Abstract
The skin microbiome, a diverse and dynamic ecosystem of microorganisms, plays a pivotal role in maintaining skin health by interacting with skin cells, immune components, and structural barriers. It is essential for skin homeostasis, immune defense, and protection against pathogenic colonization. Dysbiosis in the microbiome has been implicated in numerous dermatological conditions, including acne, eczema, psoriasis, and rosacea. Acne, the most prevalent skin condition, affects up to 85% of individuals at some point in their lives, while eczema and psoriasis impose significant public health and economic burdens. The composition of the skin microbiome varies across skin types and anatomical sites, with sebaceous, moist, and dry areas fostering distinct microbial communities. Emerging therapeutic strategies such as microbiome-targeted treatments offer novel avenues for addressing skin diseases. Among these approaches, postbiotics have gained significant attention for their safety and efficacy. Unlike probiotics, postbiotics are non-viable microbial cells or their metabolites, which reduce safety concerns while providing functional benefits such as UV protection and wound healing. This review consolidates current insights into the role of the skin microbiome in health and disease, emphasizing postbiotics as a promising therapeutic strategy by exploring the clinical and commercial potential of microbiome-based treatments, particularly postbiotics, and their ability to redefine dermatological care and improve patient outcomes.
Keywords:
skin microbiome; skin health; postbiotics; skin diseases; acne; eczema; psoriasis; dermatology; skin barrier function 1. Introduction
In recent years, the skin microbiome has garnered increasing attention in both scientific research and commercial skincare [1]. The skin is not only the body’s largest organ but also a dynamic ecosystem inhabited by trillions of microbes, including bacteria, fungi, and viruses, all of which contribute to skin health and functionality [2,3]. These microorganisms play a crucial role in maintaining the skin’s structural integrity and immune defense mechanisms by interacting with skin cells and environmental factors [4,5]. Hence, the microbiome is essential for the maintenance of skin barriers [5], and its dysbiosis has been implicated in a wide range of dermatological conditions, such as acne, eczema, and psoriasis [6]. Acne is the most prevalent skin condition, especially in adolescents, affecting up to 85% of people at some point in their lives [7]. Eczema, also known as atopic dermatitis, affects around 1–20% of the population, leading to chronic inflammation and skin barrier dysfunction [8]. Psoriasis, an autoimmune disorder, leads to scaly, itchy patches of skin, affecting about 3% of people worldwide [9]. Beyond these common conditions, rare disorders such as vitiligo (loss of skin pigmentation) and alopecia (hair loss) also impact millions of people [10].
The skin microbiome makes a significant contribution to cutaneous immune defense by preventing the colonization of pathogenic microorganisms and by supporting skin homeostasis [11,12]. However, when this delicate balance is disrupted, harmful bacteria can proliferate, worsening the symptoms—though not the root cause—of atopic dermatitis, rosacea, and seborrheic dermatitis [13]. For instance, Staphylococcus epidermidis, a commensal bacterium, produces antimicrobial peptides (AMPs) that suppress pathogens like Staphylococcus aureus while also modulating inflammatory pathways to support skin homeostasis [14]. Additionally, microbial metabolites, such as short-chain fatty acids (SCFAs) and ceramides, strengthen the skin barrier, promote hydration, and maintain an acidic pH, which is essential for preventing microbial overgrowth [15]. Further, commensal microbiome-specific T-cell responses, driven by skin-resident dendritic cells, occur without inflammation, highlighting tissue-resident cells’ ability to sense microbial changes [16]. Colonization by Staphylococcus epidermidis activates interleukin (IL)-17A+ CD8+ T cells, enhancing innate immunity and limiting pathogen invasion. Therefore, skin microbiomes have emerged as a critical therapeutic target in preventing and treating skin diseases.
The composition of the skin microbiome is affected by skin type (e.g., dry, oily, or moist), anatomical site, age, and environmental factors. Sebaceous sites, such as the forehead and back, are dominated by Cutibacterium acnes, a bacterium associated with sebum metabolism and acne pathogenesis [17]. Moist areas like the underarms harbor Corynebacterium and Staphylococcus species, while dry areas like the forearms have a higher microbial diversity, including Gram-positive and Gram-negative bacteria [18]. These variations reflect the adaptive nature of microbial communities to their local microenvironment. Overall, skin conditions, whether mild or severe, present significant challenges not only to individuals but also to public health systems globally. In the United States, the cost of managing skin diseases exceeds USD 75 billion annually, comprising direct costs like hospital visits, drugs, and over-the-counter treatments, as well as indirect costs such as loss of productivity and the psychological burden on patients [19]. Further, there are limited drugs available for the treatment of skin diseases, including anti-inflammatory agents, corticosteroids, and immunomodulators (tacrolimus, pimecrolimus, and methotrexate) [8]. Despite these advancements, available treatment options remain limited and often pose severe long-term adverse effects.
Recently, in clinical settings, therapies aimed at restoring microbial balance, such as the use of prebiotics, probiotics, and postbiotics, have shown promise in treating conditions like acne and atopic dermatitis [20,21,22]. Postbiotics represent a promising alternative for skin diseases due to their potential safety and efficacy profiles. Modulating this microbial community through microbiome-targeted approaches such as prebiotics, probiotics, and postbiotics is emerging as a promising therapeutic strategy [23]. Among these strategies, postbiotics, i.e., bioactive compounds produced by probiotic organisms, are gaining interest for their potential to support and restore skin microbial balance, offering unique advantages over prebiotics and probiotics [20,21]. Unlike probiotics, postbiotics lack live cells, which minimize safety concerns related to microbial viability, yet they retain beneficial properties that promote a healthy skin environment. Unlike prebiotics, which serve as nutrients for beneficial skin bacteria [24], postbiotics deliver immediate functional benefits without the need for live bacteria. Postbiotics, comprising non-viable microbial cells or their metabolites, have demonstrated promise in enhancing skin health by providing anti-inflammatory and antioxidant effects, strengthening the skin barrier, boosting hydration, and selectively supporting the growth of beneficial microbes while inhibiting pathogenic colonization [25,26]. This review aims to emphasize the skin microbiome and associated abnormalities. Further, we elaborated the findings on microbiome-targeted therapies in dermatology, with a primary focus on postbiotics’ role in maintaining microbial balance and supporting skin health.
2. Ontology of Skin
The skin is a complex, multilayered organ composed of epidermis, dermis, and hypodermis. These layers perform vital functions that contribute to the skin’s role as a physical barrier, thermoregulator, and sensory organ [27]. The epidermis, the outermost layer, is primarily made up of keratinocytes, cells that produce keratin, a structural protein crucial for the skin’s protective and resilient qualities [28,29]. The dermis beneath it contains connective tissue, blood vessels, nerves, and skin appendages such as hair follicles and sweat glands. The deepest layer, the hypodermis, consists of adipose tissue, which provides insulation and mechanical protecting to the body [30]. The skin microbiome is distributed throughout different layers and regions of the skin, with specific types of microorganisms adapted to different skin environments.
Epidermis: The outermost layer of the skin, particularly the stratum corneum (the top layer of the epidermis), hosts a variety of bacteria, fungi, and viruses [31,32]. This layer provides a protective barrier, and its slightly acidic pH favors the growth of certain beneficial bacteria that help defend against pathogens [32]. The diverse community of microorganisms residing on the epidermis primarily consists of bacteria, fungi, and viruses. Key species include Staphylococcus epidermidis, Propionibacterium, Corynebacterium, Micrococcus, and Malassezia, which predominantly inhabit the outermost layer of the epidermis, known as the stratum corneum [3]. Colonization by Staphylococcus epidermidis triggers the activation of IL-17A+ CD8+ T cells, which migrate to the epidermis, boost innate barrier immunity, and reduce pathogen invasion [16]. The T-cell responses specific to commensals arise from the coordinated activity of skin-resident dendritic cell subsets and occur without causing inflammation [16]. Micrococcus species are part of the normal flora of human skin and are involved in the defense against pathogenic microorganisms [33]. They produce antimicrobial substances and help maintain skin integrity by regulating the immune system. This indicates that tissue-resident cells are primed to detect and react to changes in microbial communities.
Hair follicles (HF): Microorganisms also inhibit hair follicles, which provide a protected, nutrient-rich environment. Immune cells, including Langerhans cells (LCs), are present in the skin and around appendages, closely interacting with the microbiota [34]. LCs, located in the epidermis and HF outer root sheath, capture microbial antigens and interact with skin-resident memory T cells [35]. Under steady conditions, LCs promote the activation of T regulatory cells for peripheral tolerance. During pathogen exposure, they stimulate effector memory T cells. Additionally, microbe-derived products are taken up by antigen-presenting cells, keratinocytes, or diffuse through the epithelial barrier [36]. Skin-resident CD103+ dendritic cells sense microbial shifts, particularly in response to Staphylococcus epidermidis colonization in mice [37]. Further, in anaerobic conditions, the deeper follicle regions support the growth of bacteria like Propionibacterium acnes, commonly associated with acne [12,38]. Moreover, bacteriocins are antimicrobial peptides produced by bacteria to inhibit the growth of closely related or competing bacterial species and genera. Staphylococcus epidermidis, a commensal bacterium found on human skin, produces several types of bacteriocins that specifically target and inhibit pathogens, including Staphylococcus aureus, which is associated with infections and inflammatory skin diseases [39]. Staphylococcus epidermidis also produces antimicrobial peptides (AMPs) like phenol-soluble modulins (PSMs), which have strong antimicrobial activity against Staphylococcus aureus and other pathogens [40]. These AMPs help protect against skin infections and contribute to immune modulation by activating host immune receptors. For instance, PSMs can stimulate keratinocytes to release cytokines that enhance skin immunity and reinforce the skin’s barrier function [14].
