrition and requiring nursing care increases. Thus, to achieve healthy longevity, diagnosis of oral frailty, frailty, and sarcopenia and appropriate intervention are important. Furthermore, oral frailty leads to restricted food intake and indigestion, which may contribute to constipation. The oral intake and digestion of nutrients play a crucial role in causing undernutrition in the elderly. However, the relationship between constipation severity and oral frailty in older individuals has not been thoroughly examined.
To date, few reports have evaluated oral frailty and sarcopenia as risk factors for chronic constipation. A previous cohort study of 1042 patient who visited a geriatric hospital revealed high rates of sarcopenia, frailty, and pre-frailty (21.4%, 16.5%, and 51.9%, respectively) [16].
In the present analysis, we investigated, in a multidisciplinary setting, the relationship between constipation severity and oral frailty, including sarcopenia, among a large number of elderly outpatients at an institution specializing in geriatric medicine.
2. Materials and Methods
2.1. Study Cohort
This single-center, cross-sectional analysis was conducted using baseline data obtained from the prospective cohort JUSTICE-TOKYO (JUntendo Sarcopenia regisTratIon of exploring for prediCtors and prognosis in Elderly in TOKYO) study [16]. The study involved consecutive elderly outpatients (age: ≥65 years) who visited the Juntendo Tokyo Koto Geriatric Medical Center between November 2020 and November 2021. The investigation included a 4-year follow-up, as well as annual assessments of survival, incidence of falls, hospitalization, and skeletal muscle mass. Baseline data were recorded at the time of enrollment and entered into the Research Electronic Data Capture (REDCap) system [17].
2.2. Inclusion Criteria
Outpatients aged ≥ 65 years attending Juntendo Tokyo Koto Geriatric Medical Center were eligible for enrollment in the JUSTICE-TOKYO study. Consecutive patients in the stated period were included in the study based on obtaining the following complete information from personal health records: (i) personal characteristics (age, gender, and body mass index), (ii) constipation scoring system, (iii) Oral Frailty Index-8, (iv) dual-energy X-ray absorptiometry, walking speed, and handgrip strength.
2.3. Exclusion Criteria
Patients were excluded for the following reasons: (i) inability to walk independently because of severe osteoarthritis or neuromuscular disease; (ii) immobility; (iii) delirium tremens at presentation; (iv) a history of advanced gastrointestinal cancer, inflammatory bowel disease, or an active gastroduodenal ulcer, as well as acute gastrointestinal, renal, cerebrovascular, coronary, hepatic, and respiratory events; (v) an inability to be interviewed by questionnaire; (vi) administration of enteral nutrition formulas; and (vii) a predicted life expectancy of <1 year because of malignant disease.
2.4. Measurement of Baseline Variables
Information regarding the measurement of baseline variables is provided in Table 1 [14,18,19,20,21,22,23,24,25,26,27,28,29]. Patient data were collected within 3 months following enrollment.

Table 1.
Measurement of baseline variables.
2.5. Endpoint
The Constipation Scoring System (CSS) was used as the dependent variable (endpoint) to examine factors influencing constipation severity.
2.6. Statistical Analysis
We assessed the risk factors for CSS by univariate analysis (UVA) and multivariate analysis (MVA). In the UVA, correlations between CSS and clinical parameters were calculated using Pearson’s correlation coefficients (r) for quantitative variables and correlation ratios (η) for nominal scales. In the multiple regression analysis (MRA), the CSS was employed as a dependent variable. Significant independent variables (p-value < 0.2) in the UVA were included in the MVA. Age, QOL (EuroQol-five dimensions [EQ-5D]), history of falls, day care use, handgrip strength, walking speed, comorbidities (cerebral infarction/hemorrhage, chronic hepatic disease, hypertension, atrial fibrillation, frailty, sarcopenia), use of agents (i.e., acid secretion suppressants, laxatives, and number of oral medications), oral function (Oral Frailty Index-8 (OFI-8), the 10-item Eating Assessment Tool (EAT-10), the Mini-Mental State Examination (MMSE), the 15-item Geriatric Depression Scale (GDS-15), the chronic obstructive pulmonary disease (COPD) assessment test (CAT), hypozincemia, the CONtrolling NUTritional Status (CONUT) score, the Dietary Variety Score (DVS), and QOL scores related to reflux, upper abdominal pain, fullness, constipation, and diarrhea were considered as independent variables. Risk factors for CSS were subjected to MRA via a stepwise method. A variance inflation factor ≥10 denoted multicollinearity. Pairwise deletion was applied in MRA to handle missing data and enhance statistical power. The SPSS version 28 software was used for all statistical analyses (IBM Corporation, Armonk, NY, USA). p-values < 0.05 denoted statistically significant differences.
