Microbiome and Postbiotics in Skin Health
Abstract
1. Introduction
2. Ontology of Skin
3. Commensal Microbiome and Immune Cell Interaction to Maintain Skin Health
4. Interaction of Microbiome with Skin
5. Types of Skin and Their Microbiomes
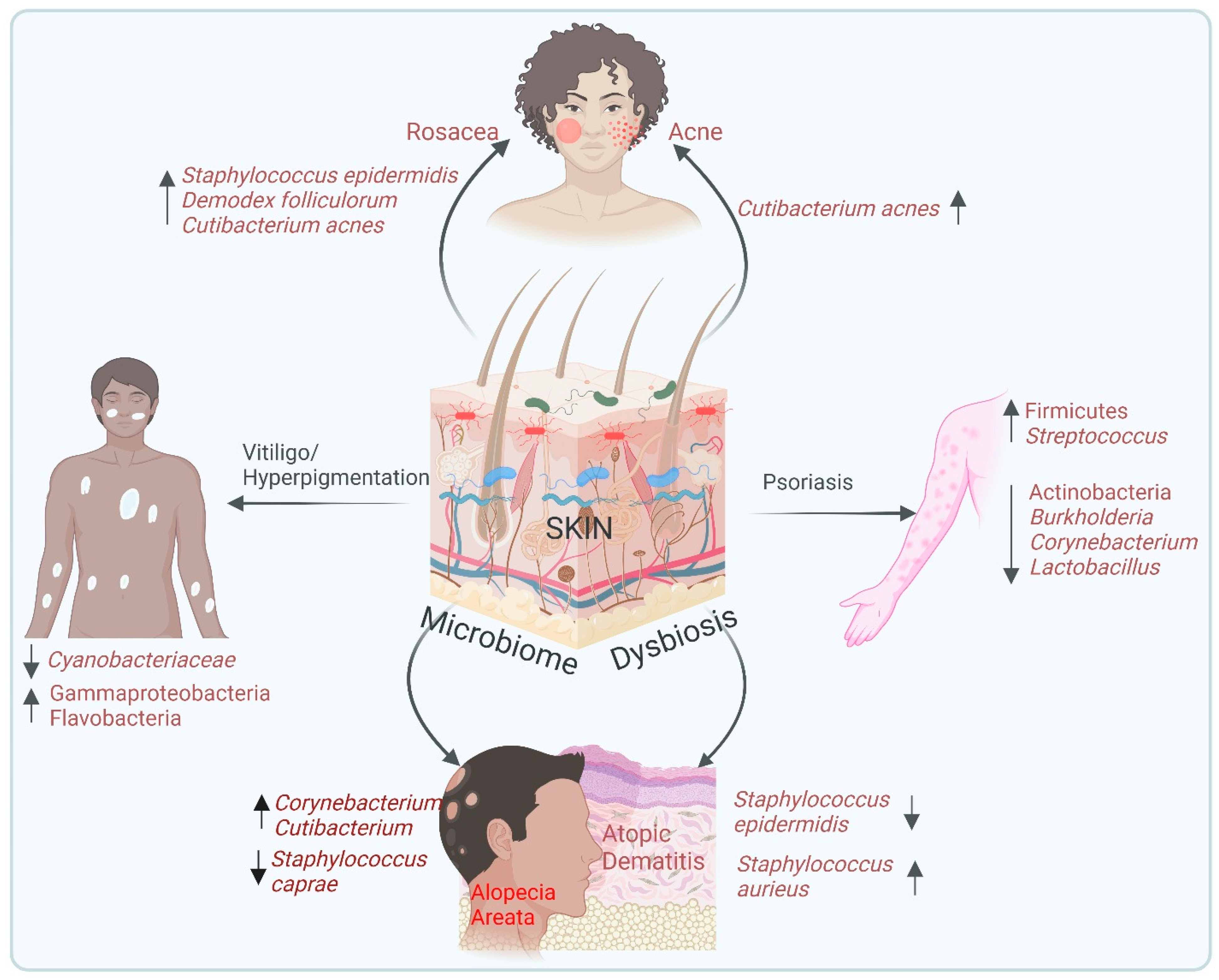
6. Microbiome and Skin Diseases
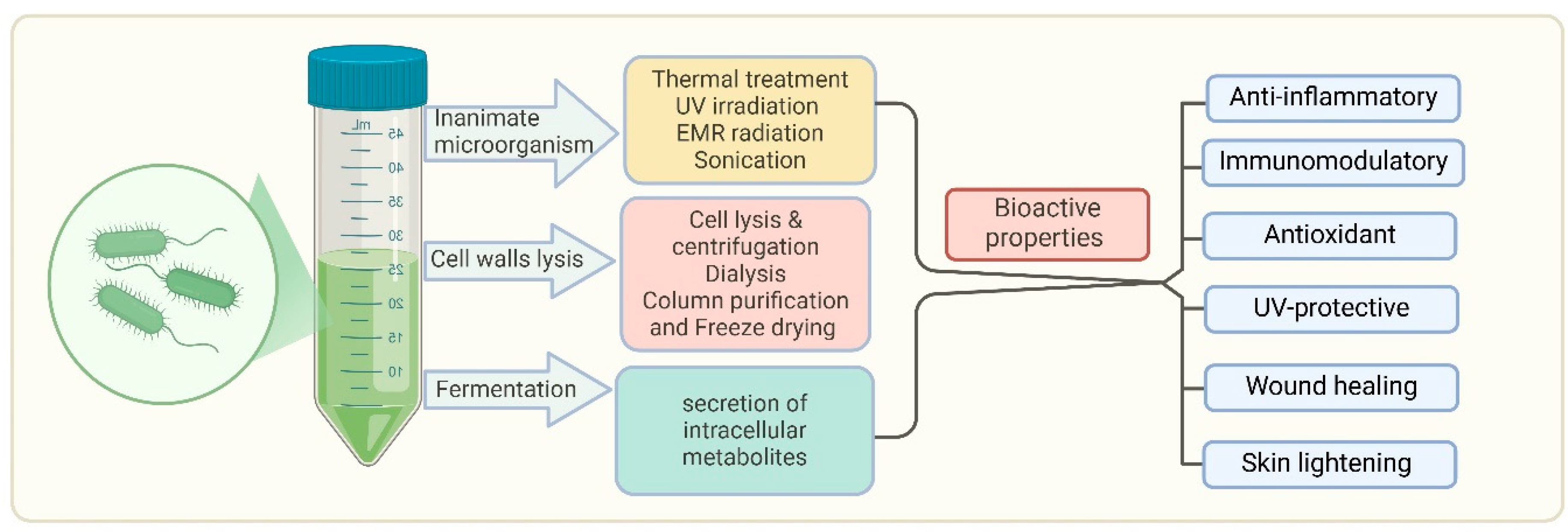
7. Postbiotics in the Treatment of Skin Diseases
8. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Alves, A.C.; Martins, S.M.d.S.B., Jr.; Belo, J.V.T.; Lemos, M.V.C.; Lima, C.E.d.M.C.; Silva, C.D.d.; Zagmignan, A.; Nascimento da Silva, L.C. Global Trends and Scientific Impact of Topical Probiotics in Dermatological Treatment and Skincare. Microorganisms 2024, 12, 2010. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [PubMed]
- Kostecka, M.; Kostecka, J.; Szwed-Gułaga, O.; Jackowska, I.; Kostecka-Jarecka, J. The impact of common acne on the well-being of young people aged 15–35 years and the influence of nutrition knowledge and diet on acne development. Nutrients 2022, 14, 5293. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Son, S.W.; Cho, S.H. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol. Res. 2016, 8, 181–190. [Google Scholar] [CrossRef]
- Bakshi, H.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Treatment of psoriasis: A comprehensive review of entire therapies. Curr. Drug Saf. 2020, 15, 82–104. [Google Scholar] [CrossRef]
- Premkumar, M.; Kalarani, I.B.; Mohammed, V.; Veerabathiran, R. An Extensive Review of Vitiligo-Associated Conditions. Int. J. Dermatol. Venereol. 2024, 7, 44–51. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Cheng, J.Y.; Gallo, R.L. Mechanisms for control of skin immune function by the microbiome. Curr. Opin. Immunol. 2021, 72, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.; Nizet, V.; Gallo, R. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cogen, A.L.; Radek, K.A.; Park, H.J.; MacLeod, D.T.; Leichtle, A.; Ryan, A.F.; Di Nardo, A.; Gallo, R.L. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Investig. Dermatol. 2010, 130, 2211–2221. [Google Scholar] [CrossRef]
- Sanford, J.A.; Zhang, L.-J.; Williams, M.R.; Gangoiti, J.A.; Huang, C.-M.; Gallo, R.L. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 2016, 1, eaah4609. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.-J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef]
- Zouboulis, C.; Boschnakow, A. Chronological ageing and photoageing of the human sebaceous gland. Clin. Exp. Dermatol. 2001, 26, 600–607. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar]
- Lim, H.W.; Collins, S.A.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef]
- Stavrakidis, K.K.S. Probiotics: Benefits on Skin Health and Therapeutical Potential. Doctoral Dissertation, Department of Chemistry and Biochemistry, Faculty of Medicine, University of Rijeka, Rijeka, Croatia, 2024. [Google Scholar]
- Nicholas-Haizelden, K.; Murphy, B.; Hoptroff, M.; Horsburgh, M.J. Bioprospecting the skin microbiome: Advances in therapeutics and personal care products. Microorganisms 2023, 11, 1899. [Google Scholar] [CrossRef]
- Gueniche, A.; Perin, O.; Bouslimani, A.; Landemaine, L.; Misra, N.; Cupferman, S.; Aguilar, L.; Clavaud, C.; Chopra, T.; Khodr, A. Advances in microbiome-derived solutions and methodologies are founding a new era in skin health and care. Pathogens 2022, 11, 121. [Google Scholar] [CrossRef]
