Proximity Proteomics Reveals USP44 Forms a Complex with BRCA2 in Neuroblastoma Cells and Is Required to Prevent Chromosome Breakage
Abstract
1. Introduction
2. Materials and Methods
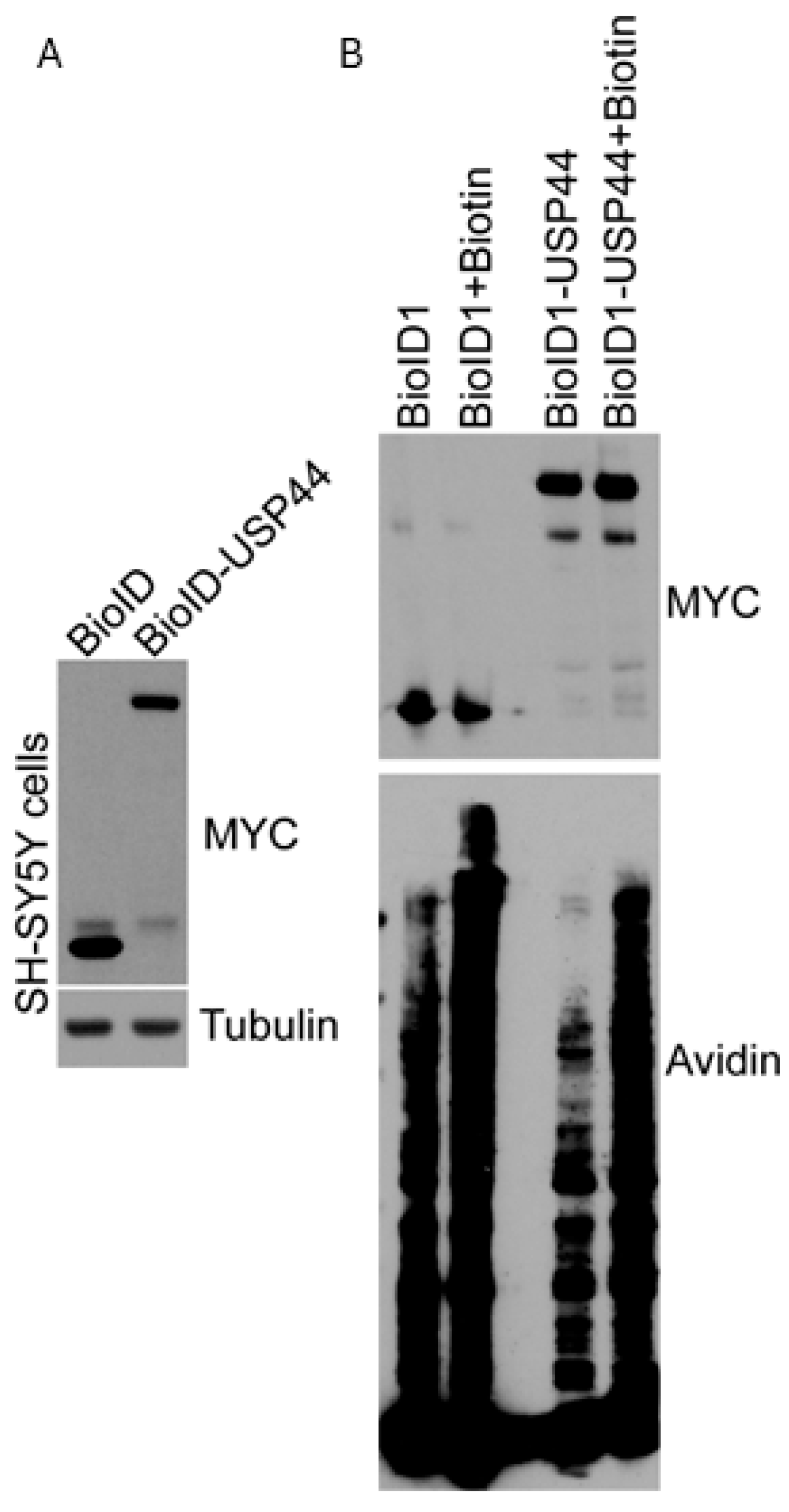
2.1. Proximity Proteomics
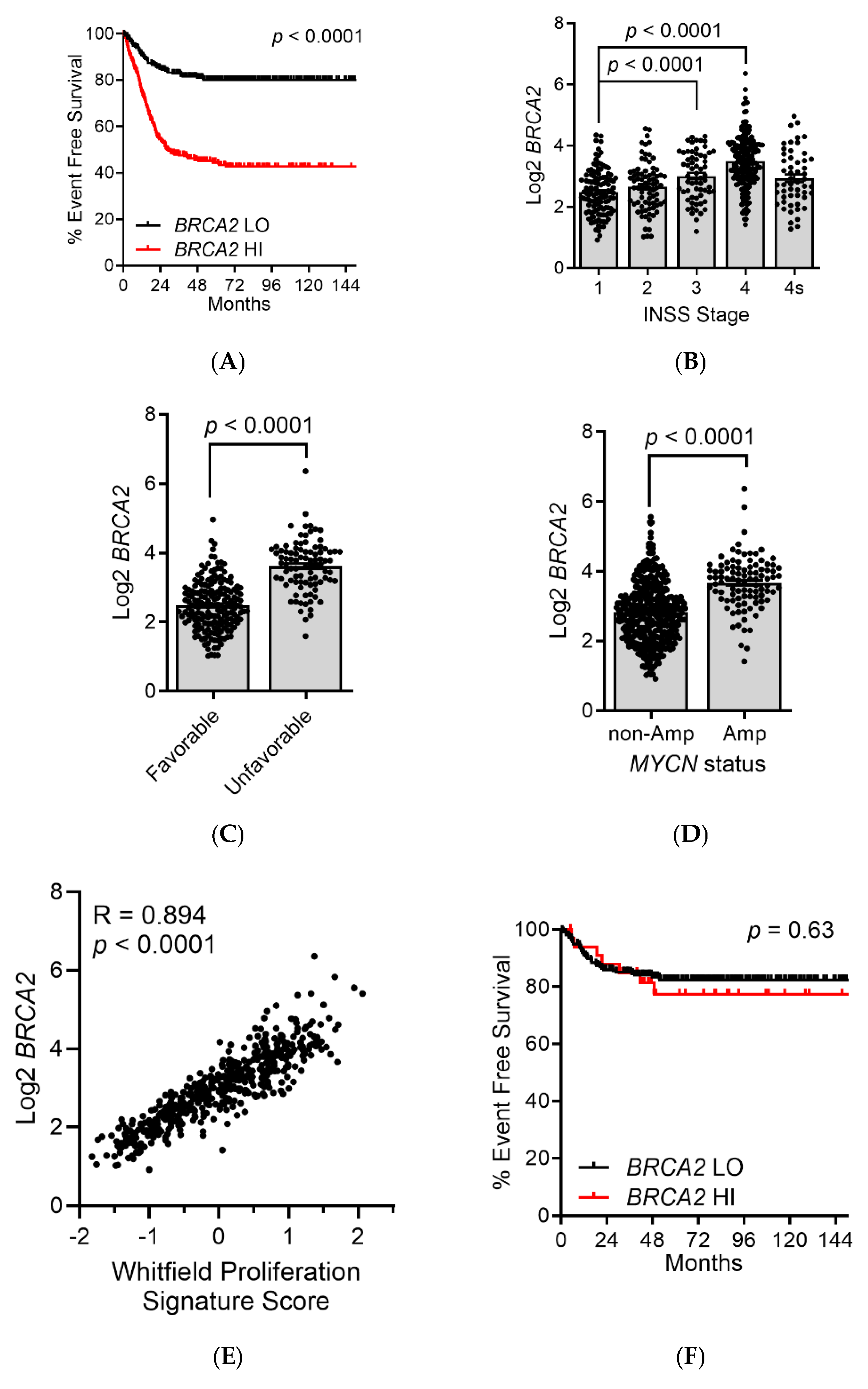
2.2. Bioinformatic Analyses and Statistics
2.3. Cell Culture, Immunoprecipitation, and Immunoreagents
2.4. Chromosome Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stegmeier, F.; Rape, M.; Draviam, V.M.; Nalepa, G.; Sowa, M.E.; Ang, X.L.; McDonald, E.R., 3rd; Li, M.Z.; Hannon, G.J.; Sorger, P.K.; et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 2007, 446, 876–881. [Google Scholar] [CrossRef]
- Ekstrom, T.L.; Hussain, S.; Bedekovics, T.; Ali, A.; Paolini, L.; Mahmood, H.; Rosok, R.M.; Koster, J.; Johnsen, S.A.; Galardy, P.J. USP44 overexpression drives a MYC-like gene expression program in neuroblastoma through epigenetic reprogramming. Mol. Cancer Res. 2024, 22, 812–825. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, B.; Sun, R.; Wang, Y.; Zhang, L.; Xu, G. USP44 accelerates the growth of T-cell acute lymphoblastic leukemia through interacting with WDR5 and repressing its ubiquitination. Int. J. Med. Sci. 2022, 19, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Lei, W. USP44 hypermethylation promotes cell proliferation and metastasis in breast cancer. Future Oncol. 2021, 17, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, Q.; Ren, W.; Yan, B.; Yi, L.; Tang, T.; Lin, H.; Zhang, Y. USP44 suppresses proliferation and enhances apoptosis in colorectal cancer cells by inactivating the Wnt/beta-catenin pathway via Axin1 deubiquitination. Cell Biol. Int. 2020, 44, 1651–1659. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, S.; Yang, H.; Deng, S.; Fan, P.; Li, M.; Jin, X. USP44 suppresses pancreatic cancer progression and overcomes gemcitabine resistance by deubiquitinating FBP1. Am. J. Cancer Res. 2019, 9, 1722–1733. [Google Scholar] [PubMed]
