Elastography in Reproductive Medicine, a Game-Changer for Diagnosing Polycystic Ovary Syndrome, Predicting Intrauterine Insemination Success, and Enhancing In Vitro Fertilization Outcomes: A Systematic Review
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Systematic Review Protocol
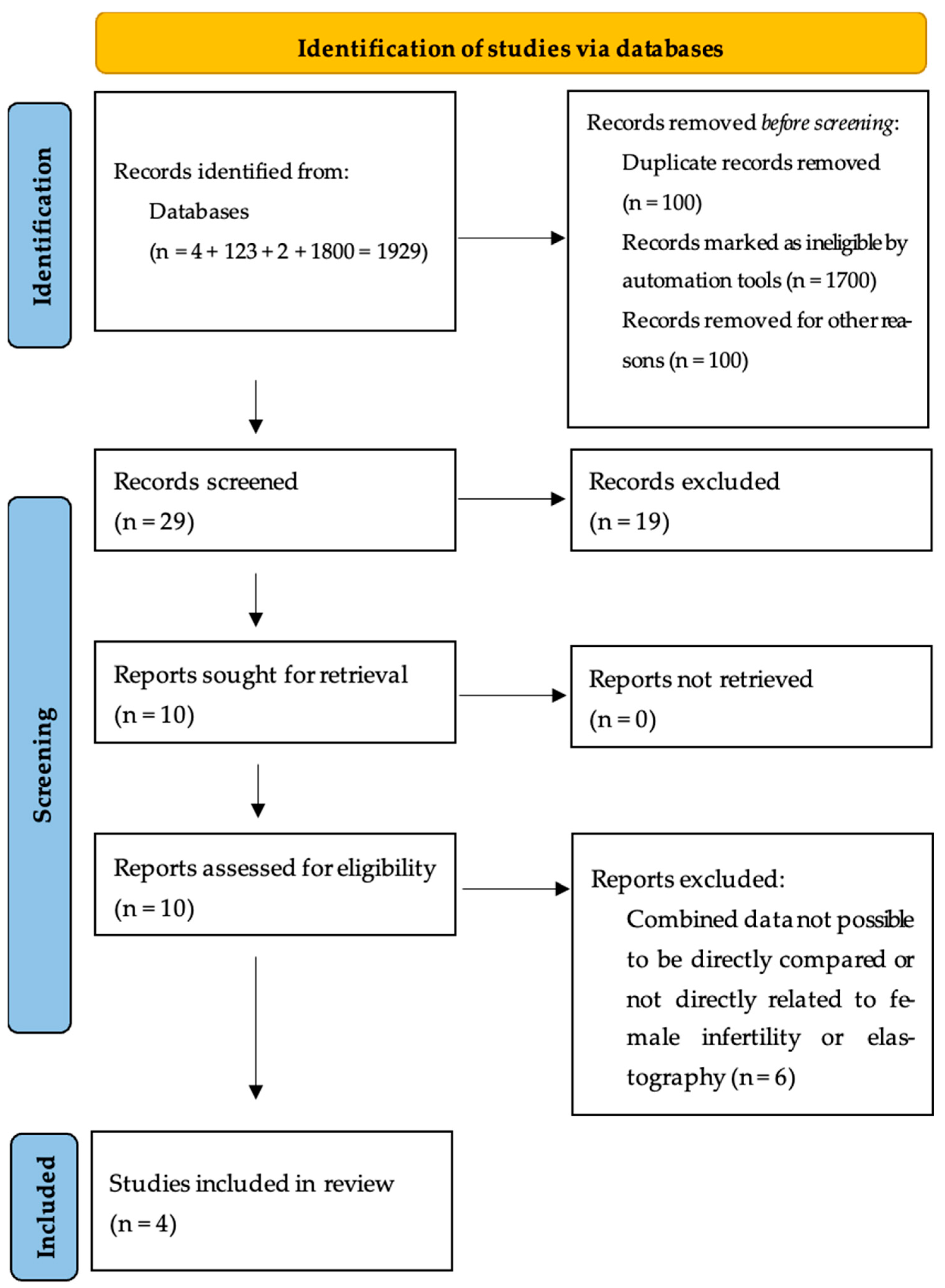
2.2. Search Strategies and Literature Selection
2.3. Eligibility Criteria
- The diagnostic accuracy of elastography in identifying ovarian stiffness in PCOS was evaluated.