- Al-Smadi, K.; Leite-Silva, V.R.; Filho, N.A.; Lopes, P.S.; Mohammed, Y. Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies. Antibiotics 2023, 12, 1698. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Sinkko, H.; Hielm-Björkman, A.; Salmela, E.; Tiira, K.; Laatikainen, T.; Mäkeläinen, S.; Kaukonen, M.; Uusitalo, L.; Hanski, I. Skin microbiota and allergic symptoms associate with exposure to environmental microbes. Proc. Natl. Acad. Sci. USA 2018, 115, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hao, Q.; Liu, S.-B.; Zhang, Q.-S.; Chen, X.-Y.; Li, S.-H.; Ran, C.; Yang, Y.-L.; Teame, T.; Zhang, Z. The positive effects of postbiotic (SWF concentration®) supplemented diet on skin mucus, liver, gut health, the structure and function of gut microbiota of common carp (Cyprinus carpio) fed with high-fat diet. Fish Shellfish Immunol. 2023, 135, 108681. [Google Scholar]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market—An update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar]
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar]
- Zhang, L.-J. Keratins in skin epidermal development and diseases. In Ling-Juan Z. Keratins in Skin Epidermal Development and Diseases; IntechOpen: London, UK, 2018. [Google Scholar]
- Menon, G.K. Skin basics; structure and function. In Lipids and Skin Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–23. [Google Scholar]
- Driskell, R.R.; Jahoda, C.A.; Chuong, C.M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 553, 427–436. [Google Scholar] [PubMed]
- Sharma, G.; Khanna, G.; Sharma, P.; Deol, P.K.; Kaur, I.P. Mechanistic role of probiotics in improving skin health. In Probiotic Research in Therapeutics; Springer: Singapore, 2022; pp. 27–47. [Google Scholar]
- Heo, Y.M.; Lee, D.-G.; Mun, S.; Kim, M.; Baek, C.; Lee, H.; Yun, S.K.; Kang, S.; Han, K. Skin benefits of postbiotics derived from Micrococcus luteus derived from human skin: An untapped potential for dermatological health. Genes. Genom. 2024, 46, 13–25. [Google Scholar] [CrossRef]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar]
- Collin, M.; Milne, P. Langerhans cell origin and regulation. Curr. Opin. Hematol. 2016, 23, 28–35. [Google Scholar]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012, 36, 873–884. [Google Scholar] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [PubMed]
- Ferreira, I.; Lopes, C.; Amaral, M. Treatment Advances for Acne Vulgaris: The Scientific Role of Cannabinoids. Cosmetics 2024, 11, 22. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar]
- Cogen, A.L.; Yamasaki, K.; Muto, J.; Sanchez, K.M.; Crotty Alexander, L.; Tanios, J.; Lai, Y.; Kim, J.E.; Nizet, V.; Gallo, R.L. Staphylococcus epidermidis antimicrobial δ-toxin (phenol-soluble modulin-γ) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS ONE 2010, 5, e8557. [Google Scholar]
- Swaney, M.H.; Nelsen, A.; Sandstrom, S.; Kalan, L.R. Sweat and sebum preferences of the human skin microbiota. Microbiol. Spectr. 2023, 11, e0418022. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal stability of the human skin microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.-H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar]
- Chen, P.; He, G.; Qian, J.; Zhan, Y.; Xiao, R. Potential role of the skin microbiota in Inflammatory skin diseases. J. Cosmet. Dermatol. 2021, 20, 400–409. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the role of langerhans cells as immune regulators within the skin. Front. Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Glatthardt, T.; Lima, R.D.; de Mattos, R.M.; Ferreira, R.B.R. Microbe interactions within the skin microbiome. Antibiotics 2024, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.H.; Ahamed, A.; Chen, K.; Lebig, E.G.G.; Petros, B.; Saeed, S.; Martins-Green, M. Skin microbiota and its role in health and disease with an emphasis on wound healing and chronic wound development. In Microbiome, Immunity, Digestive Health and Nutrition: Epidemiology, Pathophysiology, Prevention and Treatment; Academic Press: Cambridge, MA, USA, 2022; pp. 297–311. [Google Scholar]