Sebaceous (oil) glands: These glands produce sebum, an oily substance that supports certain types of bacteria and fungi, including Cutibacterium acne. Sebaceous areas, such as the scalp, face, and upper back, tend to have higher microbial density due to the abundance of lipids. As people age, sebum production gradually declines, especially after middle age [17]. This reduction in oiliness leads to less favorable conditions for Cutibacterium acnes, which explains why acnes often become less severe or even resolves as people grow older [17]. Additionally, the skin becomes dry with age, reducing the presence of lipid-dependent bacteria in hair follicles. Studies have consistently shown that the microbial communities in these distinct microenvironments differ significantly in both composition and function [41]. These variations are likely influenced by differences in the availability and utilization of nutrients by microbes, as well as the inhibitory effects of skin secretions unique to each site [42,43].
Sweat glands: Both eccrine and apocrine sweat glands contribute to the skin microbiome. Apocrine glands, particularly in areas like the armpits and groin, release a nutrient-rich sweat that supports different microbial communities, which are involved in body odor production [31]. However, the skin barrier is not solely dependent on its structural components. The resident skin microbiome, an ecosystem of bacteria, fungi, viruses, and archaea, plays a vital role in maintaining skin homeostasis and health [31]. The microbiome supports the skin by producing AMPs, regulating pH, and metabolizing sebum, the skin’s natural oil [5]. Importantly, this symbiotic relationship between skin cells and microbiota enhances the skin’s ability to protect against environmental insults, pathogens, and inflammatory stimuli [3]. The following section emphasized the skin microbiome and its interaction with different skin cells.
3. Commensal Microbiome and Immune Cell Interaction to Maintain Skin Health
The human skin is the body’s largest organ and serves as a complex and dynamic barrier that interacts with both the external environment and internal physiological systems [3]. In the skin resides a diverse community of microorganisms, collectively known as the skin microbiome, which includes bacteria, fungi, viruses, and archaea [31]. These microorganisms are essential for maintaining the health and function of the skin. They contribute to various processes, such as protecting the skin from pathogens, modulating immune responses, and maintaining the skin’s barrier function (Figure 1) [5].
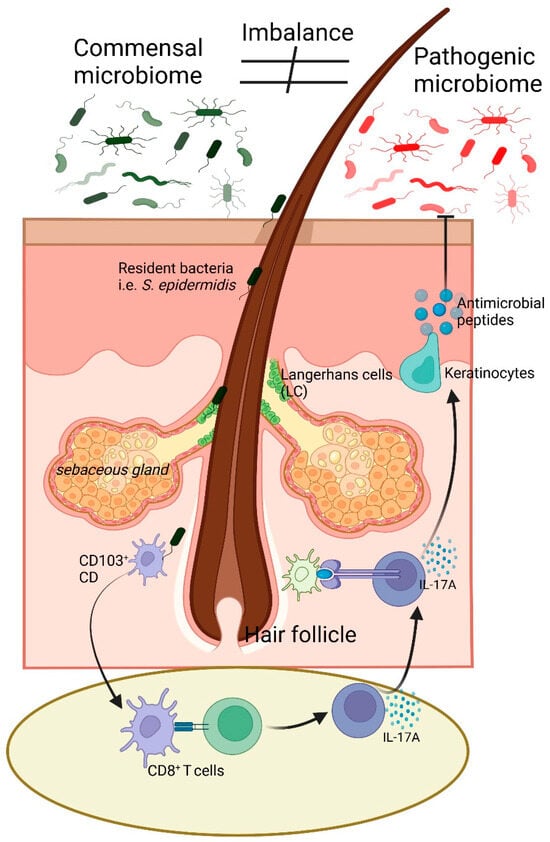
Figure 1.
Illustration of skin microbiome balance and immune response regulation. The commensal microbiome, including Staphylococcus epidermidis, supports skin homeostasis and immune balance. An imbalance can lead to a shift towards a pathogenic microbiome, triggering immune responses. LC and CD103+ dendritic cells detect microbial changes and interact with CD8+ T cells, leading to IL-17A secretion. Keratinocytes respond by producing antimicrobial peptides, contributing to pathogen defense. The sebaceous gland and hair follicle microenvironment play key roles in this process. Arrow (→) indicates direction of flow of signals, while block arrow ( ) indicates inhibitory pathway.
) indicates inhibitory pathway.
 ) indicates inhibitory pathway.
) indicates inhibitory pathway.
The interaction between the skin and its resident microbiota is a critical determinant of skin health, and disruptions to this relationship can lead to a wide range of skin disorders.
The skin microbiome varies significantly across different body sites, largely influenced by factors such as moisture levels, temperature, pH, and exposure to environmental elements [3]. Areas such as the face, hands, and scalp have distinct microbial communities and are regulated by specific environments [3]. Understanding the complex interactions between skin cells and the microbiome is essential for developing effective treatments for skin diseases and for maintaining overall skin health.
4. Interaction of Microbiome with Skin
The skin microbiome varies across different regions of the body due to differences in environmental factors such as moisture, temperature, and sebum production [3]. Sebaceous areas like the forehead and upper back are rich in lipophilic bacteria such as Cutibacterium acnes (formerly Propionibacterium acnes), which metabolizes sebum into fatty acids that nourish the microbiome while maintaining skin integrity [44]. Conversely, moist areas like the axillae and groin support the growth of bacteria such as Staphylococcus and Corynebacterium species, which are better adapted to these humid microenvironments [18]. Skin microbiota can modulate the skin’s immune system by engaging with immune cells such as Langerhans cells and dermal dendritic cells [45,46]. These interactions help maintain immune tolerance, preventing overactivation of inflammatory pathways that may contribute to conditions such as atopic dermatitis and psoriasis [16]. Additionally, the skin microbiome produces a variety of metabolites, such as SCFAs, bacteriocins, and AMPs, which contribute to pathogen inhibition and skin barrier reinforcement. These metabolites play a significant role in maintaining skin homeostasis, promoting immune tolerance, and defending against potential pathogens [47,48]. For instance, Staphylococcus epidermidis, a commensal bacterium, has been shown to produce AMPs that directly inhibit Staphylococcus aureus, a pathogen associated with various skin infections [14]. Further, SCFAs such as acetate, propionate, and butyrate are key microbial metabolites produced primarily by anaerobic bacteria like Cutibacterium acne. These SCFAs help maintain an acidic environment on the skin, which is less hospitable to many pathogenic bacteria. SCFAs also strengthen the skin’s barrier function by promoting ceramide production, which enhances hydration and structural integrity [15]. Overall, these microbial metabolites not only inhibit pathogens but also strengthen the skin barrier. For example, commensal bacteria induce the production of tight junction proteins in skin cells, which improves barrier integrity and reduces transepidermal water loss. This activity is particularly beneficial in conditions where the skin barrier is compromised, such as eczema and psoriasis [15].
5. Types of Skin and Their Microbiomes
Rough vs. smooth skin: Rough and smooth skin are differentiated by the structure and composition of the stratum corneum, the outermost layer of the epidermis. Rough skin typically has a thicker stratum corneum, characterized by an accumulation of dead skin cells and lower hydration levels, which results in a coarse texture. In contrast, smooth skin is well-hydrated, with a balanced turnover of skin cells, leading to a soft and even texture [2]. The microbiome of rough skin tends to differ from that of smooth skin due to variations in the skin’s microenvironment. Rough skin, often found in areas subjected to friction or environmental stress, may harbor a higher concentration of Gram-positive bacteria such as Corynebacterium and Staphylococcus [49]. A study demonstrated that these bacteria thrive in dry, keratinized environments. They have adapted to metabolize lipids and proteins from the outer skin layer, contributing to the removal and renewal process of dead skin cells [43]. Corynebacterium species is known for their ability to degrade long-chain fatty acids in the skin [50], a process that can lead to odor production in rough areas like armpits but also supports the removal of dead cells in dry regions. Further, rough skin areas tend to host more bacteria, which can exacerbate inflammation. For instance, an increase in the diversity of certain Staphylococcus species, like Staphylococcus epidermidis, has been correlated with mild irritation in rougher areas of the skin [43]. These bacteria, while part of the normal skin flora, may become problematic when present in excessive amounts, leading to minor infections or increased cell turnover. Therefore, the microbial diversity of rough skin could be one of the contributing factors for the development of skin sensitivity or related abnormality. Application of microbiome/their metabolites or postbiotics may reverse these changes.
In contrast to rough skin, smooth skin tends to have a more diverse microbial community, including both Gram-positive and Gram-negative bacteria, fungi, and other microbes [3]. The presence of species like Cutibacterium acne and Malassezia (yeast) is often observed in more balanced skin areas [3]. This microbial diversity supports skin homeostasis, reducing the likelihood of inflammatory or pathogenic bacterial overgrowth. The diversity of microbes on smooth skin plays a key role in maintaining the integrity of the skin barrier. A study by Belkaid and Segre demonstrated that microbial diversity enhances the skin’s defense mechanisms by preventing pathogenic organisms from dominating the skin’s ecosystem [51]. Species like Staphylococcus epidermidis, in smoother skin areas, have been shown to produce antimicrobial peptides that help fend off pathogens like Staphylococcus aureus, thus contributing to the maintenance of skin health [51].
Dark vs. light skin: The color of human skin is determined by the amount and distribution of melanin, a pigment produced by melanocytes in the epidermis [52,53]. Individuals with darker skin have more melanin and often more active melanocytes compared to those with lighter skin [52,53]. This difference in pigmentation has implications for the skin’s interaction with UV radiation, with darker skin providing more protection against sun damage [54]. The skin microbiome also varies between individuals with dark and light skin. Studies have shown that individuals with darker skin tend to have a higher abundance of Corynebacterium species [18], while those with lighter skin may harbor more Propionibacterium species [44]. These differences may be attributed to variations in sebum production, moisture levels, and other factors influenced by melanin content. Melanin itself has been shown to possess antimicrobial properties, which could also influence the composition of the skin microbiome. Therefore, regulation or transplantation of a specific microbiome can regulate the skin melanin content and could be rate limiting in a microbiome-based therapeutic approach related to melanin sensitivity.