- A correlation of uterine elasticity and contractility with pregnancy rates in IUI cycles.
- Endometrial stiffness and receptivity are assessed in relation to implantation success and clinical pregnancy rates in IVF.
- Prognostic usefulness of elastographic measures for assisted reproductive technology (ART) success was evaluated.
Exclusion Criteria
- No RTE or SWE elastography was used in the diagnostic or prognostic assessment.
- They did not report reproductive outcomes such as pregnancy rates, implantation success, or ART-related parameters.
- They did not compare conventional ultrasound, histology, or clinical results.
- They were case reports, case series, editorials, reviews, or meta-analyses.
- Male infertility was included in the absence of a female elastography examination.
- Other ultrasound methods (such as Doppler and grayscale imaging) were used instead of elastography.
- Had partial data or did not make the full-text available.
2.4. Data Extraction and Quality Assessment
| Study | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Çıracı et al. (2015) [11] | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Swierkowski-Blanchard et al. (2017) [24] | Serious | Moderate | Low | Low | Moderate | Low | Serious | Serious |
| Liu et al. (2022) [22] | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Li et al. (2024) [23] | Low | Low | Low | Low | Low | Low | Low | Low |
| Study | Year | Study Design | Population | Elastography Type | Key Findings | Risk of Bias (ROBINS-I) |
|---|---|---|---|---|---|---|
| Çıracı et al. [11] | 2015 | Prospective Cohort | Women with PCOS | RTE | Increased ovarian stiffness in PCOS patients | Moderate |
| Swierkowski-Blanchard et al. [24] | 2017 | Observational | Women undergoing IUI | 2D Elastography | Higher uterine elasticity predicts IUI success | Serious |
| Liu et al. [22] | 2022 | Retrospective Cohort | Women undergoing IVF | Conventional + Elastography | Endometrial echogenicity not predictive of implantation | Low |
| Li et al. [23] | 2024 | Prospective Study | Women with unexplained infertility | SWE | Increased endometrial stiffness linked to lower implantation rates | Low |
3. Results
3.1. Overview of Included Studies
| Author, Year | Type of Study | Number of Patients | Age (Years) | Inclusion Criteria | Exclusion Criteria | Time of Elastography | Organ of Elastography | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Çıracı et al., 2015 [11] | Prospective | 96 (48 PCOS, 48 control) | 19–36 (PCOS), 20–38 (Controls) | Women diagnosed with PCOS using Rotterdam criteria, healthy controls | Cushing syndrome, congenital adrenal hyperplasia, hyperprolactinemia, thyroid dysfunction, virilizing tumors, type 2 diabetes mellitus, medication affecting hormonal balance | 3rd day of menstrual cycle | Ovaries (for PCOS diagnosis) | Ovaries in PCOS are stiffer than normal. Mean strain ratios in PCOS group: 6.1 ± 1.4 (2.7–10.1) vs. control group: 3.3 ± 1.2 (1.7–7.2), significant difference (p < 0.001). Strain ratio cutoff of 3.8 optimized for PCOS diagnosis. |
| Swierkowski-Blanchard et al. 2017, [24] | Prospective | 100 | 32.8 | Women scheduled for IUI, at least one permeable fallopian tube, normal semen analysis | Uterine malformation, previous uterine surgery, fibroid, polyp, adenomyosis, age >42 years | Before IUI procedure | Uterus (myometrium and endometrium for IUI outcomes) | Elasticity index higher in pregnant women (2.4 ± 1.3 vs. 1.5 ± 0.7, p < 0.05), predictive threshold >1.7 (OR = 63.26). Low uterine contraction frequency < 2.8/min (OR = 0.039) and high endometrial thickness >8 mm (OR = 28.21) were predictive of pregnancy. |
| Liu et al., 2022 [22] | Retrospective | 604, controls 1812 | Matched control (±1 year) | Women undergoing IVF with non-uniform endometrial echogenicity | Obvious intrauterine lesions, severe hormonal imbalances | During controlled ovarian stimulation | Endometrium (impact of non-uniform echogenicity in IVF) | Non-uniform endometrial echogenicity does not affect IVF success rates. No significant difference in live birth rate, clinical pregnancy rate, or miscarriage rate between groups (p > 0.05). |
| Li et al., 2024 [23] | Retrospective | 111 (59 unexplained infertility, 52 controls) | UI: 21–35, NC: 22–35 | Women with unexplained infertility, normal controls | Hormonal treatment, uterine malformations, severe systemic diseases | Late proliferative and mid-secretory phase | Endometrium (shear wave elastography to assess receptivity in unexplained infertility) | E-mean (elasticity) significantly higher in the unexplained infertility group than controls (p < 0.05). SWE was effective for assessing receptivity (AUC = 0.89). Higher stiffness correlated with lower receptivity. |
3.2. Elastography in PCOS Diagnosis
- Standardize the elastographic procedure for evaluating ovarian stiffness.