- Kim, J.; Kim, B.E.; Goleva, E.; Berdyshev, E.; Bae, J.; Kim, S.; Kim, H.-y.; Lee, U.H.; Kim, M.S.; Jung, M. Alterations of epidermal lipid profiles and skin microbiome in children with atopic dermatitis. Allergy Asthma Immunol. Res. 2023, 15, 186. [Google Scholar]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Ortonne, J.P. The normal color of human skin. In The Pigmentary System: Physiology and Pathophysiology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; pp. 504–520. [Google Scholar]
- Naik, P.P.; Farrukh, S.N. Influence of ethnicities and skin color variations in different populations: A review. Ski. Pharmacol. Physiol. 2022, 35, 65–76. [Google Scholar] [CrossRef]
- Fajuyigbe, D.; Young, A.R. The impact of skin colour on human photobiological responses. Pigment. Cell Melanoma Res. 2016, 29, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. The heterogeneity and complexity of skin surface lipids in human skin health and disease. Prog. Lipid Res. 2023, 93, 101264. [Google Scholar]
- Corvec, S. Clinical and biological features of Cutibacterium (formerly Propionibacterium) avidum, an underrecognized microorganism. Clin. Microbiol. Rev. 2018, 31, e00064-17. [Google Scholar] [CrossRef]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, A.; Grice, E.A. Interactions between host factors and the skin microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Egert, M.; Simmering, R. The microbiota of the human skin. In Microbiota of the Human Body; Springer: Cham, Switzerland, 2016; pp. 61–81. [Google Scholar]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.; Chen, W.-C.; Thornton, M.; Qin, K.; Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 2007, 39, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Coenye, T.; He, L.; Kabashima, K.; Kobayashi, T.; Niemann, C.; Nomura, T.; Oláh, A.; Picardo, M.; Quist, S.R. Sebaceous immunobiology-skin homeostasis, pathophysiology, coordination of innate immunity and inflammatory response and disease associations. Front. Immunol. 2022, 13, 1029818. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the skin and gut in atopic dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef]
- Townsend, E.C.; Kalan, L.R. The dynamic balance of the skin microbiome across the lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56 (Suppl. 2), S230–S242. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Zhang, M.; Kaffenberger, B.H. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017, 153, 523–528. [Google Scholar] [CrossRef]
- Liegenfeld, S.C.; Stenzel, S.; Rembe, J.-D.; Dittmer, M.; Ramos, P.; Stuermer, E.K. Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch. Microbiol. Res. 2025, 16, 39. [Google Scholar] [CrossRef]
- White, E.K.; Grice, E.A. The wound microbiome. Cold Spring Harb. Perspect. Biol. 2023, 15, a041218. [Google Scholar]
- Luna, P.C. Skin microbiome as years go by. Am. J. Clin. Dermatol. 2020, 21 (Suppl. 1), 12–17. [Google Scholar]
- Sun, H.; Pulakat, L.; Anderson, D.W. Challenges and new therapeutic approaches in the management of chronic wounds. Curr. Drug Targets 2020, 21, 1264–1275. [Google Scholar] [PubMed]
- Jockenhöfer, F.; Chapot, V.; Stoffels-Weindorf, M.; Körber, A.; Klode, J.; Buer, J.; Küpper, B.; Roesch, A.; Dissemond, J. Bacterial spectrum colonizing chronic leg ulcers: A 10-year comparison from a German wound care center. J. Dtsch. Dermatol. Ges. 2014, 12, 1121–1127. [Google Scholar]
- Ak, M. A comprehensive review of acne vulgaris. J. Clin. Pharm. 2019, 1, 17–45. [Google Scholar]
- Cavallo, I.; Sivori, F.; Truglio, M.; De Maio, F.; Lucantoni, F.; Cardinali, G.; Pontone, M.; Bernardi, T.; Sanguinetti, M.; Capitanio, B. Skin dysbiosis and Cutibacterium acnes biofilm in inflammatory acne lesions of adolescents. Sci. Rep. 2022, 12, 21104. [Google Scholar]