Oily vs. dry skin: Oily skin is characterized by the over-production of sebum, while dry skin has insufficient sebum production, leading to a lack of moisture and a compromised skin barrier [55]. The microbiomes of oily and dry skin differ significantly due to these variations in the skin’s lipid content. It has been reported that oily skin tends to have higher levels of Cutibacterium acnes (formerly Propionibacterium acnes), a bacterium that metabolizes sebum into free fatty acids [56]. These fatty acids can have antimicrobial effects, shaping the composition of the microbiome. Additionally, the abundance of sebum on oily skin can create a favorable environment for lipophilic fungi such as Malassezia [57]. In contrast, dry skin is more prone to colonization by bacteria like Staphylococcus epidermidis, which can thrive in lower-lipid environments [58]. The reduced microbial diversity on dry skin can make it more susceptible to irritation and inflammation.
Sweaty vs. less sweaty skin: Sweaty skin is typically found in areas with higher densities of sweat glands, such as the armpits, palms, and feet. Sweat, composed of water, salts, and organic compounds, creates a moist environment that influences the composition of the skin microbiome [59]. Moist environments promote the growth of bacteria like Corynebacterium and Staphylococcus, which are commonly found in sweat-prone areas. Less sweaty skin, on the other hand, tends to have lower moisture levels and a different microbial composition, and it has been demonstrated that these areas may harbor more Actinobacteria and Firmicutes, which thrive in drier conditions [60]. Therefore, differences in microbial composition between sweaty and less sweaty skin can influence the skin’s overall function and susceptibility to infections, as sweat can also act as a medium for the spread of pathogenic microorganisms if not regulated properly by the microbiome.
Male vs. female skin: The skin of males and females exhibits physiological differences, largely influenced by hormonal factors. Male skin tends to be thicker and produces more sebum due to the higher levels of androgens, such as testosterone [61]. This increased sebum production creates a favorable environment for sebaceous gland-associated bacteria like Cutibacterium acnes [62]. Additionally, the higher density of sweat glands in males contributes to a microbiome dominated by moisture-loving bacteria like Corynebacterium [63]. Female skin, in contrast, is typically thinner and may be more sensitive to hormonal fluctuations, particularly those related to the menstrual cycle [58]. These hormonal changes can influence both sebum production and the composition of the skin microbiome [58]. Females may experience shifts in their skin’s microbial community over time, with increases in Staphylococcus species during periods of lower sebum production [64]. The interplay between hormones, sebum, and the microbiome in males and females creates distinct microbial environments that influence skin health and susceptibility to conditions like acne and eczema.
6. Microbiome and Skin Diseases
Dysbiosis and skin disease: Human skin, though resilient, is susceptible to a wide array of diseases that range from mild, cosmetic concerns to more severe conditions that significantly impair quality of life [65]. Skin disorders are among the most common health complaints globally, with billions spent annually on treatments and consultations [66]. Disruptions in the composition of the skin microbiome, known as dysbiosis in skin, have been linked to several inflammatory diseases, including acne, eczema and psoriasis [58], as well as other rare disorders like vitiligo, melanoma, and alopecia. In this context, various studies have shown that manipulating the microbiome can regulate skin health. For example, germ-free mouse models, which lack a commensal microbiome, exhibit heightened susceptibility to inflammatory skin conditions, but colonization with specific commensal bacteria such as S. epidermidis in these models restores immune balance and enhances skin integrity [37]. Despite this advancement, many skin diseases remain inadequately treated due to our limited understanding of their underlying pathophysiology and the ineffectiveness or adverse side effects of current treatments. Further, understanding how the skin microbiome contributes to skin diseases opens up new avenues for therapeutic interventions that target microbial balance rather than just addressing symptoms. The following sections provide an in-depth look at the relationship between the microbiome and specific skin diseases, as shown in Figure 2. Further, the skin microbiome plays a vital role in wound healing by maintaining a balance between pathogenic and non-pathogenic microbes [67,68]. While beneficial microbes such as Staphylococcus epidermidis and Cutibacterium acnes support immune modulation and prevent biofilm formation, pathogenic bacteria like Staphylococcus aureus and Pseudomonas aeruginosa contribute to inflammation and tissue damage [67,68]. Wound colonization is influenced by factors such as immune status, genetic predisposition, environment, and lifestyle [69]. Acute wounds, including those from trauma or burns, typically host a diverse microbial population comprising Gram-positive and Gram-negative bacteria [70]; whereas chronic wounds, such as diabetic foot ulcers, often harbor a stable pathogenic community dominated by Staphylococcus aureus, Pseudomonas aeruginosa, Corynebacterium, and anaerobes [67,71]. The presence of biofilms in chronic wounds further complicates healing by promoting inflammation and delaying tissue regeneration [67]. Therefore, understanding the wound microbiome is crucial for developing targeted therapeutic strategies to enhance healing and prevent complications.
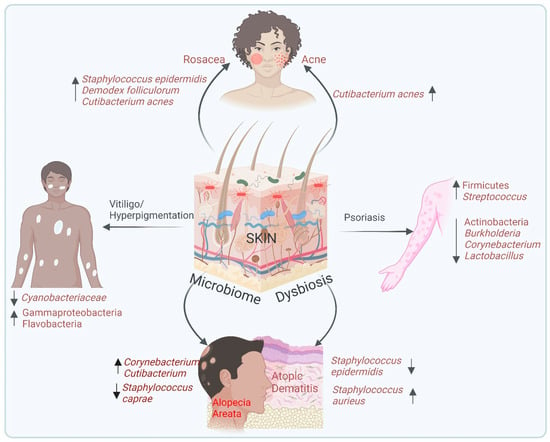
Figure 2.
Skin microbiome dysbiosis in different dermatological abnormalities. This illustration highlights the association between skin microbiome dysbiosis and various dermatological conditions. Acne occurs due to an increase in the abundance of Cutibacterium acne. Rosacea causes increased levels of Staphylococcus epidermidis, Demodex folliculorum, and Cutibacterium acne. Further, during psoriasis, there is an enrichment of Firmicutes and Streptococcus and a reduction in actinobacteria, Burkholderia, Corynebacterium and Lactobacillus. Vitiligo/hyperpigmentation causes a reduction in the diversity of Cyanobacteriaceae and Flavobacteria. Atopic dermatitis is due to the dysregulation of the microbiome with increased Staphylococcus aureus and decreased Staphylococcus epidermidis. Moreover, the alopecia areata expresses high levels of Corynebacterium and Cutibacterium with a reduction in Staphylococcus caprae. This figure underscores the significance of microbial balance in maintaining skin health and that of its perturbation as a contributing factor to disease pathogenesis.
Acne: Acne is characterized by the formation of pimples, blackheads, whiteheads, and cysts, primarily on the face, chest, and back [72]. Cutibacterium acnes (formerly Propionibacterium acnes) is the primary bacterium implicated in acne development [56,73]. This microorganism increases in the oily environment of sebaceous glands and induces inflammation by interacting with Toll-like receptors (TLRs) on keratinocytes, leading to the release of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α [74]. Additionally, Cutibacterium acnes contribute to inflammation and host tissue damage by producing various virulence factors, including lipases, proteases, and Christine–Atkins–Munch-Petersen (CAMP) factors [75]. A study revealed that acne-associated Cutibacterium acnes strains significantly increased the production of inflammatory cytokines—specifically interferon (IFN)-γ and IL-17—in peripheral blood mononuclear cells [76]. Another study by Agak et al. emphasized the increased expression of IFN-γ and IL-17 in acne lesions [77]. Overall, an elevated level of Cutibacterium acnes enhanced the expression of filaggrin and integrin, contributing to the abnormal adhesion and differentiation of keratinocytes [78]. Moreover, acne-associated Cutibacterium acnes strains exhibited significantly higher expression of an adhesion protein, a cell surface hydrolase, compared to strains linked to healthy skin. This indicates the distinct functional roles of Cutibacterium acnes strains in modulating host immune responses and acne pathogenesis. These findings highlight the critical role of the microbiome in regulating acne. Therefore, a balanced skin microbiome is essential to prevent the overgrowth of pathogenic strains, suggesting that microbial modulation may be a promising approach to managing acne.
Eczema (Atopic Dermatitis): Eczema is a chronic inflammatory skin condition characterized by red, itchy, and inflamed skin [79]. The disease often manifests in childhood and can persist into adulthood, significantly impacting quality of life. The exact cause of eczema is multifactorial, involving genetic predisposition, environmental triggers, and immune dysregulation [79]. In recent years, the role of the skin microbiome in eczema has garnered significant attention. One of the hallmark features of eczema is a reduction in microbial diversity, particularly a decrease in beneficial bacteria such as Staphylococcus epidermidis and an overgrowth of Staphylococcus aureus [80]. Staphylococcus aureus can exacerbate the inflammatory response in eczema by colonizing the skin and producing toxins that further damage the skin barrier [81]. Studies have shown that the presence of Staphylococcus aureus on the skin correlates with increased disease severity as the bacterium promotes immune activation and the release of pro-inflammatory cytokines like IL-4, IL-13, and IL-31 [82]. Staphylococcus aureus can aggravate the severity of eczema by releasing various virulence factors such as superantigens (SAgs). Mechanistically, SAgs bind to the major histocompatibility complex class II (MHC-II) molecules on antigen-presenting cells (APCs), including skin keratinocytes, and to the β-chains of T cell receptors (TCRs) [83]. This results in the non-specific activation of T cells, triggering systemic inflammation through the release of pro-inflammatory cytokines such as TNF-α and IL-1β [84]. Overall, in eczema, reduced microbial diversity and the overgrowth of Staphylococcus aureus exacerbate the disease by intensifying inflammation and disrupting the skin barrier. Therefore, findings suggest that the regulation of Staphylococcus aureus could present a viable therapeutic approach to treating or preventing eczema.