- Set universal cutoffs for strain ratio measurements.
- Determine the repeatability and interobserver reliability of RTE in PCOS diagnosis.
- Investigate the link between ovarian stiffness and metabolic markers in PCOS patients (insulin resistance, obesity, and inflammation).
3.3. Predictive Value of Elastography in IUI Success
- At least one permeable fallopian tube, as determined by hysterosalpingography (HSG) or sonohysterography.
- Normal sperm analysis for the male partner.
- No known uterine abnormalities (such as fibroids, adenomyosis, or congenital deformities).
- Prioritize patients with greater elasticity indices and less uterine contractions when choosing IUI candidates.
- Change treatment tactics (for example, using uterine relaxants in patients with frequent contractions).
- Improve counseling by giving patients more precise success rate projections based on uterine biomechanical parameters.
- Investigate the effect of hormone stimulation on uterine biomechanics and how elasticity measures change between IUI treatments (natural vs. stimulated cycles).
- Evaluate interobserver variability in elastographic readings to ensure reliability in clinical situations.
- Determine if elastography-guided IUI selection increases overall pregnancy rates throughout numerous treatment cycles.
3.4. Endometrial Elastography in IVF Cycles
4. Discussion
4.1. Mechanistic Explanation of Findings
4.2. Comparison with Other Imaging Modalities
4.3. Clinical Implications and Future Directions
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nik Hazlina, N.H.; Norhayati, M.N.; Shaiful Bahari, I.; Nik Muhammad Arif, N.A. Worldwide prevalence, risk factors and psychological impact of infertility among women: A systematic review and meta-analysis. BMJ Open 2022, 12, e057132. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Zhang, W.-D.; Yuan, B.; Zhang, J.-B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Kawai, M.; Kano, T.; Kikkawa, F.; Maeda, O.; Oguchi, H.; Tomoda, Y. Transvaginal Doppler ultrasound with color flow imaging in the diagnosis of ovarian cancer. Obs. Gynecol. 1992, 79, 163–167. [Google Scholar]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. BioMed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, X.; Qin, L.; Wu, Y.; Li, H. Application of strain elastography ultrasound to the endometrium of normal women. BMC Med. Imaging 2024, 24, 153. [Google Scholar] [CrossRef]
- Lacconi, V.; Massimiani, M.; Carriero, I.; Bianco, C.; Ticconi, C.; Pavone, V.; Alteri, A.; Muzii, L.; Rago, R.; Pisaturo, V.; et al. When the Embryo Meets the Endometrium: Identifying the Features Required for Successful Embryo Implantation. Int. J. Mol. Sci. 2024, 25, 2834. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Ciraci, S.; Tan, S.; Ozcan, A.S.; Aslan, A.; Keskin, H.L.; Ates, O.F.; Akcay, Y.; Arslan, H. Contribution of real-time elastography in diagnosis of polycystic ovary syndrome. Diagn. Interv. Radiol. 2015, 21, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Amargant, F.; Manuel, S.L.; Tu, Q.; Parkes, W.S.; Rivas, F.; Zhou, L.T.; Rowley, J.E.; Villanueva, C.E.; Hornick, J.E.; Shekhawat, G.S.; et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020, 19, e13259. [Google Scholar] [CrossRef] [PubMed]
- Javedani Masroor, M.; Younesi Asl, L.; Sarchami, N. The Effect of Uterine Contractions on Fertility Outcomes in Frozen Embryo Transfer Cycles: A Cohort Study. J. Reprod. Infertil. 2023. [Google Scholar] [CrossRef]