- Mawardi, P.; Ardiani, I.; Primisawitri, P.P.; Nareswari, A. Dual role of Cutibacterium acnes in acne vulgaris pathophysiology. Bali Med. J. 2021, 10, 486–490. [Google Scholar]
- Nakatsuji, T.; Tang, D.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: Potential targets for inflammatory acne treatment. PLoS ONE 2011, 6, e14797. [Google Scholar]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, 1953. [Google Scholar]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.-H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.P.; Leccia, M.T.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.; Vâță, D.; Alexa, A.-I.; Trandafir, L.; Patrașcu, A.-I.; Hâncu, M.F.; Gheucă-Solovăstru, L. Atopic Dermatitis—Beyond the Skin. Diagnostics 2021, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus aureus and the cutaneous microbiota biofilms in the pathogenesis of atopic dermatitis. Microorganisms 2019, 7, 301. [Google Scholar] [CrossRef]
- Gomez-Casado, C.; Unger, Z.; Olah, P.; Homey, B. Microbiome in Atopic Dermatitis: Is It All About Staphylococcus aureus? Curr. Treat. Options Allergy 2023, 10, 351–363. [Google Scholar]
- Özdemіr, E.; Öksüz, L. Effect of Staphylococcus aureus colonization and immune defects on the pathogenesis of atopic dermatitis. Arch. Microbiol. 2024, 206, 1–18. [Google Scholar]
- Proft, T.; Fraser, J.D. Bacterial superantigens. Clin. Exp. Immunol. 2003, 133, 299–306. [Google Scholar]
- Kim, K.H.; Han, J.H.; Chung, J.H.; Cho, K.H.; Eun, H.C. Role of staphylococcal superantigen in atopic dermatitis: Influence on keratinocytes. J. Korean Med. Sci. 2006, 21, 315–323. [Google Scholar]
- Kuchekar, A.B.; Pujari, R.R.; Kuchekar, S.B.; Dhole, S.N.; Mule, P.M.; Vidyapeeth, B.; Wadi, B. Psoriasis: A comprehensive review. Int. J. Pharm. Life Sci. 2011, 2, 857–877. [Google Scholar]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and microbiota: A systematic review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Visser, M.J.; Kell, D.B.; Pretorius, E. Bacterial dysbiosis and translocation in psoriasis vulgaris. Front. Cell. Infect. Microbiol. 2019, 9, 7. [Google Scholar]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.-h.; Strober, B.E.; Pei, Z.; Blaser, M.J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE 2008, 3, e2719. [Google Scholar] [CrossRef]
- Teng, Y.; Xie, W.; Tao, X.; Liu, N.; Yu, Y.; Huang, Y.; Xu, D.; Fan, Y. Infection-provoked psoriasis: Induced or aggravated. Exp. Ther. Med. 2021, 21, 567. [Google Scholar] [PubMed]
- Ruiz-Romeu, E.; Ferran, M.; Giménez-Arnau, A.; Bugara, B.; Lipert, B.; Jura, J.; Florencia, E.F.; Prens, E.P.; Celada, A.; Pujol, R.M. MCPIP1 RNase is aberrantly distributed in psoriatic epidermis and rapidly induced by IL-17A. J. Investig. Dermatol. 2016, 136, 1599–1607. [Google Scholar] [PubMed]
- Chang, H.-W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar]
- Macleod, T.; Bridgewood, C.; Hyde, I.; Heague, M.; Helliwell, P.; Stacey, M.; Wittmann, M. Molecular and cellular regulation of psoriatic inflammation. Clin. Sci. 2022, 136, 935–952. [Google Scholar]
- Bhatiaa, M.; Kumara, M.; Pareek, B.; Sonib, S. Skin & its ailments: A systemic. In Contemporary Advances in Science & Technology Volume-IV: Medicinal Chemistry & Pharmaceutical Sciences; Notion Press: Chennai, India, 2022; Volume 4, p. 57. [Google Scholar]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the microbiome: A systematic review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar]
- Rainer, B.M.; Thompson, K.G.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Bui, J.; Fischer, A.H.; Pasieka, H.B.; Garza, L.A.; Kang, S. Characterization and analysis of the skin microbiota in rosacea: A case–control study. Am. J. Clin. Dermatol. 2020, 21, 139–147. [Google Scholar]