Psoriasis: Psoriasis is a chronic autoimmune condition that affects approximately 2–3% of the global population. It is characterized by thick, scaly patches of skin that can appear on different parts of the body, which includes the scalp, knees, and lower back [85]. Psoriatic skin lesions exhibit an enrichment of bacteria from phyla of Firmicutes, whereas bacteria from phyla of actinobacteria are notably under-represented compared to healthy and non-lesional skin [86].
Furthermore, Streptococcus, Staphylococcus, Corynebacterium, and Propionibacterium were identified in lesional skin [87]. Compared to healthy individuals, those with psoriasis often have lower microbial diversity and an enrichment of Streptococcus and reduction in Burkholderia, Corynebacterium, and Lactobacillus species [2,88,89]. In particular, Streptococcus pyogenes has been implicated in triggering and exacerbating psoriasis by inducing immune responses that promote the proliferation of keratinocytes and inflammatory cells [90,91]. Additionally, Chang et al. showed that a reduction in immunoregulatory bacteria like Staphylococcus epidermidis and Cutibacterium acnes leads to increased colonization of Staphylococcus aureus, which aggravates cutaneous inflammation through the T helper type-17 (Th17) axis [92]. This condition is driven by the hyperactivation of the immune system that leads to the rapid turnover of skin cells. Although psoriasis has a strong genetic component, environmental factors, including microbial dysbiosis, play a critical role in its pathogenesis [93]. Research on the microbiome in psoriasis has revealed significant alterations in the skin’s microbial communities.
Rosacea: Rosacea is a persistent inflammatory skin condition that mainly impacts the face, leading to symptoms such as redness, visible blood vessels, and, in some instances, small, red, pus-filled bumps [94]. While the precise cause of rosacea remains unclear, it is believed to result from a combination of genetic factors, immune system dysfunction, and environmental triggers. Recently, skin microbiomes have emerged as a key player in rosacea pathogenesis. Individuals with rosacea often show dysbiosis in their skin microbiomes, particularly an overgrowth of the skin mite Demodex folliculorum, which can provoke an inflammatory response [95]. Additionally, studies have found that Cutibacterium acnes and Staphylococcus epidermidis are more abundant on the skin of rosacea patients, potentially contributing to the condition’s characteristic inflammation [96,97]. The interactions between these microorganisms and the immune system can exacerbate rosacea symptoms, leading to the release of pro-inflammatory cytokines and activation of immune cells to the affected areas. Targeting microbiome through therapies that reduce dysbiosis offer new treatment opportunities for individuals with rosacea.
Sunburn: Sunburn is caused by overexposure to ultraviolet (UV) radiation, leading to damage to the skin’s outer layers, inflammation, and pain [98]. While sunburn is an acute injury, repeated or severe sunburns can increase the risk of skin cancer and premature skin aging [99]. UV radiation not only affects skin cells but also alters the composition of the skin microbiome [100]. UV radiation can reduce the diversity of the skin microbiome, making the skin more vulnerable to harmful bacteria that can exacerbate inflammation and hinder the healing process. In healthy skin, the microbiome plays a protective role by modulating immune responses and promoting tissue repair [100]. Microbiome-based treatments, such as postbiotic formulations, are being explored as potential strategies to promote faster recovery from sunburn by enhancing the skin’s microbial diversity and reducing inflammation [101]. These treatments aim to restore the skin microbiome’s protective function, mitigating UV-induced damage and accelerating healing. Recently, Ácsová et al. demonstrated that Lactococcus Ferment Lysate and Bifida Ferment Lysate inhibit the synthetic DPPH (2,2-diphenyl-1-picrylhydrazyl) radical and have a sun-protective capbility with SPF (sun protective factor) 4-5 against UVB radiation [102]. Similarly, Kimoto-Nira et al. demonstrated that oral intake of heat-killed cells of the Lactococcus lactis strain H61 reduced the dehydration of the skin and enhanced skin elasticity [103]. Moreover, fermented milk from the Lactobacillus helveticus strain showed therapeutic potential for UVB-exposed skin by reducing lipid peroxidation and melanin formation [104]. Therefore, postbiotics-based skin formulation could be a promising strategy for sunburn protection and some age-related skin property alterations, such as changes to the melanin content.
Vitiligo and hyperpigmentation: Vitiligo is a persistent skin condition characterized by the loss of pigmentation due to damage of melanocytes [105]. This causes white patches to develop on various parts of the body. Vitiligo is recognized as an autoimmune disorder in which the immune system erroneously attacks melanocytes, though the exact mechanisms remain unclear [105]. Topical corticosteroids (TCSs) and topical calcineurin inhibitors (TCIs) are mainly used for the treatment of many types of vitiligo [106]. However, long-term use of these treatments has serious side effects; therefore, there is a need for alternative treatment strategies. Hyperpigmentation is a condition characterized by darkened patches of skin caused by an overproduction of melanin, often following inflammation or injury [107]. Emerging research has indicated a potential role in the skin microbiome in vitiligo’s pathogenesis. Studies have shown that individuals with vitiligo often exhibit dysbiosis, or microbial imbalance, particularly on depigmented areas of the skin [108]. This microbial imbalance may influence immune responses, further exacerbating melanocyte destruction and accelerating depigmentation [108]. In vitiligo patients, research indicates a decrease in the diversity of skin microbes, with studies showing a reduction in beneficial bacteria like Corynebacteriaceae, while potentially seeing an increase in bacteria like Gammaproteobacteria and Flavobacteria, suggesting an imbalance in the skin microbiome within affected vitiligo patches; essentially, the microbial diversity on vitiligo-affected skin tends to decrease compared to that of healthy skin. Therefore, alterations in skin bacteria have been observed to correlate with inflammation, suggesting that microbial shifts could act as additional triggers for autoimmunity in vitiligo patients [109].
Alopecia areata (AA): Alopecia areata causes abrupt patches of hair loss, usually on the scalp. The disorder results in the loss of hair follicles. Numerous studies have demonstrated the significant potential of using platelet-rich plasma (PRP) against AA [110]. However, due to its low stability and the lack of standardization of the production process, topical applications are difficult. This limitation has been resolved by Rinaldi et al., who used bioactive peptides obtained from biotechnological tools and combined them with postbiotics [111]. Numerous investigations into the scalp microbiomes of AA patients have yielded conflicting results. For instance, Won et al. investigated severe instances of AA and observed a decline in Staphylococcus caprae from the Firmicutes phylum and a notable rise in bacteria from the Actinobacteria group, including Corynebacterium and Cutibacterium [112]. On the other hand, Juhasz et al. found that individuals with AA had more Clostridia, a different class of Firmicutes, on their scalps [113]. Furthermore, studies have demonstrated that patients with AA have higher levels of Neisseria and Anaerococcus in their epidermis, while Candidatus aquiluna and Staphylococcus epidermidis are lower in their dermis [114,115]. Thus, these findings are providing a window for research into AA in context to microbiome regulation for the purpose of developing a better treatment strategy.
Overall, based on the evidence, we can conclude that disruption of microbial diversity is one of the key factors underpinning the regulation of skin-related abnormalities. Therefore, microbiome modulators could be a better strategy to employ. Thus, in the following section, we elaborate the microbiome modulator in the treatment of skin diseases.
7. Postbiotics in the Treatment of Skin Diseases
Postbiotics refer to bioactive compounds. These components can modulate immune responses, enhance skin barrier function, and reduce inflammation, making them valuable tools in skincare and dermatological treatments. Viable cells can be deactivated using various mechanical approaches, such as sonication, heat treatment, electromagnetic radiation, high-pressure processes, or ultraviolet light exposure [116]. From a technological perspective, postbiotics provide significant advantages over live probiotics, particularly in terms of stability and safety [117]. Postbiotics reduce the risk of infections or adverse immune reactions, making them particularly beneficial for individuals with weakened immune systems or compromised skin barriers [117]. The production and bioactive properties of postbiotics are shown in Figure 3.
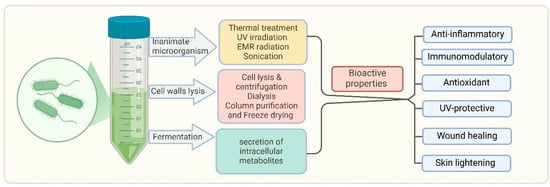
Figure 3.
Production and bioactive properties of postbiotics. This diagram illustrates the process of obtaining bioactive properties from microorganisms through various treatments such as thermal treatment, UV irradiation, EMR radiation, sonication, cell wall lysis, and fermentation. Fermentation leads to the secretion of intracellular metabolites that contribute to postbiotic activity. Bioactive properties include diverse therapeutic effects against acne, rosacea, psoriasis, atopic dermatitis, and vitiligo/hyperpigmentation. It also shows immunomodulatory, UV-protective, and wound healing properties. This highlights the promising therapeutic potential of postbiotics in maintaining health and addressing skin-related issues.
This makes them particularly relevant for use in topical formulations for individuals with sensitive or diseased skin.
There are various examples of preclinical and clinical evidence implicating the therapeutic potential of postbiotics, as shown in Table 1.

Table 1.
Preclinical and clinical studies on postbiotics in skin health.