- Hooker, A.B.; De Leeuw, R.A.; Emanuel, M.H.; Mijatovic, V.; Brolmann, H.A.M.; Huirne, J.A.F. The link between intrauterine adhesions and impaired reproductive performance: A systematic review of the literature. BMC Pregnancy Childbirth 2022, 22, 837. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Lyu, G. Advances in the clinical application of ultrasound elastography in uterine imaging. Insights Imaging 2022, 13, 141. [Google Scholar] [CrossRef]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef]
- Ultrasonography in infertility. In Ultrasonography in Reproductive Medicine and Infertility; Rizk, B.R.M.B., Ed.; Cambridge University Press: Cambridge, UK, 2010; pp. 67–192. ISBN 978-0-511-77685-4. [Google Scholar]
- Zhang, C.; Chen, C.; Wang, J.; Wang, Y.; Wen, S.; Cao, Y.; Qian, W. An endometrial receptivity scoring system basing on the endometrial thickness, volume, echo, peristalsis, and blood flow evaluated by ultrasonography. Front. Endocrinol. 2022, 13, 907874. [Google Scholar] [CrossRef]
- Zaniker, E.J.; Zhang, M.; Hughes, L.; La Follette, L.; Atazhanova, T.; Trofimchuk, A.; Babayev, E.; Duncan, F.E. Shear wave elastography to assess stiffness of the human ovary and other reproductive tissues across the reproductive lifespan in health and disease. Biol. Reprod. 2024, 110, 1100–1114. [Google Scholar] [CrossRef]
- Karalis, V.D. The Integration of Artificial Intelligence into Clinical Practice. Appl. Biosci. 2024, 3, 14–44. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Liu, W.; Qu, H.; Du, Y.; Ma, J.; Lv, J.; Yan, L. The newly non-uniform endometrial echogenicity on transvaginal ultrasound do not impact in vitro fertilization and embryo transfer success: A retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, L.; Zhang, Z.; Zou, H.; He, M.; Qin, M.; Wang, H. Evaluation of endometrial receptivity in women with unexplained infertility by shear wave elastography. Insights Imaging 2024, 15, 85. [Google Scholar] [CrossRef]
- Swierkowski-Blanchard, N.; Boitrelle, F.; Alter, L.; Selva, J.; Quibel, T.; Torre, A. Uterine contractility and elastography as prognostic factors for pregnancy after intrauterine insemination. Fertil. Steril. 2017, 107, 961–968.e3. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.T. Polycystic Ovarian Syndrome: Diagnosis and Management. Clin. Med. Res. 2004, 2, 13–27. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Seifer, D.B. Women with PCOS who undergo IVF: A comprehensive review of therapeutic strategies for successful outcomes. Reprod. Biol. Endocrinol. 2023, 21, 70. [Google Scholar] [CrossRef]
- He, Y.; Deng, S.; Wang, Y.; Wang, X.; Huang, Q.; Cheng, J.; Wang, D.; Lyu, G. Evaluation of ovarian stiffness and its biological mechanism using shear wave elastography in polycystic ovary syndrome. Sci. Rep. 2025, 15, 585. [Google Scholar] [CrossRef]
- Ma, H.; Yang, Z.; Wang, Y.; Song, H.; Zhang, F.; Yang, L.; Yan, N.; Zhang, S.; Cai, Y.; Li, J. The Value of Shear Wave Elastography in Predicting the Risk of Endometrial Cancer and Atypical Endometrial Hyperplasia. J. Ultrasound Med. 2021, 40, 2441–2448. [Google Scholar] [CrossRef]
- Sun, C.; Yang, X.; Wang, T.; Cheng, M.; Han, Y. Ovarian Biomechanics: From Health to Disease. Front. Oncol. 2022, 11, 744257. [Google Scholar] [CrossRef]