- Woo, Y.R.; Lee, S.H.; Cho, S.H.; Lee, J.D.; Kim, H.S. Characterization and analysis of the skin microbiota in rosacea: Impact of systemic antibiotics. J. Clin. Med. 2020, 9, 185. [Google Scholar] [CrossRef]
- Robinson, J.; Begum, R.; Maqbool, M. Ultraviolet Radiation: Benefits, Harms, Protection. Introd. Non-Ioniz. Radiat. 2023, 2, 62. [Google Scholar]
- Merin, K.; Shaji, M.; Kameswaran, R. A review on sun exposure and skin diseases. Indian. J. Dermatol. 2022, 67, 625. [Google Scholar] [PubMed]
- Burns, E.M.; Ahmed, H.; Isedeh, P.N.; Kohli, I.; Van Der Pol, W.; Shaheen, A.; Muzaffar, A.F.; Al-Sadek, C.; Foy, T.M.; Abdelgawwad, M.S. Ultraviolet radiation, both UVA and UVB, influences the composition of the skin microbiome. Exp. Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzka, A.; Micallef, M.P.; Biazzo, M.; Podrini, C. The Role of the Skin Microbiome in Acne: Challenges and Future Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 11422. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Martiniaková, S. Efficacy of postbiotics against free radicals and UV radiation. Chem. Pap. 2022, 76, 2357–2364. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [PubMed]
- Rong, J.; Shan, C.; Liu, S.; Zheng, H.; Liu, C.; Liu, M.; Jin, F.; Wang, L. Skin resistance to UVB-induced oxidative stress and hyperpigmentation by the topical use of Lactobacillus helveticus NS8-fermented milk supernatant. J. Appl. Microbiol. 2017, 123, 511–523. [Google Scholar]
- Leung, A.K.; Lam, J.M.; Leong, K.F.; Hon, K.L. Vitiligo: An updated narrative review. Curr. Pediatr. Rev. 2021, 17, 76–91. [Google Scholar] [CrossRef]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.; Jouary, T. Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar]
- Kaufman, B.P.; Aman, T.; Alexis, A.F. Postinflammatory hyperpigmentation: Epidemiology, clinical presentation, pathogenesis and treatment. Am. J. Clin. Dermatol. 2018, 19, 489–503. [Google Scholar] [CrossRef]
- Ganju, P.; Nagpal, S.; Mohammed, M.; Nishal Kumar, P.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar]
- Ni, Q.; Ye, Z.; Wang, Y.; Chen, J.; Zhang, W.; Ma, C.; Li, K.; Liu, Y.; Liu, L.; Han, Z. Gut microbial dysbiosis and plasma metabolic profile in individuals with vitiligo. Front. Microbiol. 2020, 11, 592248. [Google Scholar]
- Giordano, S.; Romeo, M.; Di Summa, P.; Lankinen, P. A meta-analysis on evidence of platelet-rich plasma for androgenetic alopecia. Int. J. Trichology 2018, 10, 1–10. [Google Scholar]
- Rinaldi, F.; Trink, A.; Pinto, D. Efficacy of postbiotics in a PRP-like cosmetic product for the treatment of alopecia area Celsi: A randomized double-blinded parallel-group study. Dermatol. Ther. 2020, 10, 483–493. [Google Scholar]
- Won, E.J.; Jang, H.H.; Park, H.; Kim, S.J. A potential predictive role of the scalp microbiome profiling in patients with alopecia areata: Staphylococcus caprae, Corynebacterium, and Cutibacterium species. Microorganisms 2022, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, M.; Chen, S.; Khosrovi-Eghbal, A.; Ekelem, C.; Landaverde, Y.; Baldi, P.; Mesinkovska, N.A. Characterizing the skin and gut microbiome of alopecia areata patients. SKIN J. Cutan. Med. 2020, 4, 23–30. [Google Scholar]
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp bacterial shift in Alopecia areata. PLoS ONE 2019, 14, e0215206. [Google Scholar]