3. Results
3.1. Clinical Characteristics
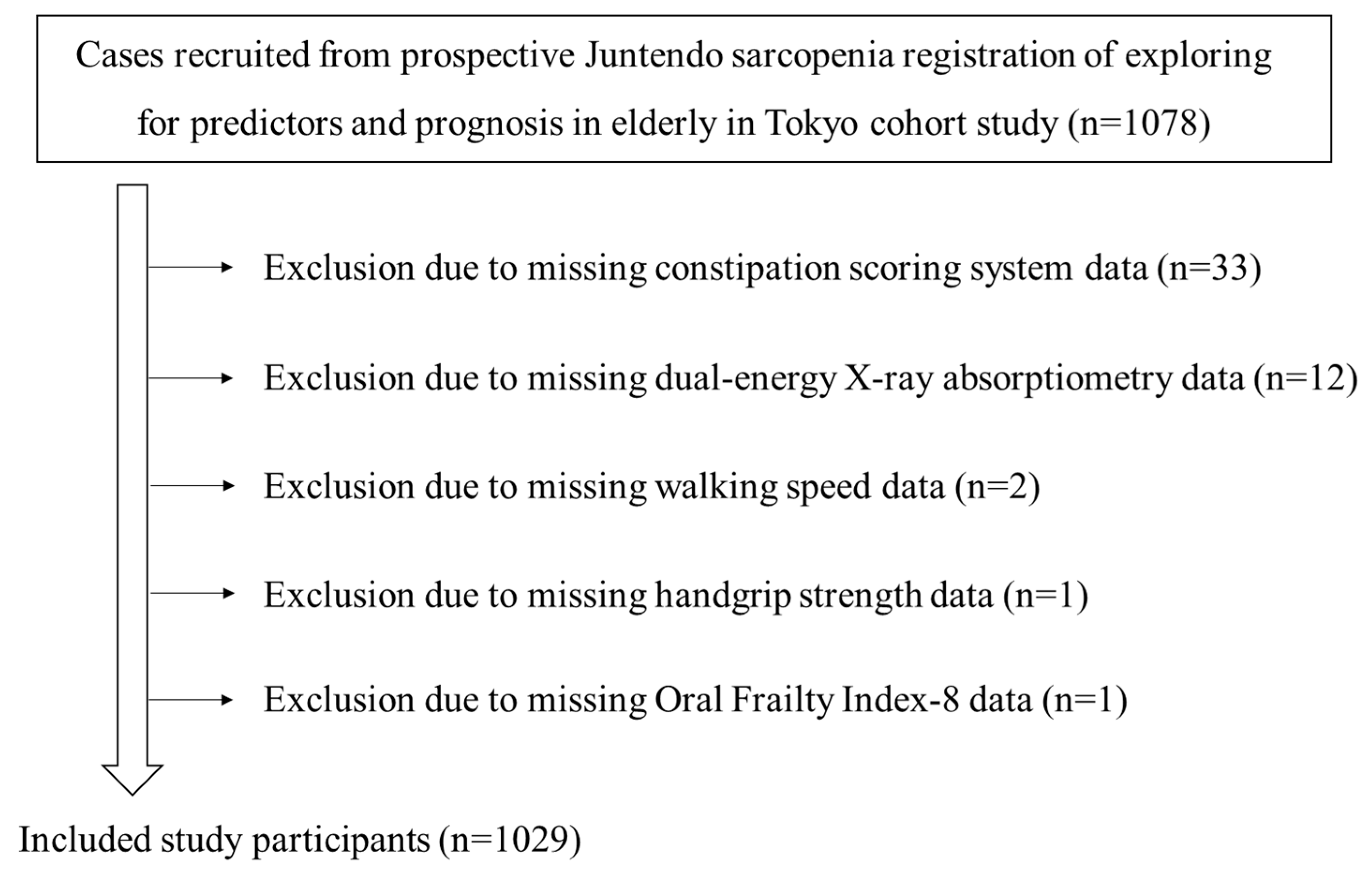
The clinical characteristics of the 1029 subjects (male: n = 450; female: n = 579; mean age: 78.3 ± 6.1 years; mean body mass index: 22.9 ± 3.9 kg/m2) are shown in Table 2. The participant recruitment flow diagram is shown in Figure 1. Among the eligible subjects, 562 and 226 were users of acid secretion suppressants and laxatives, respectively. The mean score of the CSS was 3.4.