- Raja-Khan, N.; Urbanek, M.; Rodgers, R.J.; Legro, R.S. The Role of TGF-β in Polycystic Ovary Syndrome. Reprod. Sci. 2014, 21, 20–31. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De La Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Stanziano, A.; Bianchi, F.P.; Caringella, A.M.; Cantatore, C.; D’Amato, A.; Vitti, A.; Cortone, A.; Vitagliano, A.; D’Amato, G. The use of real time strain endometrial elastosonography plus endometrial thickness and vascularization flow index to predict endometrial receptivity in IVF treatments: A pilot study. BMC Med. Imaging 2023, 23, 130. [Google Scholar] [CrossRef]
- Khalil, R.A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem. Pharmacol. 2013, 86, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Khalil, R.A. Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 148, pp. 87–165. ISBN 978-0-12-812776-6. [Google Scholar]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Jiao, Y.; Xue, N.; Zou, C.; Shui, X.; Wang, H.; Hu, C. Assessment of early damage of endometrium after artificial abortion by shear wave elastography. Insights Imaging 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, G. Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int. J. Mol. Sci. 2023, 24, 6992. [Google Scholar] [CrossRef]
- Frank, M.; Schäfer, S.; Möllers, M.; Falkenberg, M.; Braun, J.; Möllmann, U.; Strube, F.; Fruscalzo, A.; Amler, S.; Klockenbusch, W.; et al. Importance of Transvaginal Elastography in the Diagnosis of Uterine Fibroids and Adenomyosis. Ultraschall Med. 2015, 37, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, S.; Wang, L.; Wang, Y.; Lu, S.; Li, R. Endometrial Elasticity is an Ultrasound Marker for Predicting Clinical Pregnancy Outcomes after Embryo Transfer. Reprod. Sci. 2025, 32, 64–73. [Google Scholar] [CrossRef]
- Fijardo, M.; Kwan, J.Y.Y.; Bissey, P.-A.; Citrin, D.E.; Yip, K.W.; Liu, F.-F. The clinical manifestations and molecular pathogenesis of radiation fibrosis. eBioMedicine 2024, 103, 105089. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Thaker, N.; Dhande, R.; Parihar, P. Role of Transvaginal Sonography in the Diagnosis of Female Infertility: A Comprehensive Review. Cureus 2023, 15, e50048. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Ciliberti, G.; De Chiara, B.; Conte, E.; Franchin, L.; Musella, F.; Vitale, E.; Piroli, F.; Cangemi, S.; Cornara, S.; et al. Advancements and applications of artificial intelligence in cardiovascular imaging: A comprehensive review. Eur. Heart J.-Imaging Methods Pract. 2024, 2, qyae136. [Google Scholar] [CrossRef] [PubMed]
- Petanovski, Z.; Kostova, E.P. Everyday Practice of 2D/3D Vaginal Ultrasound in Reproductive Gynecology. Donald Sch. J. Ultrasound Obstet. Gynecol. 2020, 14, 97–116. [Google Scholar] [CrossRef]
- Di Michele, S.; Fulghesu, A.M.; Pittui, E.; Cordella, M.; Sicilia, G.; Mandurino, G.; D’Alterio, M.N.; Vitale, S.G.; Angioni, S. Ultrasound Assessment in Polycystic Ovary Syndrome Diagnosis: From Origins to Future Perspectives—A Comprehensive Review. Biomedicines 2025, 13, 453. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Dwivedi, A.N.D.; Ganesh, V.; Shukla, R.C.; Jain, M.; Kumar, I. Colour Doppler evaluation of uterine and ovarian blood flow in patients of polycystic ovarian disease and post-treatment changes. Clin. Radiol. 2020, 75, 772–779. [Google Scholar] [CrossRef]