Postbiotics have been incorporated into a variety of skincare products, including moisturizers, serums, and cleansers, aimed at improving skin health by restoring microbial balance and enhancing skin resilience [128,129,130]. The use of postbiotics in dermatology is supported by a growing body of evidence from both preclinical and clinical studies, which have demonstrated their potential in treating a range of skin conditions from acne to eczema. For instance, lipoteichoic acid (LTA), a key component of the cell wall in Gram-positive bacteria, has shown inhibitory effects on melanogenesis [118]. LTA derived from Lactobacillus plantarum suppressed melanogenesis in B16-F10 cells by decreasing cellular tyrosinase activity and downregulating the expression of tyrosinase in a dose-dependent manner [118]. In clinical trials, Majeed et al. demonstrated that LactoSporin, a postbiotic derived from Bacillus coagulans, significantly decreased oiliness, pimples, and redness associated with acne, marking it as a candidate for acne treatment [119]. The potential efficacy of postbiotics in reducing acne-related issues could be attributed to their ability to inhibit the enzyme 5-alpha-reductase, as demonstrated in in vitro studies [119]. This enzyme is crucial in the production of hormones that stimulate sebaceous gland activity, creating an environment conducive to the growth of Cutibacterium acne, the bacterium often linked to acne development [131]. By inhibiting 5-alpha reductase, postbiotics may help manage sebum production, thus reducing one of the primary factors that support acne formation. Further, Chung et al. developed a multi-strain postbiotic complex (PC) combining Lactobacillus helveticus HY7801 and Lactococcus lactis HY449 [120]. This complex exhibited antibacterial effects against Staphylococcus aureus and Cutibacterium acnes, while also modulating inflammatory cytokines and hyaluronic acid, which contribute to the anti-inflammatory response in skin cells [120].
Further, torii et al. demonstrated that oral administration of L-92 derived from Lactobacillus acidophilus significantly improved atopic dermatitis in Japanese children by regulating the T helper type-1/T helper type-2 (Th1/Th2) immune axis [121]. Similarly, Lima et al. demonstrated the efficacy of orally administered Lactobacillus-derived postbiotics in atopic dermatitis [132]. Moreover, Kim et al. investigated the synergistic effects of combining lactic acid bacteria postbiotics with Smilax china L. extract through a cofermentation process, focusing on their potential therapeutic application for atopic dermatitis [133]. This study introduced MB-2006, a fermented product developed by cofermenting Smilax china L. extract with Lactobacillus acidophilus (LAC) and Lactobacillus rhamnosus (LRH), and compared its efficacy to that of the heat-killed probiotics LAC and LRH alone [133]. Their findings demonstrated that MB-2006 was significantly more effective than LAC or LRH alone in reducing pro-inflammatory markers, such as IL-4 and thymic stromal lymphopoietin (TSLP), and in inhibiting the NF-κB pathway and the activation of atopic dermatitis-like HaCaT keratinocyte cells [133]. Furthermore, Lactobacillus-derived postbiotics have demonstrated benefits in contact dermatitis models, where they reduced pro-inflammatory cytokines and enhanced skin barrier integrity. Lactobacillus rhamnosus GG-derived postbiotics, for instance, reduced inflammation in allergic dermatitis models by modulating immune pathways and strengthening skin barriers [134].
A recent study demonstrated the anti-inflammatory potential of Malassezia-derived lipid metabolites such as poly unsaturated fatty acid (PUFA). Malassezin, a natural indole derivative, is produced by the skin-resident fungus Malassezia furfur and has been linked with the skin depigmentation disorder pityriasis versicolor [122]. Research indicates that topical application of isolated Malassezin can reduce epidermal discoloration, such as hyperpigmentation caused by photoaging [123], likely due to its selective apoptotic effects on primary human melanocytes. As a unique agent derived from the skin microflora, Malassezin is now available as a commercial ingredient for skin-whitening products, targeting hyperpigmented areas while preserving the pigmentation of normal skin [123].
Likewise, the opportunistic skin pathogen Cutibacterium acne produces propionic acid, which holds promise as a safe and effective alternative for treating hyperpigmentation [124]. Another compound, pityriacitrin, an indole alkaloid derived from Malassezia furfur, has demonstrated strong UV-protective properties [125]. The UV-absorbing properties of pityriacitrin impact various bacterial and fungal members of the human skin microbiota and show potential for use as postbiotic ingredients in commercial sun protection products [126]. Based on the above findings, it can be postulated that postbiotics, which have the ability to reduce hyperpigmentation and inflammation, could represent a promising approach to treating vitiligo; however, further research is required in order to fully understand the microbiome’s role in vitiligo and to develop effective microbiome-based therapies.
Further, microbiome-targeted therapies are being explored as potential treatments for AA. By increasing proliferation and cell migration, these therapies aim to regulate hair growth. For example, a double-blind, randomized study involving 160 participants was conducted to assess the effectiveness of a gel formulation containing postbiotics for the topical treatment of AA. Among the patients treated, approximately 47.5% experienced complete hair regrowth, 13.75% showed partial regrowth, and 6.25% reported no response [111]. Key mediators of the epithelial cell proliferation and migration of human keratinocytes also increased the expression transforming growth factor beta-1 (TGF-β1), vascular endothelial growth factor A (VEGF-A), fibroblast growth factor 7 (FGF7), and IL-8 genes [135]. In addition, both compounds modulate the expression of filaggrin, involucrin, β-defensin 2, and TNF-α genes [136]. Based on this evidence, it can be speculated that postbiotics could provide a new avenue for treatment strategies.
Recent advancements in microbiome research have opened the door to a variety of interventions that modulate skin microbiomes for improved health and cosmetic outcomes. Among these, postbiotics, prebiotic, and other microbiome modulators have gained considerable attention for their ability to promote skin barrier integrity, reduce inflammation, and treat skin diseases. These microbiome-based therapies aim to restore or maintain the natural balance of the skin’s microbial communities, which is crucial for overall skin health. In the following section, we emphasized the role of postbiotics, prebiotics, and other modulators in skin health, along with their applications in skincare products and treatments.
Inactivated bacteria (postbiotics) and microbial metabolites as postbiotics in skin health: Inactivated probiotics, also known as non-viable probiotics, are bacterial strains that have been inactivated or killed but still retain their beneficial properties. A study by Dimarzio et al. demonstrated the antiaging effect of peptides derived from Streptococcus thermophilus S244 [137]. The mechanism involves an increase in the ceramide level and hydration of forearm. Further, heat-killed Lactiplantibacillus plantarum have been reported to have an antiwrinkle and UV-protective effect. Moreover, it has been shown to reduce the skin melanin level [138]. Therefore, it can be effective for the treatment of hyperpigmentation. Another study by Kim et al. demonstrated the skin barrier-enhancing effect of dermabiotics derived from Lactiplantibacillus plantarum [127]. Further, CLS02021 (postbiotic), through topical application, increased skin elasticity and reduced both wrinkles and pore size [139]. Inactivated cells of the bacteria belonging to members of the Lactobacillaceae family promote anti-inflammatory responses, reduce skin sensitivity, and improve moisture retention when applied topically. Preclinical studies have demonstrated the efficacy of dead bacteria in enhancing skin health. For example, non-viable Lactobacillus reuteri has been shown to reduce skin inflammation and promote the healing of wounds [140]. In clinical settings, dead probiotics have been used in the treatment of conditions such as acne. In this context, Bae et al. demonstrated that heat-killed Pediococcus acidilactici (LM1013) inhibits biofilm formation by Cutibacterium acnes [141]. Another clinical study showed the efficacy of cofermented postbiotics of TYCA06/AP-32/CP-9/collagen with respect to their ability to combat acne vulgaris [142]. Furthermore, rice flour fermented with Lactobacillus paracasei CBA L74 was found to be effective in the treatment of atopic dermatitis in infants [143]. Although several dead probiotics have been found effective against skin disease, their mechanistic proof is still missing, which provides scope for further extensive research. Microbial metabolites, another class of postbiotics, are compounds produced by live microorganisms during their metabolic activities. For instance, SCFAs such as butyrate and acetate are known for their anti-inflammatory and immune-modulating properties against dermatitis [144]. Additionally, butyrate reduces skin inflammation and dermal IL-33 expression caused by Staphylococcus aureus by inhibiting histone deacetylase [145]. Further, Trompette et al. demonstrated that gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation, improving epidermal barrier integrity and ultimately limiting early allergen sensitization and disease development [146]. Moreover, a postbiotic derived from Lactobacillus reuteri PTCC1655 has been shown to enhance wound healing by modulating the inflammatory phase, increasing collagen and elastin deposition, and promoting angiogenesis [147]. Additionally, gene expression assays revealed that this postbiotic accelerates healing by upregulating both inflammatory mediators (IL-6 and TNF-α) and anti-inflammatory mediators (TGF-β and VEGF) [147]. Furthermore, a study by Golkar et al. evaluated the wound healing efficacy of three topical cold cream formulations containing postbiotics from Lactobacillus fermentum, Lactobacillus reuteri, and Bacillus subtilis species [148]. All of the formulations significantly accelerated wound healing compared to controls, with the Bacillus subtilis cream demonstrating superior results [148]. These findings suggest that postbiotic-infused creams hold promise as effective wound healing treatments (see Table 2 and Table 3).

Table 2.
Preclinical and clinical studies on dead probiotics in skin health.

Table 3.
Studies on microbial metabolites in skin health.
In the context of skin health, microbial metabolites can improve skin hydration, reduce inflammation, and promote wound healing. For example, lactate, a metabolite produced by Lactobacillus species, helps maintain skin moisture by enhancing the production of ceramides in the epidermis [151,152]. Similarly, a tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor [153].
8. Conclusions and Future Perspective
This review has explored the essential role of skin microbiomes in maintaining skin health and the pathogenesis of various skin diseases. Our understanding of the skin microbiome has opened new avenues for therapeutic interventions, with postbiotics, dead probiotics, and microbiome metabolites showing great promise in both skincare and dermatology. Postbiotics and dead probiotics have shown potential in inhibiting inflammation and the immune–inflammation axis and treating conditions such as acne, eczema, and wounds. Microbiome metabolites, i.e., SCFAs such as acetate, butyrate, and lactate, also offer promising therapeutic avenues for treating and preventing skin diseases. Despite significant progress, there are several unanswered questions that require more preclinical studies to understand the precise mechanisms through which postbiotics and prebiotics modulate the skin microbiome and promote skin health and wound healing. Future research should focus on elucidating the molecular pathways through which microbiome modulators influence immune responses, skin barrier function, and microbial composition. Understanding these mechanisms will facilitate the development of more targeted and effective treatments. While there is growing evidence from clinical trials supporting the efficacy of microbiome-based therapies, larger, long-term studies are required to validate their safety and effectiveness across diverse populations.