Table 2.
Clinical characteristics of study patients (n = 1029).

Figure 1.
Flow diagram of study participants.
3.2. Correlation Between CSS and Various Clinical Parameters (UVA)
Table 3 shows the correlation between CSS and variables in the UVA. Significant differences were recorded in age, QOL (EQ-5D), history of falls, day care use, frailty, handgrip strength, walking speed, sarcopenia, laxatives, number of oral medications, OFI-8, EAT-10, MMSE, GDS-15, QOL scores related to reflux, upper abdominal pain, fullness, constipation, and diarrhea, CAT, CONUT score, and DVS.

Table 3.
Correlation between constipation scoring system score and various clinical parameters.
3.3. Association Between CSS and Other Variables (MRA)
Based on the MRA, the variables of OFI-8, EAT-10, sarcopenia, laxative use, the QOL score related to constipation, the QOL score related to diarrhea, and the CAT were linked to the CSS (Table 4).

Table 4.
Association between constipation scoring system score and other variables in multiple regression analysis.
4. Discussion
Herein, we present the first large cross-sectional analysis investigating risk factors associated with constipation severity (including oral frailty) in elderly outpatients. Our results clarified that OFI-8, EAT-10, sarcopenia, laxative use, QOL scores related to constipation and diarrhea, and the CAT were related to the CSS. Elderly patients with constipation may also have oral frailty, swallowing dysfunction, sarcopenia, or COPD. The relationship between constipation and oral frailty, sarcopenia, or COPD in elderly individuals may apply to diagnosis and treatment. In the future, early intervention for these conditions may be beneficial for the clinical management of constipation.
In our study, OFI-8 and EAT-10, as measures of oral function, were associated with the CSS. Oral frailty significantly impacts the progression of frailty [15], and is correlated with physical frailty [30]. It has become clear that a deterioration in the oral environment, decreased bite force and tongue pressure, a reduced number of teeth, and decreased saliva secretion have a significant impact on the progression of frailty and sarcopenia. The progression of oral frailty may reduce mechanical digestion through chewing and stirring, as well as chemical digestion, and may affect digestive and absorption disorders. We previously reported that frailty is associated with CSS scores [31]. Oral frailty has been identified as a precursor to frailty. Moreover, impaired oral and swallowing function can cause indigestion and reduced nutritional status. These observations suggest that this may be associated with frailty as well as constipation. In this study, the GDS-15 was significantly correlated with the CSS, suggesting that various factors, such as depressive symptoms from a psychological perspective, and living alone or economic hardship from a social perspective, may affect constipation. Lin et al. reported that depression was associated with oral frailty and physical frailty [32]. Therefore, when treating constipation in elderly individuals with oral frailty and frailty, comprehensive multidisciplinary support is required. The present findings indicate that improving oral frailty may lead to improved constipation, and prompt diagnosis of oral frailty is essential.
Sarcopenia was also significantly associated with the CSS. In previous research, we demonstrated that sarcopenia was associated with the CSS retrospectively [33]. Of 310 outpatients aged ≥65 years, 83 (26.8%) had sarcopenia, and the CSS score was significantly higher in the group with sarcopenia versus that without (4.9 ± 4.9 vs. 3.6 ± 3.6, respectively, p = 0.009). MRA revealed that the CSS score was significantly associated with sarcopenia, the Izumo scale QOL score related to constipation, the use of laxatives and intestinal motility regulators, and the Bristol Stool Form Scale (BSFS) score. The results of this study support this evidence. In a cross-sectional study, Park et al. assessed the relationship between constipation and sarcopenia in 1278 community-dwelling elderly individuals [34]. Logistic regression analysis demonstrated that the incidence of functional constipation was significantly higher in the group with sarcopenia versus that without (odds ratio: 2.02; 95% confidence interval: 1.34–3.04). An association between low physical activity and chronic constipation has also been reported in other studies [35]. It has been shown that constipation is related to sarcopenia and, in geriatric care, it is necessary to understand and improve constipation, as well as the overall condition of the body, including the nutritional status and muscle weakness. In elderly individuals with sarcopenia, the severity of constipation is thought to increase due to various factors, including decreased appetite, decreased physical activity, decreased intestinal function, and the effects of polypharmacy [2,36]. Decreased function of the pelvic floor muscles is a cause of functional fecal evacuation disorders [37]. Furthermore, weakened abdominal muscles suppress the increase in intra-abdominal pressure that accompanies straining. Therefore, it is important to prevent muscle weakness throughout the body, including improving the function of the abdominal and pelvic floor muscles.