- Gómez-Arias, P.J.; Gay-Mimbrera, J.; Rivera-Ruiz, I.; Aguilar-Luque, M.; Juan-Cencerrado, M.; Mochón-Jiménez, C.; Gómez-García, F.; Sánchez-González, S.; Ortega-Hernández, A.; Gómez-Garre, D. Association Between Scalp Microbiota Imbalance, Disease Severity, and Systemic Inflammatory Markers in Alopecia Areata. Dermatol. Ther. 2024, 14, 2971–2986. [Google Scholar]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, H.; Jung, B.J.; You, G.E.; Jang, S.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits melanogenesis in B16F10 mouse melanoma cells. Mol. Cells 2015, 38, 163–170. [Google Scholar]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.; Shah, K.; Beede, K. Novel topical application of a postbiotic, LactoSporin®, in mild to moderate acne: A randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics 2020, 7, 70. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lee, H.; Kim, M.; Lee, J.W.; Saeed, M.; Lee, H.; Jung, S.-H.; Shim, J.-J.; Lee, J.-L.; Heo, K. Development and metabolic profiling of a postbiotic complex exhibiting antibacterial activity against skin microorganisms and anti-inflammatory effect on human keratinocytes. Food Sci. Biotechnol. 2022, 31, 1325–1334. [Google Scholar]
- Torii, S.; Torii, A.; Itoh, K.; Urisu, A.; Terada, A.; Fujisawa, T.; Yamada, K.; Suzuki, H.; Ishida, Y.; Nakamura, F. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int. Arch. Allergy Immunol. 2011, 154, 236–245. [Google Scholar] [PubMed]
- Saunte, D.M.; Gaitanis, G.; Hay, R.J. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112. [Google Scholar]
- Grimes, P.; Bhawan, J.; Howell, M.; Desai, S.; Coryell, E.; Einziger, M.; Simpson, A.; Yaroshinsky, A.; McCraw, T. Histopathological Changes Induced by Malassezin: A Novel Natural Microbiome Indole for Treatment of Facial Hyperpigmentation. J. Drugs Dermatol. 2022, 21, 141–145. [Google Scholar]
- Kao, H.-J.; Wang, Y.-H.; Keshari, S.; Yang, J.J.; Simbolon, S.; Chen, C.-C.; Huang, C.-M. Propionic acid produced by Cutibacterium acnes fermentation ameliorates ultraviolet B-induced melanin synthesis. Sci. Rep. 2021, 11, 11980. [Google Scholar]
- Mayser, P.; Schäfer, U.; Krämer, H.-J.; Irlinger, B.; Steglich, W. Pityriacitrin–an ultraviolet-absorbing indole alkaloid from the yeast Malassezia furfur. Arch. Dermatol. Res. 2002, 294, 131–134. [Google Scholar]
- Machowinski, A.; Krämer, H.J.; Hort, W.; Mayser, P. Pityriacitrin–a potent UV filter produced by Malassezia furfur and its effect on human skin microflora. Mycoses 2006, 49, 388–392. [Google Scholar]
- Kim, K.M.; Song, J.-W.; Lee, C.-W.; Kim, D.-S.; Sohn, J.; Lee, S. Skin Barrier-Enhancing Effects of Dermabiotics HDB with Regulation of Skin Microbiota. J. Microbiol. Biotechnol. 2023, 34, 65. [Google Scholar]
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863–3872. [Google Scholar]
- Im, A.-R.; Lee, B.; Kang, D.-J.; Chae, S. Skin moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food 2018, 21, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-J.; Iwasa, M.; Han, K.-I.; Kim, W.-J.; Tang, Y.; Hwang, Y.J.; Chae, J.R.; Han, W.C.; Shin, Y.-S.; Kim, E.-K. Heat-killed Enterococcus faecalis EF-2001 ameliorates atopic dermatitis in a murine model. Nutrients 2016, 8, 146. [Google Scholar] [CrossRef]
- Lambrechts, I.A.; de Canha, M.N.; Lall, N. Exploiting medicinal plants as possible treatments for acne vulgaris. In Medicinal Plants for Holistic Health and Well-Being; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–143. [Google Scholar]
- Lima, M.; Paulino, L.C. Oral postbiotics as a therapeutic strategy for atopic dermatitis: A systematic review of randomized controlled trials. J. Am. Nutr. Assoc. 2024, 43, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Cho, M.; Kang, D.-J. Anti-Inflammatory Response of New Postbiotics in TNF-α/IFN-γ-Induced Atopic Dermatitis-like HaCaT Keratinocytes. Curr. Issues Mol. Biol. 2024, 46, 6100–6111. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; McBain, A.J.; Cruickshank, S.M.; O’Neill, C.A. Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl. Environ. Microbiol. 2014, 80, 5773–5781. [Google Scholar] [CrossRef]