Author Contributions
S.K.P., L.L. and H.Y. conducted the literature search and wrote and formatted the manuscript. D.Y. and S.J. reviewed the manuscript and provided corrections. All authors have read and agreed to the published version of the manuscript.
Funding
There was no specific grant provided for this review article from public, private, or nonprofit funding organizations. Yadav’s lab would like to acknowledge the generous support from the National Institutes of Health, the National of Institute of Aging (R56AG069676, R56AG064075, RF1AG071762, R21AG072379, U01AG076928; R21AG085881), and the Ed and Ethel Moore Alzheimer’s Disease Research Program of the Florida Department of Health (22A17). Additionally, they are thankful for resources provided by the University of South Florida (USF) Center for Microbiome Research, the Microbiomes Institute, the Center for Excellence in Aging and Brain Repair, the Department of Neurosurgery and Brain Repair, and the USF Morsani College of Medicine.
Conflicts of Interest
Hariom Yadav is cofounder and chief scientific officer of Postbiotics Inc. and BiomAge Inc. He is also cofounder of MusB LLC, MusB Research LLC and MeraBiome Inc. with Shalini Jain. However, they have no conflicts of interest with respect to the work/literature review presented in this manuscript.
References
- Alves, A.C.; Martins, S.M.d.S.B., Jr.; Belo, J.V.T.; Lemos, M.V.C.; Lima, C.E.d.M.C.; Silva, C.D.d.; Zagmignan, A.; Nascimento da Silva, L.C. Global Trends and Scientific Impact of Topical Probiotics in Dermatological Treatment and Skincare. Microorganisms 2024, 12, 2010. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [PubMed]
- Kostecka, M.; Kostecka, J.; Szwed-Gułaga, O.; Jackowska, I.; Kostecka-Jarecka, J. The impact of common acne on the well-being of young people aged 15–35 years and the influence of nutrition knowledge and diet on acne development. Nutrients 2022, 14, 5293. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Son, S.W.; Cho, S.H. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol. Res. 2016, 8, 181–190. [Google Scholar] [CrossRef]
- Bakshi, H.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Treatment of psoriasis: A comprehensive review of entire therapies. Curr. Drug Saf. 2020, 15, 82–104. [Google Scholar] [CrossRef]
- Premkumar, M.; Kalarani, I.B.; Mohammed, V.; Veerabathiran, R. An Extensive Review of Vitiligo-Associated Conditions. Int. J. Dermatol. Venereol. 2024, 7, 44–51. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Cheng, J.Y.; Gallo, R.L. Mechanisms for control of skin immune function by the microbiome. Curr. Opin. Immunol. 2021, 72, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.; Nizet, V.; Gallo, R. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cogen, A.L.; Radek, K.A.; Park, H.J.; MacLeod, D.T.; Leichtle, A.; Ryan, A.F.; Di Nardo, A.; Gallo, R.L. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Investig. Dermatol. 2010, 130, 2211–2221. [Google Scholar] [CrossRef]
- Sanford, J.A.; Zhang, L.-J.; Williams, M.R.; Gangoiti, J.A.; Huang, C.-M.; Gallo, R.L. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 2016, 1, eaah4609. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.-J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef]
- Zouboulis, C.; Boschnakow, A. Chronological ageing and photoageing of the human sebaceous gland. Clin. Exp. Dermatol. 2001, 26, 600–607. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar]
- Lim, H.W.; Collins, S.A.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef]
- Stavrakidis, K.K.S. Probiotics: Benefits on Skin Health and Therapeutical Potential. Doctoral Dissertation, Department of Chemistry and Biochemistry, Faculty of Medicine, University of Rijeka, Rijeka, Croatia, 2024. [Google Scholar]
- Nicholas-Haizelden, K.; Murphy, B.; Hoptroff, M.; Horsburgh, M.J. Bioprospecting the skin microbiome: Advances in therapeutics and personal care products. Microorganisms 2023, 11, 1899. [Google Scholar] [CrossRef]
- Gueniche, A.; Perin, O.; Bouslimani, A.; Landemaine, L.; Misra, N.; Cupferman, S.; Aguilar, L.; Clavaud, C.; Chopra, T.; Khodr, A. Advances in microbiome-derived solutions and methodologies are founding a new era in skin health and care. Pathogens 2022, 11, 121. [Google Scholar] [CrossRef]
- Al-Smadi, K.; Leite-Silva, V.R.; Filho, N.A.; Lopes, P.S.; Mohammed, Y. Innovative Approaches for Maintaining and Enhancing Skin Health and Managing Skin Diseases through Microbiome-Targeted Strategies. Antibiotics 2023, 12, 1698. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Sinkko, H.; Hielm-Björkman, A.; Salmela, E.; Tiira, K.; Laatikainen, T.; Mäkeläinen, S.; Kaukonen, M.; Uusitalo, L.; Hanski, I. Skin microbiota and allergic symptoms associate with exposure to environmental microbes. Proc. Natl. Acad. Sci. USA 2018, 115, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hao, Q.; Liu, S.-B.; Zhang, Q.-S.; Chen, X.-Y.; Li, S.-H.; Ran, C.; Yang, Y.-L.; Teame, T.; Zhang, Z. The positive effects of postbiotic (SWF concentration®) supplemented diet on skin mucus, liver, gut health, the structure and function of gut microbiota of common carp (Cyprinus carpio) fed with high-fat diet. Fish Shellfish Immunol. 2023, 135, 108681. [Google Scholar]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market—An update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar]
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar]
- Zhang, L.-J. Keratins in skin epidermal development and diseases. In Ling-Juan Z. Keratins in Skin Epidermal Development and Diseases; IntechOpen: London, UK, 2018. [Google Scholar]
- Menon, G.K. Skin basics; structure and function. In Lipids and Skin Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–23. [Google Scholar]
- Driskell, R.R.; Jahoda, C.A.; Chuong, C.M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 553, 427–436. [Google Scholar] [PubMed]
- Sharma, G.; Khanna, G.; Sharma, P.; Deol, P.K.; Kaur, I.P. Mechanistic role of probiotics in improving skin health. In Probiotic Research in Therapeutics; Springer: Singapore, 2022; pp. 27–47. [Google Scholar]
- Heo, Y.M.; Lee, D.-G.; Mun, S.; Kim, M.; Baek, C.; Lee, H.; Yun, S.K.; Kang, S.; Han, K. Skin benefits of postbiotics derived from Micrococcus luteus derived from human skin: An untapped potential for dermatological health. Genes. Genom. 2024, 46, 13–25. [Google Scholar] [CrossRef]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar]
- Collin, M.; Milne, P. Langerhans cell origin and regulation. Curr. Opin. Hematol. 2016, 23, 28–35. [Google Scholar]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012, 36, 873–884. [Google Scholar] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [PubMed]
- Ferreira, I.; Lopes, C.; Amaral, M. Treatment Advances for Acne Vulgaris: The Scientific Role of Cannabinoids. Cosmetics 2024, 11, 22. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar]
- Cogen, A.L.; Yamasaki, K.; Muto, J.; Sanchez, K.M.; Crotty Alexander, L.; Tanios, J.; Lai, Y.; Kim, J.E.; Nizet, V.; Gallo, R.L. Staphylococcus epidermidis antimicrobial δ-toxin (phenol-soluble modulin-γ) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS ONE 2010, 5, e8557. [Google Scholar]
- Swaney, M.H.; Nelsen, A.; Sandstrom, S.; Kalan, L.R. Sweat and sebum preferences of the human skin microbiota. Microbiol. Spectr. 2023, 11, e0418022. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal stability of the human skin microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.-H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar]
- Chen, P.; He, G.; Qian, J.; Zhan, Y.; Xiao, R. Potential role of the skin microbiota in Inflammatory skin diseases. J. Cosmet. Dermatol. 2021, 20, 400–409. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the role of langerhans cells as immune regulators within the skin. Front. Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Glatthardt, T.; Lima, R.D.; de Mattos, R.M.; Ferreira, R.B.R. Microbe interactions within the skin microbiome. Antibiotics 2024, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.H.; Ahamed, A.; Chen, K.; Lebig, E.G.G.; Petros, B.; Saeed, S.; Martins-Green, M. Skin microbiota and its role in health and disease with an emphasis on wound healing and chronic wound development. In Microbiome, Immunity, Digestive Health and Nutrition: Epidemiology, Pathophysiology, Prevention and Treatment; Academic Press: Cambridge, MA, USA, 2022; pp. 297–311. [Google Scholar]
- Kim, J.; Kim, B.E.; Goleva, E.; Berdyshev, E.; Bae, J.; Kim, S.; Kim, H.-y.; Lee, U.H.; Kim, M.S.; Jung, M. Alterations of epidermal lipid profiles and skin microbiome in children with atopic dermatitis. Allergy Asthma Immunol. Res. 2023, 15, 186. [Google Scholar]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Ortonne, J.P. The normal color of human skin. In The Pigmentary System: Physiology and Pathophysiology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; pp. 504–520. [Google Scholar]
- Naik, P.P.; Farrukh, S.N. Influence of ethnicities and skin color variations in different populations: A review. Ski. Pharmacol. Physiol. 2022, 35, 65–76. [Google Scholar] [CrossRef]