- Singh, M.; Acharya, N.; Shukla, S.; Shrivastava, D.; Sharma, G. Comparative study of endometrial & subendometrial angiogenesis in unexplained infertile versus normal fertile women. Indian J. Med. Res. 2021, 154, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Li, Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod. Biol. Endocrinol. 2012, 10, 100. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Ren, X.; Huang, B.; Zhu, G.; Yang, W.; Jin, L. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine 2018, 97, e9689. [Google Scholar] [CrossRef]
- Huang, B.; Lu, D.; Kong, Y.; Ma, L. Successful live birth of thin endometrium: A case report. Medicine 2024, 103, e37399. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, H.K.; Kim, S.K. Doppler ultrasound investigation of female infertility. Obs. Gynecol. Sci. 2023, 66, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Celli, V.; Dolciami, M.; Ninkova, R.; Ercolani, G.; Rizzo, S.; Porpora, M.; Catalano, C.; Manganaro, L. MRI and Adenomyosis: What Can Radiologists Evaluate? Int. J. Environ. Res. Public Health 2022, 19, 5840. [Google Scholar] [CrossRef]
- Wang, S.; Duan, H. The role of the junctional zone in the management of adenomyosis with infertility. Front. Endocrinol. 2023, 14, 1246819. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Horne, A.W.; Gibson, D.A.; Roberts, N.; Saunders, P.T.K. Endometriosis: Recent advances that could accelerate diagnosis and improve care. Trends Mol. Med. 2024, 30, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Paluch, Ł.; Nawrocka-Laskus, E.; Wieczorek, J.; Mruk, B.; Frel, M.; Walecki, J. Use of Ultrasound Elastography in the Assessment of the Musculoskeletal System. Pol. J. Radiol. 2016, 81, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Byenfeldt, M.; Elvin, A.; Fransson, P. On Patient Related Factors and Their Impact on Ultrasound-Based Shear Wave Elastography of the Liver. Ultrasound Med. Biol. 2018, 44, 1606–1615. [Google Scholar] [CrossRef]
- Che, D.; Wei, H.; Yang, Z.; Zhang, Y.; Ma, S.; Zhou, X. Application of transvaginal sonographic elastography to distinguish endometrial cancer from benign masses. Am. J. Transl. Res. 2019, 11, 1049–1057. [Google Scholar]
- Lentscher, J.A.; Decherney, A.H. Clinical Presentation and Diagnosis of Polycystic Ovarian Syndrome. Clin. Obstet. Gynecol. 2021, 64, 3–11. [Google Scholar] [CrossRef]
- Re, C.; Mignini Renzini, M.; Rodriguez, A.; Dal Canto, M.; Buccheri, M.; Sacchi, S.; Bartolucci, S.; Fadini, R.; La Marca, A. From a circle to a sphere: The ultrasound imaging of ovarian follicle with 2D and 3D technology. Gynecol. Endocrinol. 2019, 35, 184–189. [Google Scholar] [CrossRef]
- Huniadi, A.; Bimbo-Szuhai, E.; Botea, M.; Zaha, I.; Beiusanu, C.; Pallag, A.; Stefan, L.; Bodog, A.; Șandor, M.; Grierosu, C. Fertility Predictors in Intrauterine Insemination (IUI). J. Pers. Med. 2023, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 202–223. [Google Scholar] [CrossRef]
- Langendijk, P.; Bouwman, E.G.; Kidson, A.; Kirkwood, R.N.; Soede, N.M.; Kemp, B. Role of myometrial activity in sperm transport through the genital tract and in fertilization in sows. Reproduction 2002, 123, 683–690. [Google Scholar]
- Sun, B.; Yeh, J. Non-Invasive and Mechanism-Based Molecular Assessment of Endometrial Receptivity During the Window of Implantation: Current Concepts and Future Prospective Testing Directions. Front. Reprod. Health 2022, 4, 863173. [Google Scholar] [CrossRef]