- Park, J.M.; Lee, J.E.; Park, C.M.; Kim, J.H. USP44 Promotes the Tumorigenesis of Prostate Cancer Cells through EZH2 Protein Stabilization. Mol. Cells 2019, 42, 17–27. [Google Scholar] [CrossRef]
- Bernardes, V.F.; Odell, E.W.; Gomez, R.S.; Gomes, C.C. DNA Aneuploidy in Malignant Salivary Gland Neoplasms is Independent of USP44 Protein Expression. Braz. Dent. J. 2017, 28, 148–151. [Google Scholar] [CrossRef]
- Liu, T.; Sun, B.; Zhao, X.; Li, Y.; Zhao, X.; Liu, Y.; Yao, Z.; Gu, Q.; Dong, X.; Shao, B.; et al. USP44+ Cancer Stem Cell Subclones Contribute to Breast Cancer Aggressiveness by Promoting Vasculogenic Mimicry. Mol. Cancer Ther. 2015, 14, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Sloane, M.A.; Wong, J.W.; Perera, D.; Nunez, A.C.; Pimanda, J.E.; Hawkins, N.J.; Sieber, O.M.; Bourke, M.J.; Hesson, L.B.; Ward, R.L. Epigenetic inactivation of the candidate tumor suppressor USP44 is a frequent and early event in colorectal neoplasia. Epigenetics 2014, 9, 1092–1100. [Google Scholar] [CrossRef]
- Zhang, Y.; Foreman, O.; Wigle, D.A.; Kosari, F.; Vasmatzis, G.; Salisbury, J.L.; van Deursen, J.; Galardy, P.J. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J. Clin. Investig. 2012, 122, 4362–4374. [Google Scholar] [CrossRef]
- Zhang, Y.; van Deursen, J.; Galardy, P.J. Overexpression of ubiquitin specific protease 44 (USP44) induces chromosomal instability and is frequently observed in human T-cell leukemia. PLoS ONE 2011, 6, e23389. [Google Scholar] [CrossRef]
- Zhang, Y.; Mandemaker, I.K.; Matsumoto, S.; Foreman, O.; Holland, C.P.; Lloyd, W.R.; Sugasawa, K.; Vermeulen, W.; Marteijn, J.A.; Galardy, P.J. USP44 Stabilizes DDB2 to Facilitate Nucleotide Excision Repair and Prevent Tumors. Front. Cell Dev. Biol. 2021, 9, 663411. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Atanassov, B.S.; Li, W.; Zhang, Y.; Florens, L.; Mohan, R.D.; Galardy, P.J.; Washburn, M.P.; Workman, J.L.; Dent, S.Y.R. USP44 Is an Integral Component of N-CoR that Contributes to Gene Repression by Deubiquitinating Histone H2B. Cell Rep. 2016, 17, 2382–2393. [Google Scholar] [CrossRef]