Moreover, our study showed that the CSS was correlated with abdominal symptoms. The data demonstrated that the QOL scores related to constipation and laxative use were positively associated, whereas the QOL score related to diarrhea was negatively associated with CSS. We previously reported that the CSS was associated with the QOL score related to constipation and the BSFS score [33]. The feeling of incomplete evacuation or hard stools that continue for days could affect QOL, while constipation-related symptoms may include reduced appetite and limited activity in the elderly. The CSS was also associated with laxative use. Yamamoto et al. reported that individuals who had hard stools, used multiple laxatives, or spent more money on treatment had low QOL scores [38]. High constipation severity may lead to increased use of laxatives, which might affect QOL. The QOL score related to diarrhea was positively correlated, but negatively associated with CSS. This finding may be attributed to laxative use by many patients and high diarrhea scores in some patients, despite having constipation. Based on previous reports, low BSFS scores are associated with CSS [33]; in an MVA including various confounding factors, QOL scores related to diarrhea were thought to be negatively associated with CSS.
Regarding pulmonary function, the CAT was associated with CSS. In a systematic review and meta-analysis, 15.5–34% of patients with COPD had sarcopenia [39]. In addition, COPD was correlated or associated with constipation symptoms [40,41]. Kagiali et al. revealed that CAT scores were higher in frail patients versus non-frail patients [42]. In our opinion, COPD-related decreased activity and muscle weakness might influence the severity of constipation.
In a systematic review and meta-analysis of data on chronic idiopathic constipation in the community, female sex and age were identified as risk factors for constipation [1]. This review focused on individuals aged ≥15 years, reporting that the risk of constipation increased with age. In contrast, the present analysis did not detect a significant association between sex and constipation. This discrepancy may be caused by differences in the characteristics of our study population, which primarily comprised elderly individuals (mean age: 78.3 years), and the fact that the research was conducted in a university hospital specializing in geriatric medicine. In studies involving a broader general population, including younger adults, age might emerge as a more prominent risk factor. Additionally, while sex has been identified as a risk factor in the general population, with females being more susceptible, our findings indicated no significant association between sex and constipation risk. According to an overview of the 2022 Basic Survey on National Living Conditions by the Ministry of Health, Labor and Welfare in Japan [43], the prevalence of self-reported constipation was 27.5% and 43.7% among males and females, respectively (68.1% and 73.8% for those aged ≥65 years, respectively). While females generally exhibited higher rates of self-reported constipation, the sex disparity narrowed with advancing age, as the prevalence among males increased significantly in the older population. This trend suggests that the lack of sex association observed in this study may be attributed to the age distribution of the population, predominantly comprising elderly individuals.
The current analysis had several limitations. Firstly, the study subjects were limited to outpatients from a single institution specializing in geriatric medicine. Secondly, we did not take into account other background variables, such as exercise routines, dietary patterns, occupations, education level, and marital status. This limits the generalizability of the findings to older individuals; moreover, the results may have been overestimated due to unhealthy subject bias. Thirdly, this study did not evaluate oral dysfunction or oral bacteria. In the future, collaboration with dentists will be necessary in order to examine oral function.
5. Conclusions
In conclusion, this study revealed that a high oral frailty score, impaired swallowing (EAT-10), sarcopenia, laxative use, a high QOL score related to constipation, a low QOL score related to diarrhea, and COPD assessment (CAT) were associated with CSS. Intervention studies are warranted to assess the effect of improvements in these factors on chronic constipation.
Author Contributions
Conceptualization, D.A., K.S., T.T. and K.M. (Katsumi Miyauchi); data curation, T.T., H.K., T.K. and O.N.; formal analysis, T.T., Y.N. and N.Y.; funding acquisition, T.T., A.N., K.S., K.M. (Katsumi Miyauchi) and K.M. (Kei Matsuno); investigation, D.A., K.S., K.M. (Kei Matsuno) and K.M. (Katsumi Miyauchi); methodology, T.T., D.A., K.S., K.M. (Katsumi Miyauchi), M.H., M.S., A.N. and K.M. (Kei Matsuno); project administration, D.A. and T.T.; resources, D.A., O.N., T.T., K.S., K.M. (Kei Matsuno) and H.I.; software, Y.N. and N.Y.; supervision, D.A., A.N., K.M. (Kei Matsuno) and K.M. (Katsumi Miyauchi); validation, O.N., T.T., K.S. and A.N.; visualization, T.T. and D.A.; writing—original draft preparation, T.T., D.A, H.K., S.O., T.K. and O.N.; writing—review and editing, T.T., O.N., D.A., K.S. and K.M. (Katsumi Miyauchi); review of the manuscript, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (JSPS KAKENHI) (grant numbers: 21K11633, 16K09042, 22K10317, and 23K10854).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of The Juntendo Tokyo Koto Geriatric Medical Center (approval number: G20-0011; approval date: 25 August 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, due to privacy and ethical restrictions.