- Pinto, D.; Marzani, B.; Minervini, F.; Calasso, M.; Giuliani, G.; Gobbetti, M.; De Angelis, M. Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1, FGF7, VEGF-A and IL-8 genes. Peptides 2011, 32, 1815–1824. [Google Scholar] [CrossRef]
- Marzani, B.; Pinto, D.; Minervini, F.; Calasso, M.; Di Cagno, R.; Giuliani, G.; Gobbetti, M.; De Angelis, M. The antimicrobial peptide pheromone P lantaricin A increases antioxidant defenses of human keratinocytes and modulates the expression of filaggrin, involucrin, β-defensin 2 and tumor necrosis factor-α genes. Exp. Dermatol. 2012, 21, 665–671. [Google Scholar] [CrossRef]
- Dimarzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Chou, C.-H.; Chiang, Y.-J.; Lin, C.-G.; Lee, C.-H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114. [Google Scholar] [CrossRef]
- Catic, T.; Pehlivanovic, B.; Pljakic, N.; Balicevac, A. The moisturizing efficacy of a proprietary dermo-cosmetic product (cls02021) versus placebo in a 4-week application period. Med. Arch. 2022, 76, 108. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway. Stem Cell Res. Ther. 2019, 10, 243. [Google Scholar] [PubMed]
- Bae, W.Y.; Jung, W.H.; Lee, Y.J.; Shin, S.L.; An, Y.K.; Kim, T.R.; Sohn, M. Heat-treated Pediococcus acidilactici LM1013-mediated inhibition of biofilm formation by Cutibacterium acnes and its application in acne vulgaris: A single-arm clinical trial. J. Cosmet. Dermatol. 2023, 22, 3125–3134. [Google Scholar] [PubMed]
- Ho, H.H.; Chen, C.W.; Yi, T.H.; Huang, Y.F.; Kuo, Y.W.; Lin, J.H.; Chen, J.F.; Tsai, S.Y.; Chan, L.P.; Liang, C.H. Novel application of a Co-Fermented postbiotics of TYCA06/AP-32/CP-9/collagen in the improvement of acne vulgaris—A randomized clinical study of efficacy evaluation. J. Cosmet. Dermatol. 2022, 21, 6249–6260. [Google Scholar]
- D’Auria, E.; Panelli, S.; Lunardon, L.; Pajoro, M.; Paradiso, L.; Beretta, S.; Loretelli, C.; Tosi, D.; Perini, M.; Bedogni, G. Rice flour fermented with Lactobacillus paracasei CBA L74 in the treatment of atopic dermatitis in infants: A randomized, double-blind, placebo-controlled trial. Pharmacol. Res. 2021, 163, 105284. [Google Scholar] [PubMed]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar]
- Luo, C.-H.; Lai, A.C.-Y.; Chang, Y.-J. Butyrate inhibits Staphylococcus aureus-aggravated dermal IL-33 expression and skin inflammation through histone deacetylase inhibition. Front. Immunol. 2023, 14, 1114699. [Google Scholar]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Santo Domingo, J.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef]
- Jamaran, S.; Jafari, P.; Marjani, A.; Akbari, N.; Feizabad, M.M. Novel wound dressing based on postbiotic/chitosan film accelerates cutaneous wound healing. Jundishapur J. Microbiol. 2021, 14, e120806. [Google Scholar]
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A novel effective formulation of bioactive compounds for wound healing: Preparation, in vivo characterization, and comparison of various postbiotics cold creams in a rat model. Evid.-Based Complement. Altern. Med. 2021, 2021, 8577116. [Google Scholar]
- Kumar, A.; Green, K.M.; Rawat, M. A comprehensive overview of postbiotics with a special focus on discovery techniques and clinical applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [PubMed]
- Tokudome, Y. Influence of oral administration of lactic acid bacteria metabolites on skin barrier function and water content in a murine model of atopic dermatitis. Nutrients 2018, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Tamane, T.; Tokudome, Y. Effect of lactic fermentation products on human epidermal cell differentiation, ceramide content, and amino acid production. Ski. Pharmacol. Physiol. 2021, 34, 103–114. [Google Scholar]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [PubMed]
 ) indicates inhibitory pathway.