- Fajuyigbe, D.; Young, A.R. The impact of skin colour on human photobiological responses. Pigment. Cell Melanoma Res. 2016, 29, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. The heterogeneity and complexity of skin surface lipids in human skin health and disease. Prog. Lipid Res. 2023, 93, 101264. [Google Scholar]
- Corvec, S. Clinical and biological features of Cutibacterium (formerly Propionibacterium) avidum, an underrecognized microorganism. Clin. Microbiol. Rev. 2018, 31, e00064-17. [Google Scholar] [CrossRef]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, A.; Grice, E.A. Interactions between host factors and the skin microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Egert, M.; Simmering, R. The microbiota of the human skin. In Microbiota of the Human Body; Springer: Cham, Switzerland, 2016; pp. 61–81. [Google Scholar]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.; Chen, W.-C.; Thornton, M.; Qin, K.; Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 2007, 39, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Coenye, T.; He, L.; Kabashima, K.; Kobayashi, T.; Niemann, C.; Nomura, T.; Oláh, A.; Picardo, M.; Quist, S.R. Sebaceous immunobiology-skin homeostasis, pathophysiology, coordination of innate immunity and inflammatory response and disease associations. Front. Immunol. 2022, 13, 1029818. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the skin and gut in atopic dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef]
- Townsend, E.C.; Kalan, L.R. The dynamic balance of the skin microbiome across the lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56 (Suppl. 2), S230–S242. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Zhang, M.; Kaffenberger, B.H. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017, 153, 523–528. [Google Scholar] [CrossRef]
- Liegenfeld, S.C.; Stenzel, S.; Rembe, J.-D.; Dittmer, M.; Ramos, P.; Stuermer, E.K. Pathogenic and Non-Pathogenic Microbes in the Wound Microbiome—How to Flip the Switch. Microbiol. Res. 2025, 16, 39. [Google Scholar] [CrossRef]
- White, E.K.; Grice, E.A. The wound microbiome. Cold Spring Harb. Perspect. Biol. 2023, 15, a041218. [Google Scholar]
- Luna, P.C. Skin microbiome as years go by. Am. J. Clin. Dermatol. 2020, 21 (Suppl. 1), 12–17. [Google Scholar]
- Sun, H.; Pulakat, L.; Anderson, D.W. Challenges and new therapeutic approaches in the management of chronic wounds. Curr. Drug Targets 2020, 21, 1264–1275. [Google Scholar] [PubMed]
- Jockenhöfer, F.; Chapot, V.; Stoffels-Weindorf, M.; Körber, A.; Klode, J.; Buer, J.; Küpper, B.; Roesch, A.; Dissemond, J. Bacterial spectrum colonizing chronic leg ulcers: A 10-year comparison from a German wound care center. J. Dtsch. Dermatol. Ges. 2014, 12, 1121–1127. [Google Scholar]
- Ak, M. A comprehensive review of acne vulgaris. J. Clin. Pharm. 2019, 1, 17–45. [Google Scholar]
- Cavallo, I.; Sivori, F.; Truglio, M.; De Maio, F.; Lucantoni, F.; Cardinali, G.; Pontone, M.; Bernardi, T.; Sanguinetti, M.; Capitanio, B. Skin dysbiosis and Cutibacterium acnes biofilm in inflammatory acne lesions of adolescents. Sci. Rep. 2022, 12, 21104. [Google Scholar]
- Mawardi, P.; Ardiani, I.; Primisawitri, P.P.; Nareswari, A. Dual role of Cutibacterium acnes in acne vulgaris pathophysiology. Bali Med. J. 2021, 10, 486–490. [Google Scholar]
- Nakatsuji, T.; Tang, D.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: Potential targets for inflammatory acne treatment. PLoS ONE 2011, 6, e14797. [Google Scholar]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, 1953. [Google Scholar]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.-H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.P.; Leccia, M.T.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.; Vâță, D.; Alexa, A.-I.; Trandafir, L.; Patrașcu, A.-I.; Hâncu, M.F.; Gheucă-Solovăstru, L. Atopic Dermatitis—Beyond the Skin. Diagnostics 2021, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus aureus and the cutaneous microbiota biofilms in the pathogenesis of atopic dermatitis. Microorganisms 2019, 7, 301. [Google Scholar] [CrossRef]
- Gomez-Casado, C.; Unger, Z.; Olah, P.; Homey, B. Microbiome in Atopic Dermatitis: Is It All About Staphylococcus aureus? Curr. Treat. Options Allergy 2023, 10, 351–363. [Google Scholar]
- Özdemіr, E.; Öksüz, L. Effect of Staphylococcus aureus colonization and immune defects on the pathogenesis of atopic dermatitis. Arch. Microbiol. 2024, 206, 1–18. [Google Scholar]
- Proft, T.; Fraser, J.D. Bacterial superantigens. Clin. Exp. Immunol. 2003, 133, 299–306. [Google Scholar]
- Kim, K.H.; Han, J.H.; Chung, J.H.; Cho, K.H.; Eun, H.C. Role of staphylococcal superantigen in atopic dermatitis: Influence on keratinocytes. J. Korean Med. Sci. 2006, 21, 315–323. [Google Scholar]
- Kuchekar, A.B.; Pujari, R.R.; Kuchekar, S.B.; Dhole, S.N.; Mule, P.M.; Vidyapeeth, B.; Wadi, B. Psoriasis: A comprehensive review. Int. J. Pharm. Life Sci. 2011, 2, 857–877. [Google Scholar]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and microbiota: A systematic review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Visser, M.J.; Kell, D.B.; Pretorius, E. Bacterial dysbiosis and translocation in psoriasis vulgaris. Front. Cell. Infect. Microbiol. 2019, 9, 7. [Google Scholar]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.-h.; Strober, B.E.; Pei, Z.; Blaser, M.J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE 2008, 3, e2719. [Google Scholar] [CrossRef]
- Teng, Y.; Xie, W.; Tao, X.; Liu, N.; Yu, Y.; Huang, Y.; Xu, D.; Fan, Y. Infection-provoked psoriasis: Induced or aggravated. Exp. Ther. Med. 2021, 21, 567. [Google Scholar] [PubMed]
- Ruiz-Romeu, E.; Ferran, M.; Giménez-Arnau, A.; Bugara, B.; Lipert, B.; Jura, J.; Florencia, E.F.; Prens, E.P.; Celada, A.; Pujol, R.M. MCPIP1 RNase is aberrantly distributed in psoriatic epidermis and rapidly induced by IL-17A. J. Investig. Dermatol. 2016, 136, 1599–1607. [Google Scholar] [PubMed]
- Chang, H.-W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar]
- Macleod, T.; Bridgewood, C.; Hyde, I.; Heague, M.; Helliwell, P.; Stacey, M.; Wittmann, M. Molecular and cellular regulation of psoriatic inflammation. Clin. Sci. 2022, 136, 935–952. [Google Scholar]
- Bhatiaa, M.; Kumara, M.; Pareek, B.; Sonib, S. Skin & its ailments: A systemic. In Contemporary Advances in Science & Technology Volume-IV: Medicinal Chemistry & Pharmaceutical Sciences; Notion Press: Chennai, India, 2022; Volume 4, p. 57. [Google Scholar]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the microbiome: A systematic review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar]
- Rainer, B.M.; Thompson, K.G.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Bui, J.; Fischer, A.H.; Pasieka, H.B.; Garza, L.A.; Kang, S. Characterization and analysis of the skin microbiota in rosacea: A case–control study. Am. J. Clin. Dermatol. 2020, 21, 139–147. [Google Scholar]
- Woo, Y.R.; Lee, S.H.; Cho, S.H.; Lee, J.D.; Kim, H.S. Characterization and analysis of the skin microbiota in rosacea: Impact of systemic antibiotics. J. Clin. Med. 2020, 9, 185. [Google Scholar] [CrossRef]
- Robinson, J.; Begum, R.; Maqbool, M. Ultraviolet Radiation: Benefits, Harms, Protection. Introd. Non-Ioniz. Radiat. 2023, 2, 62. [Google Scholar]
- Merin, K.; Shaji, M.; Kameswaran, R. A review on sun exposure and skin diseases. Indian. J. Dermatol. 2022, 67, 625. [Google Scholar] [PubMed]
- Burns, E.M.; Ahmed, H.; Isedeh, P.N.; Kohli, I.; Van Der Pol, W.; Shaheen, A.; Muzaffar, A.F.; Al-Sadek, C.; Foy, T.M.; Abdelgawwad, M.S. Ultraviolet radiation, both UVA and UVB, influences the composition of the skin microbiome. Exp. Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzka, A.; Micallef, M.P.; Biazzo, M.; Podrini, C. The Role of the Skin Microbiome in Acne: Challenges and Future Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 11422. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Martiniaková, S. Efficacy of postbiotics against free radicals and UV radiation. Chem. Pap. 2022, 76, 2357–2364. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [PubMed]
- Rong, J.; Shan, C.; Liu, S.; Zheng, H.; Liu, C.; Liu, M.; Jin, F.; Wang, L. Skin resistance to UVB-induced oxidative stress and hyperpigmentation by the topical use of Lactobacillus helveticus NS8-fermented milk supernatant. J. Appl. Microbiol. 2017, 123, 511–523. [Google Scholar]
- Leung, A.K.; Lam, J.M.; Leong, K.F.; Hon, K.L. Vitiligo: An updated narrative review. Curr. Pediatr. Rev. 2021, 17, 76–91. [Google Scholar] [CrossRef]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.; Jouary, T. Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar]
- Kaufman, B.P.; Aman, T.; Alexis, A.F. Postinflammatory hyperpigmentation: Epidemiology, clinical presentation, pathogenesis and treatment. Am. J. Clin. Dermatol. 2018, 19, 489–503. [Google Scholar] [CrossRef]
- Ganju, P.; Nagpal, S.; Mohammed, M.; Nishal Kumar, P.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar]