Acknowledgments
We thank the study subjects for their cooperation, and Hisafumi Yamagata, Maiko Suzuki, Ryota Kanemaru, Tomoko Yamada, Yurina Sugita, Ran Matsudaira, Mutsuko Hatanaka, and Ryoko Yamaguchi for their administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suares, N.C.; Ford, A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Orozco, J.F.; Foxx-Orenstein, A.E.; Sterler, S.M.; Stoa, J.M. Chronic constipation in the elderly. Am. J. Gastroenterol. 2012, 107, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Tomita, T.; Oshima, T.; Toyoshima, F.; Yamasaki, T.; Okugawa, T.; Kondo, T.; Kono, T.; Tozawa, K.; Ikehara, H.; et al. Prevalence and Self-Recognition of Chronic Constipation: Results of an Internet Survey. J. Neurogastroenterol. Motil. 2016, 22, 677–685. [Google Scholar] [CrossRef]
- Wald, A.; Scarpignato, C.; Kamm, M.A.; Mueller-Lissner, S.; Helfrich, I.; Schuijt, C.; Bubeck, J.; Limoni, C.; Petrini, O. The burden of constipation on quality of life: Results of a multinational survey. Aliment. Pharmacol. Ther. 2007, 26, 227–236. [Google Scholar] [CrossRef]
- Belsey, J.; Greenfield, S.; Candy, D.; Geraint, M. Systematic review: Impact of constipation on quality of life in adults and children. Aliment. Pharmacol. Ther. 2010, 31, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Kazumori, K.; Baba, K.; Zhao, X.; Chen, Y.; Miwa, H. Impact of chronic constipation on health-related quality of life and work productivity in Japan. J. Gastroenterol. Hepatol. 2021, 36, 1529–1537. [Google Scholar] [CrossRef]
- Honkura, K.; Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis 2016, 246, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Rey, E.; Locke, G.R., III; Schleck, C.D.; Baum, C.; Zinsmeister, A.R.; Talley, N.J. Chronic constipation and co-morbidities: A prospective population-based nested case-control study. United Eur. Gastroenterol. J. 2016, 4, 142–151. [Google Scholar] [CrossRef]
- Chang, J.Y.; Locke, G.R., III; McNally, M.A.; Halder, S.L.; Schleck, C.D.; Zinsmeister, A.R.; Talley, N.J. Impact of functional gastrointestinal disorders on survival in the community. Am. J. Gastroenterol. 2010, 105, 822–832. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019, 281, 114–120. [Google Scholar] [CrossRef]
- Passmore, A.P. Economic aspects of pharmacotherapy for chronic constipation. Pharmacoeconomics 1995, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Matsuno, K.; Asaoka, D.; Sugano, K.; Takahashi, K.; Miyauchi, K. Rationale and design of Juntendo Sarcopenia Registration to explore the predictors and prognosis of sarcopenia and frailty in the elderly in TOKYO (JUSTICE-TOKYO). Geriatr. Gerontol. Int. 2024, 24, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Soen, S.; Fukunaga, M.; Sugimoto, T.; Sone, T.; Fujiwara, S.; Endo, N.; Gorai, I.; Shiraki, M.; Hagino, H.; Hosoi, T.; et al. Diagnostic criteria for primary osteoporosis: Year 2012 revision. J. Bone Miner. Metab. 2013, 31, 247–257. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirano, H.; Ohara, Y.; Nishimoto, M.; Iijima, K. Oral Frailty Index-8 in the risk assessment of new-onset oral frailty and functional disability among community-dwelling older adults. Arch. Gerontol. Geriatr. 2021, 94, 104340. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, K.; Sugishita, M.; Hemmi, I.; Asada, T.; Tanigawa, T. A Validity and Reliability Study of the Japanese Version of the Geriatric Depression Scale 15 (GDS-15-J). Clin. Gerontol. 2017, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, E.; Yamashita, N.; Katsube, T.; Kushiyama, Y.; Suetsugu, H.; Furuta, K.; Kinoshita, Y. Abdominal symptom-related QOL in individuals visiting an outpatient clinic and those attending an annual health check. Intern. Med. 2011, 50, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Kumagai, S.; Watanabe, S.; Shibata, H.; Amano, H.; Fujiwara, Y.; Shinkai, S.; Yoshida, H.; Suzuki, T.; Yukawa, H.; Yasumura, S.; et al. Effects of dietary variety on declines in high-level functional capacity in elderly people living in a community. Nihon Koshu Eisei Zasshi 2003, 50, 1117–1124. [Google Scholar]