- Kim, D.I.; Birendra, K.C.; Zhu, W.; Motamedchaboki, K.; Doye, V.; Roux, K.J. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc. Natl. Acad. Sci. USA 2014, 111, E2453–E2461. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Foreman, O.; Perkins, S.L.; Witzig, T.E.; Miles, R.R.; van Deursen, J.; Galardy, P.J. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia 2010, 24, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Bedekovics, T.; Liu, Q.; Hu, W.; Jeon, H.; Johnson, S.H.; Vasmatzis, G.; May, D.G.; Roux, K.J.; Galardy, P.J. UCH-L1 bypasses mTOR to promote protein biosynthesis and is required for MYC-driven lymphomagenesis in mice. Blood 2018, 132, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, M.L.; George, L.K.; Grant, G.D.; Perou, C.M. Common markers of proliferation. Nat. Rev. Cancer 2006, 6, 99–106. [Google Scholar] [CrossRef]
- Kim, J.M.; Parmar, K.; Huang, M.; Weinstock, D.M.; Ruit, C.A.; Kutok, J.L.; D’Andrea, A.D. Inactivation of murine Usp1 results in genomic instability and a fanconi anemia phenotype. Dev. Cell 2009, 16, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.J.; Kim, D.I.; Burke, B. BioID: A screen for protein-protein interactions. Curr. Protoc. Protein Sci. 2013, 74, 19–23. [Google Scholar] [CrossRef]
- Sondka, Z.; Bamford, S.; Cole, C.G.; Ward, S.A.; Dunham, I.; Forbes, S.A. The COSMIC Cancer Gene Census: Describing genetic dysfunction across all human cancers. Nat. Rev. Cancer 2018, 18, 696–705. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Bach, M.; Winkelmann, G.; Luhrmann, R. 20S small nuclear ribonucleoprotein U5 shows a surprisingly complex protein composition. Proc. Natl. Acad. Sci. USA 1989, 86, 6038–6042. [Google Scholar] [CrossRef]
- Riabov Bassat, D.; Visanpattanasin, S.; Vorlander, M.K.; Fin, L.; Phillips, A.W.; Plaschka, C. Structural basis of human U5 snRNP late biogenesis and recycling. Nat. Struct. Mol. Biol. 2024, 31, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Brandina, I.; Peter, D.; Lagad, S.; Fraudeau, A.; Portell-Montserrat, J.; Tholen, J.; Zhao, J.; Galej, W.P. Structure of the human 20S U5 snRNP. Nat. Struct. Mol. Biol. 2024, 31, 752–756. [Google Scholar] [CrossRef]