- Ni, Q.; Ye, Z.; Wang, Y.; Chen, J.; Zhang, W.; Ma, C.; Li, K.; Liu, Y.; Liu, L.; Han, Z. Gut microbial dysbiosis and plasma metabolic profile in individuals with vitiligo. Front. Microbiol. 2020, 11, 592248. [Google Scholar]
- Giordano, S.; Romeo, M.; Di Summa, P.; Lankinen, P. A meta-analysis on evidence of platelet-rich plasma for androgenetic alopecia. Int. J. Trichology 2018, 10, 1–10. [Google Scholar]
- Rinaldi, F.; Trink, A.; Pinto, D. Efficacy of postbiotics in a PRP-like cosmetic product for the treatment of alopecia area Celsi: A randomized double-blinded parallel-group study. Dermatol. Ther. 2020, 10, 483–493. [Google Scholar]
- Won, E.J.; Jang, H.H.; Park, H.; Kim, S.J. A potential predictive role of the scalp microbiome profiling in patients with alopecia areata: Staphylococcus caprae, Corynebacterium, and Cutibacterium species. Microorganisms 2022, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, M.; Chen, S.; Khosrovi-Eghbal, A.; Ekelem, C.; Landaverde, Y.; Baldi, P.; Mesinkovska, N.A. Characterizing the skin and gut microbiome of alopecia areata patients. SKIN J. Cutan. Med. 2020, 4, 23–30. [Google Scholar]
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp bacterial shift in Alopecia areata. PLoS ONE 2019, 14, e0215206. [Google Scholar]
- Gómez-Arias, P.J.; Gay-Mimbrera, J.; Rivera-Ruiz, I.; Aguilar-Luque, M.; Juan-Cencerrado, M.; Mochón-Jiménez, C.; Gómez-García, F.; Sánchez-González, S.; Ortega-Hernández, A.; Gómez-Garre, D. Association Between Scalp Microbiota Imbalance, Disease Severity, and Systemic Inflammatory Markers in Alopecia Areata. Dermatol. Ther. 2024, 14, 2971–2986. [Google Scholar]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, H.; Jung, B.J.; You, G.E.; Jang, S.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits melanogenesis in B16F10 mouse melanoma cells. Mol. Cells 2015, 38, 163–170. [Google Scholar]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.; Shah, K.; Beede, K. Novel topical application of a postbiotic, LactoSporin®, in mild to moderate acne: A randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics 2020, 7, 70. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lee, H.; Kim, M.; Lee, J.W.; Saeed, M.; Lee, H.; Jung, S.-H.; Shim, J.-J.; Lee, J.-L.; Heo, K. Development and metabolic profiling of a postbiotic complex exhibiting antibacterial activity against skin microorganisms and anti-inflammatory effect on human keratinocytes. Food Sci. Biotechnol. 2022, 31, 1325–1334. [Google Scholar]
- Torii, S.; Torii, A.; Itoh, K.; Urisu, A.; Terada, A.; Fujisawa, T.; Yamada, K.; Suzuki, H.; Ishida, Y.; Nakamura, F. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int. Arch. Allergy Immunol. 2011, 154, 236–245. [Google Scholar] [PubMed]
- Saunte, D.M.; Gaitanis, G.; Hay, R.J. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112. [Google Scholar]
- Grimes, P.; Bhawan, J.; Howell, M.; Desai, S.; Coryell, E.; Einziger, M.; Simpson, A.; Yaroshinsky, A.; McCraw, T. Histopathological Changes Induced by Malassezin: A Novel Natural Microbiome Indole for Treatment of Facial Hyperpigmentation. J. Drugs Dermatol. 2022, 21, 141–145. [Google Scholar]
- Kao, H.-J.; Wang, Y.-H.; Keshari, S.; Yang, J.J.; Simbolon, S.; Chen, C.-C.; Huang, C.-M. Propionic acid produced by Cutibacterium acnes fermentation ameliorates ultraviolet B-induced melanin synthesis. Sci. Rep. 2021, 11, 11980. [Google Scholar]
- Mayser, P.; Schäfer, U.; Krämer, H.-J.; Irlinger, B.; Steglich, W. Pityriacitrin–an ultraviolet-absorbing indole alkaloid from the yeast Malassezia furfur. Arch. Dermatol. Res. 2002, 294, 131–134. [Google Scholar]
- Machowinski, A.; Krämer, H.J.; Hort, W.; Mayser, P. Pityriacitrin–a potent UV filter produced by Malassezia furfur and its effect on human skin microflora. Mycoses 2006, 49, 388–392. [Google Scholar]
- Kim, K.M.; Song, J.-W.; Lee, C.-W.; Kim, D.-S.; Sohn, J.; Lee, S. Skin Barrier-Enhancing Effects of Dermabiotics HDB with Regulation of Skin Microbiota. J. Microbiol. Biotechnol. 2023, 34, 65. [Google Scholar]
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863–3872. [Google Scholar]
- Im, A.-R.; Lee, B.; Kang, D.-J.; Chae, S. Skin moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food 2018, 21, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-J.; Iwasa, M.; Han, K.-I.; Kim, W.-J.; Tang, Y.; Hwang, Y.J.; Chae, J.R.; Han, W.C.; Shin, Y.-S.; Kim, E.-K. Heat-killed Enterococcus faecalis EF-2001 ameliorates atopic dermatitis in a murine model. Nutrients 2016, 8, 146. [Google Scholar] [CrossRef]
- Lambrechts, I.A.; de Canha, M.N.; Lall, N. Exploiting medicinal plants as possible treatments for acne vulgaris. In Medicinal Plants for Holistic Health and Well-Being; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–143. [Google Scholar]
- Lima, M.; Paulino, L.C. Oral postbiotics as a therapeutic strategy for atopic dermatitis: A systematic review of randomized controlled trials. J. Am. Nutr. Assoc. 2024, 43, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Cho, M.; Kang, D.-J. Anti-Inflammatory Response of New Postbiotics in TNF-α/IFN-γ-Induced Atopic Dermatitis-like HaCaT Keratinocytes. Curr. Issues Mol. Biol. 2024, 46, 6100–6111. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; McBain, A.J.; Cruickshank, S.M.; O’Neill, C.A. Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl. Environ. Microbiol. 2014, 80, 5773–5781. [Google Scholar] [CrossRef]
- Pinto, D.; Marzani, B.; Minervini, F.; Calasso, M.; Giuliani, G.; Gobbetti, M.; De Angelis, M. Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1, FGF7, VEGF-A and IL-8 genes. Peptides 2011, 32, 1815–1824. [Google Scholar] [CrossRef]
- Marzani, B.; Pinto, D.; Minervini, F.; Calasso, M.; Di Cagno, R.; Giuliani, G.; Gobbetti, M.; De Angelis, M. The antimicrobial peptide pheromone P lantaricin A increases antioxidant defenses of human keratinocytes and modulates the expression of filaggrin, involucrin, β-defensin 2 and tumor necrosis factor-α genes. Exp. Dermatol. 2012, 21, 665–671. [Google Scholar] [CrossRef]
- Dimarzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Chou, C.-H.; Chiang, Y.-J.; Lin, C.-G.; Lee, C.-H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114. [Google Scholar] [CrossRef]
- Catic, T.; Pehlivanovic, B.; Pljakic, N.; Balicevac, A. The moisturizing efficacy of a proprietary dermo-cosmetic product (cls02021) versus placebo in a 4-week application period. Med. Arch. 2022, 76, 108. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway. Stem Cell Res. Ther. 2019, 10, 243. [Google Scholar] [PubMed]
- Bae, W.Y.; Jung, W.H.; Lee, Y.J.; Shin, S.L.; An, Y.K.; Kim, T.R.; Sohn, M. Heat-treated Pediococcus acidilactici LM1013-mediated inhibition of biofilm formation by Cutibacterium acnes and its application in acne vulgaris: A single-arm clinical trial. J. Cosmet. Dermatol. 2023, 22, 3125–3134. [Google Scholar] [PubMed]
- Ho, H.H.; Chen, C.W.; Yi, T.H.; Huang, Y.F.; Kuo, Y.W.; Lin, J.H.; Chen, J.F.; Tsai, S.Y.; Chan, L.P.; Liang, C.H. Novel application of a Co-Fermented postbiotics of TYCA06/AP-32/CP-9/collagen in the improvement of acne vulgaris—A randomized clinical study of efficacy evaluation. J. Cosmet. Dermatol. 2022, 21, 6249–6260. [Google Scholar]
- D’Auria, E.; Panelli, S.; Lunardon, L.; Pajoro, M.; Paradiso, L.; Beretta, S.; Loretelli, C.; Tosi, D.; Perini, M.; Bedogni, G. Rice flour fermented with Lactobacillus paracasei CBA L74 in the treatment of atopic dermatitis in infants: A randomized, double-blind, placebo-controlled trial. Pharmacol. Res. 2021, 163, 105284. [Google Scholar] [PubMed]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar]
- Luo, C.-H.; Lai, A.C.-Y.; Chang, Y.-J. Butyrate inhibits Staphylococcus aureus-aggravated dermal IL-33 expression and skin inflammation through histone deacetylase inhibition. Front. Immunol. 2023, 14, 1114699. [Google Scholar]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Santo Domingo, J.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef]
- Jamaran, S.; Jafari, P.; Marjani, A.; Akbari, N.; Feizabad, M.M. Novel wound dressing based on postbiotic/chitosan film accelerates cutaneous wound healing. Jundishapur J. Microbiol. 2021, 14, e120806. [Google Scholar]
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A novel effective formulation of bioactive compounds for wound healing: Preparation, in vivo characterization, and comparison of various postbiotics cold creams in a rat model. Evid.-Based Complement. Altern. Med. 2021, 2021, 8577116. [Google Scholar]
- Kumar, A.; Green, K.M.; Rawat, M. A comprehensive overview of postbiotics with a special focus on discovery techniques and clinical applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [PubMed]
- Tokudome, Y. Influence of oral administration of lactic acid bacteria metabolites on skin barrier function and water content in a murine model of atopic dermatitis. Nutrients 2018, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Tamane, T.; Tokudome, Y. Effect of lactic fermentation products on human epidermal cell differentiation, ceramide content, and amino acid production. Ski. Pharmacol. Physiol. 2021, 34, 103–114. [Google Scholar]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).