- Kusunoki, H.; Hasegawa, Y.; Nagasawa, Y.; Shojima, K.; Yamazaki, H.; Mori, T.; Tsuji, S.; Wada, Y.; Tamaki, K.; Nagai, K.; et al. Oral Frailty and Its Relationship with Physical Frailty in Older Adults: A Longitudinal Study Using the Oral Frailty Five-Item Checklist. Nutrients 2024, 17, 17. [Google Scholar] [CrossRef]
- Asaoka, D.; Takeda, T.; Inami, Y.; Abe, D.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Komori, H.; Akazawa, Y.; et al. The Association Between Frailty and Abdominal Symptoms: A Hospital-Based Cross-Sectional Study. Intern. Med. 2020, 59, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Huang, S.S.; Yen, C.W.; Kabasawa, Y.; Lee, C.H.; Huang, H.L. Physical Frailty and Oral Frailty Associated with Late-Life Depression in Community-Dwelling Older Adults. J. Pers. Med. 2022, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, D.; Takeda, T.; Inami, Y.; Abe, D.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Komori, H.; Akazawa, Y.; et al. Association between the severity of constipation and sarcopenia in elderly adults: A single-center university hospital-based, cross-sectional study. Biomed. Rep. 2021, 14, 2. [Google Scholar] [CrossRef]
- Park, H.; Lim, J.; Baek, J.Y.; Lee, E.; Jung, H.W.; Jang, I.Y. Status of Constipation and Its Association with Sarcopenia in Older Adults: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 11083. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B. Associations between physical activity and constipation in adult Americans: Results from the National Health and Nutrition Examination Survey. Neurogastroenterol. Motil. 2020, 32, e13789. [Google Scholar] [CrossRef] [PubMed]
- Lucak, S.; Lunsford, T.N.; Harris, L.A. Evaluation and Treatment of Constipation in the Geriatric Population. Clin. Geriatr. Med. 2021, 37, 85–102. [Google Scholar] [CrossRef]
- Rao, S.S.; Bharucha, A.E.; Chiarioni, G.; Felt-Bersma, R.; Knowles, C.; Malcolm, A.; Wald, A. Anorectal Disorders. Gastroenterology 2016, 150, 1430–1442.e4. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ohashi, W.; Yamaguchi, Y.; Igari, H.; Koshino, A.; Sugiyama, T.; Nagao, K.; Tamura, Y.; Izawa, S.; Mano, M.; et al. Factors Associated with Defecation Satisfaction among Japanese Adults with Chronic Constipation. J. Clin. Med. 2024, 13, 3216. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Osadnik, C.; Phu, S.; Morita, A.A.; Duque, G.; Probst, V.S. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1164–1176. [Google Scholar] [CrossRef]
- Gürsoy Coşkun, G.; Andac-Ozturk, S.; Arslan Ulukan, Z. Comparison of constipation and nutritional status with disease-related parameters in chronic obstructive pulmonary disease patients. Int. J. Clin. Pract. 2021, 75, e14451. [Google Scholar] [CrossRef]
- Shen, Z.; Qiu, B.; Chen, L.; Zhang, Y. Common gastrointestinal diseases and chronic obstructive pulmonary disease risk: A bidirectional Mendelian randomization analysis. Front. Genet. 2023, 14, 1256833. [Google Scholar] [CrossRef]
- Kagiali, S.; Inal-Ince, D.; Cakmak, A.; Calik-Kutukcu, E.; Saglam, M.; Vardar-Yagli, N.; Tekerlek, H.; Sonbahar-Ulu, H.; Arikan, H.; Bozdemir-Ozel, C.; et al. Daily living activities, exercise capacity, cognition, and balance in COPD patients with and without frailty. Ir. J. Med. Sci. 2022, 191, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare. 2022. Available online: https://www.mhlw.go.jp/english/database/db-hss/cslc-report2022.html (accessed on 26 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).