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 2023, 13, 1061766. [Google Scholar] [CrossRef]
- Brattain, L.J.; Telfer, B.A.; Dhyani, M.; Grajo, J.R.; Samir, A.E. Machine learning for medical ultrasound: Status, methods, and future opportunities. Abdom. Radiol. 2018, 43, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Hew, Y.; Kutuk, D.; Duzcu, T.; Ergun, Y.; Basar, M. Artificial Intelligence in IVF Laboratories: Elevating Outcomes Through Precision and Efficiency. Biology 2024, 13, 988. [Google Scholar] [CrossRef] [PubMed]
- Oglat, A.A.; Abukhalil, T. Ultrasound Elastography: Methods, Clinical Applications, and Limitations: A Review Article. Appl. Sci. 2024, 14, 4308. [Google Scholar] [CrossRef]
- Zanelli, S.; Agnoletti, D.; Alastruey, J.; Allen, J.; Bianchini, E.; Bikia, V.; Boutouyrie, P.; Bruno, R.M.; Climie, R.; Djeldjli, D.; et al. Developing technologies to assess vascular ageing: A roadmap from VascAgeNet. Physiol. Meas. 2024, 45, 121001. [Google Scholar] [CrossRef]
- Liberto, J.M.; Chen, S.-Y.; Shih, I.-M.; Wang, T.-H.; Wang, T.-L.; Pisanic, T.R. Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review. Cancers 2022, 14, 2885. [Google Scholar] [CrossRef]
- Horwood, G.; Flaxman, T.; McInnes, M.; McLean, L.; Singh, S.S. Ultrasound Elastography in Benign Gynecology: A Scoping Review. Reprod. Sci. 2024, 31, 2508–2522. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voros, C.; Varthaliti, A.; Mavrogianni, D.; Athanasiou, D.; Athanasiou, A.; Athanasiou, A.; Papahliou, A.-M.; Zografos, C.G.; Topalis, V.; Kondili, P.; et al. Elastography in Reproductive Medicine, a Game-Changer for Diagnosing Polycystic Ovary Syndrome, Predicting Intrauterine Insemination Success, and Enhancing In Vitro Fertilization Outcomes: A Systematic Review. Biomedicines 2025, 13, 784. https://doi.org/10.3390/biomedicines13040784
Voros C, Varthaliti A, Mavrogianni D, Athanasiou D, Athanasiou A, Athanasiou A, Papahliou A-M, Zografos CG, Topalis V, Kondili P, et al. Elastography in Reproductive Medicine, a Game-Changer for Diagnosing Polycystic Ovary Syndrome, Predicting Intrauterine Insemination Success, and Enhancing In Vitro Fertilization Outcomes: A Systematic Review. Biomedicines. 2025; 13(4):784. https://doi.org/10.3390/biomedicines13040784
Chicago/Turabian StyleVoros, Charalampos, Antonia Varthaliti, Despoina Mavrogianni, Diamantis Athanasiou, Antonia Athanasiou, Aikaterini Athanasiou, Anthi-Maria Papahliou, Constantinos G. Zografos, Vasileios Topalis, Panagiota Kondili, and et al. 2025. "Elastography in Reproductive Medicine, a Game-Changer for Diagnosing Polycystic Ovary Syndrome, Predicting Intrauterine Insemination Success, and Enhancing In Vitro Fertilization Outcomes: A Systematic Review" Biomedicines 13, no. 4: 784. https://doi.org/10.3390/biomedicines13040784
APA StyleVoros, C., Varthaliti, A., Mavrogianni, D., Athanasiou, D., Athanasiou, A., Athanasiou, A., Papahliou, A.-M., Zografos, C. G., Topalis, V., Kondili, P., Darlas, M., Sina, S., Daskalaki, M. A., Antsaklis, P., Loutradis, D., & Daskalakis, G. (2025). Elastography in Reproductive Medicine, a Game-Changer for Diagnosing Polycystic Ovary Syndrome, Predicting Intrauterine Insemination Success, and Enhancing In Vitro Fertilization Outcomes: A Systematic Review. Biomedicines, 13(4), 784. https://doi.org/10.3390/biomedicines13040784