) indicates inhibitory pathway.
 ) indicates inhibitory pathway.
) indicates inhibitory pathway.
| Model/Population | Postbiotic Type | Outcome | References |
|---|---|---|---|
| In vitro study using B16F10 mouse melanoma cells | Lipoteichoic acid (LTA) derived from Lactiplantibacillusplantarum | Inhibits melanogenesis and reduces cellular tyrosinase activity; reduces expression of tyrosinase family members | [118] |
| Open-label, randomized monocentric study in humans with mild to moderate acne | LactoSporin (derived from Bacillus coagulans) | Reduces oiliness, acne, and redness around acne | [119] |
| In vitro study using human keratinocytes (HaCaT cells) | Postbiotic complex (PC) from Lactobacillus helveticus HY7801 and Lactococcus lactis HY449 | Antibacterial effects against Staphylococcus aureus and Cutibacterium acne; shows anti-inflammatory activity by modulating inflammatory cytokines; increases hyaluronic acid levels in keratinocytes | [120] |
| Japanese children with atopic dermatitis | L-92 (derived from Lactobacillus acidophilus) | Significantly improves atopic dermatitis by regulating the Th1/Th2 immune axis | [121] |
| Human skin | Malassezin (from Malassezia furfur) | Associated with pityriasis versicolor | [122] |
| Human skin, in vitro | Malassezin | Decreased epidermal discoloration; reduced photoaging-induced hyperpigmentation; apoptotic effects on primary human melanocytes | [123] |
| Human skin | Propionic acid (from Cutibacterium acnes) | Potential non-toxic solution for hyperpigmentation | [124] |
| Yeast, bacteria, and humans | Pityriacitrin (from Malassezia furfur) | Potent UV-protective properties | [125] |
| Bacterial and fungal skin microflora | Pityriacitrin (yeast) | UV-absorbing effects on skin microflora | [126] |
| HDB lysate | Lactiplantibacillus plantarum | Enhanced skin hydration; reduced transepidermal water loss (TEWL); diminished skin redness on face | [127] |
| Microbial Source | Model/Population | Postbiotic Type | Outcomes | References |
|---|---|---|---|---|
| Epidermidibacterium Keratini (EPI-7) | Healthy women | Ferment filtrate | Significantly enhances skin tone and skin microbiome diversity | [141] |
| Pediococcus acidilactici LM1013 | Patients diagnosed with acne vulgaris | Heat-treated | Inhibites acne vulgaris | [149] |
| Lactobacillus acidophilus TYCA06*, Ligilactobacillus salivarius AP-32, and Bifidobacterium animalis subsp. lactis CP-9 | Patients diagnosed with acne vulgaris | Collagen cofermentation | Ameliorates redness and inflammation | [142] |
| Peptides from Streptococcus thermophilus S244 | Aging population | Sonicated | Increases skin hydration and boost ceramide levels in the skin on forearm | [137] |
| GMNL6 from Lactiplantibacillus plantarum | Aged population | Heat-killed | Increases skin hydration and reduces wrinkles; improves skin texture, tone, and UV-induced spots; reduces skin redness and melanin levels on face | [138] |
| L. casei AN177, Lactiplantibacillus plantarum AN057, S. thermophilus AN157 | Aged population | Cofermented metabolites CLS02021 | Improves skin hydration and elasticity; reduces pore size and wrinkle depth on face | [139] |
| Population | Metabolites | Outcomes | References |
|---|---|---|---|
| Adults with dry skin | Lactate | Improves skin hydration and reduced dryness and treatment of moderate xerosis | [150] |
| Hapten-sensitized mice | Butyrate and acetate | Effective against dermatitis | [144] |
| Atopic dermatitis-like mouse model | Butyrate | Prevents skin inflammation | [145] |
| Atopic dermatitis-like skin inflammation | SCFAs | Increase skin barrier integrity by promoting keratinocyte and prevent allergic inflammation | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and Postbiotics in Skin Health. Biomedicines 2025, 13, 791. https://doi.org/10.3390/biomedicines13040791
Prajapati SK, Lekkala L, Yadav D, Jain S, Yadav H. Microbiome and Postbiotics in Skin Health. Biomedicines. 2025; 13(4):791. https://doi.org/10.3390/biomedicines13040791
Chicago/Turabian StylePrajapati, Santosh Kumar, Lalitha Lekkala, Dhananjay Yadav, Shalini Jain, and Hariom Yadav. 2025. "Microbiome and Postbiotics in Skin Health" Biomedicines 13, no. 4: 791. https://doi.org/10.3390/biomedicines13040791
APA StylePrajapati, S. K., Lekkala, L., Yadav, D., Jain, S., & Yadav, H. (2025). Microbiome and Postbiotics in Skin Health. Biomedicines, 13(4), 791. https://doi.org/10.3390/biomedicines13040791