- Bertazzon, M.; Hurtado-Pico, A.; Plaza-Sirvent, C.; Schuster, M.; Preussner, M.; Kuropka, B.; Liu, F.; Kirsten, A.Z.A.; Schmitt, X.J.; Konig, B.; et al. The nuclear GYF protein CD2BP2/U5-52K is required for T cell homeostasis. Front. Immunol. 2024, 15, 1415839. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, B.; Shimamura, A.; Moreau, L.; Baldinger, S.; Hag-alshiekh, M.; Bostrom, B.; Sencer, S.; D’Andrea, A.D. Association of biallelic BRCA2/FANCD1 mutations with spontaneous chromosomal instability and solid tumors of childhood. Blood 2004, 103, 2554–2559. [Google Scholar] [CrossRef]
- Howlett, N.G.; Taniguchi, T.; Olson, S.; Cox, B.; Waisfisz, Q.; De Die-Smulders, C.; Persky, N.; Grompe, M.; Joenje, H.; Pals, G.; et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 2002, 297, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Jensen, S.C.; Roux, K.J. Identifying Protein-Protein Associations at the Nuclear Envelope with BioID. Methods Mol. Biol. 2016, 1411, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, P.; Barbi, J.; Huang, Q.; Yang, E.; Bai, Y.; Nie, J.; Gao, Y.; Tao, J.; Lu, Y.; et al. The deubiquitinase USP44 promotes Treg function during inflammation by preventing FOXP3 degradation. EMBO Rep. 2020, 21, e50308. [Google Scholar] [CrossRef]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Sims, A.E.; Spiteri, E.; Sims, R.J., 3rd; Arita, A.G.; Lach, F.P.; Landers, T.; Wurm, M.; Freund, M.; Neveling, K.; Hanenberg, H.; et al. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat. Struct. Mol. Biol. 2007, 14, 564–567. [Google Scholar] [CrossRef]
- Smogorzewska, A.; Matsuoka, S.; Vinciguerra, P.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Ballif, B.A.; Gygi, S.P.; Hofmann, K.; D’Andrea, A.D.; et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell 2007, 129, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Nijman, S.M.; Huang, T.T.; Dirac, A.M.; Brummelkamp, T.R.; Kerkhoven, R.M.; D’Andrea, A.D.; Bernards, R. The Deubiquitinating Enzyme USP1 Regulates the Fanconi Anemia Pathway. Mol. Cell 2005, 17, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hua, L.; Tang, H.; Bao, Z.; Xu, X.; Zhu, H.; Wang, S.; Jiapaer, Z.; Bhatia, R.; Dunn, I.F.; et al. CBX7 reprograms metabolic flux to protect against meningioma progression by modulating the USP44/c-MYC/LDHA axis. J. Mol. Cell Biol. 2024, 15, mjad057. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yang, X.; Shi, K.; Zhang, Y.; Shi, X.; Wang, J.; Wang, Y.; Chenyan, A.; Shan, J.; Wang, Y.; et al. MAD2 activates IGF1R/PI3K/AKT pathway and promotes cholangiocarcinoma progression by interfering USP44/LIMA1 complex. Oncogene 2023, 42, 3344–3357. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Hussain, S.; Bedekovics, T.; Jeon, R.H.; May, D.G.; Roux, K.J.; Galardy, P.J. Proximity Proteomics Reveals USP44 Forms a Complex with BRCA2 in Neuroblastoma Cells and Is Required to Prevent Chromosome Breakage. Biomedicines 2024, 12, 2901. https://doi.org/10.3390/biomedicines12122901
Ali A, Hussain S, Bedekovics T, Jeon RH, May DG, Roux KJ, Galardy PJ. Proximity Proteomics Reveals USP44 Forms a Complex with BRCA2 in Neuroblastoma Cells and Is Required to Prevent Chromosome Breakage. Biomedicines. 2024; 12(12):2901. https://doi.org/10.3390/biomedicines12122901
Chicago/Turabian StyleAli, Asma, Sajjad Hussain, Tibor Bedekovics, Raymond H. Jeon, Danielle G. May, Kyle J. Roux, and Paul J. Galardy. 2024. "Proximity Proteomics Reveals USP44 Forms a Complex with BRCA2 in Neuroblastoma Cells and Is Required to Prevent Chromosome Breakage" Biomedicines 12, no. 12: 2901. https://doi.org/10.3390/biomedicines12122901
APA StyleAli, A., Hussain, S., Bedekovics, T., Jeon, R. H., May, D. G., Roux, K. J., & Galardy, P. J. (2024). Proximity Proteomics Reveals USP44 Forms a Complex with BRCA2 in Neuroblastoma Cells and Is Required to Prevent Chromosome Breakage. Biomedicines, 12(12), 2901. https://doi.org/10.3390/biomedicines12122901






