PDH Inhibition in Drosophila Ameliorates Sensory Dysfunction Induced by Vincristine Treatment in the Chemotherapy-Induced Peripheral Neuropathy Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains
2.2. Drug Treatments
2.3. Larval Thermal Nociception Assays
2.4. Analysis of C4da Neuron Dendrites
2.5. Measurement of Mitochondrial ROS (mtROS) of C4da Neurons
2.6. Measurement of Mitophagy Levels
2.7. Quantitative RT-PCR
2.8. Measurement of PDH Activity
2.9. Statistical Analyses
2.10. Genotypes
3. Results
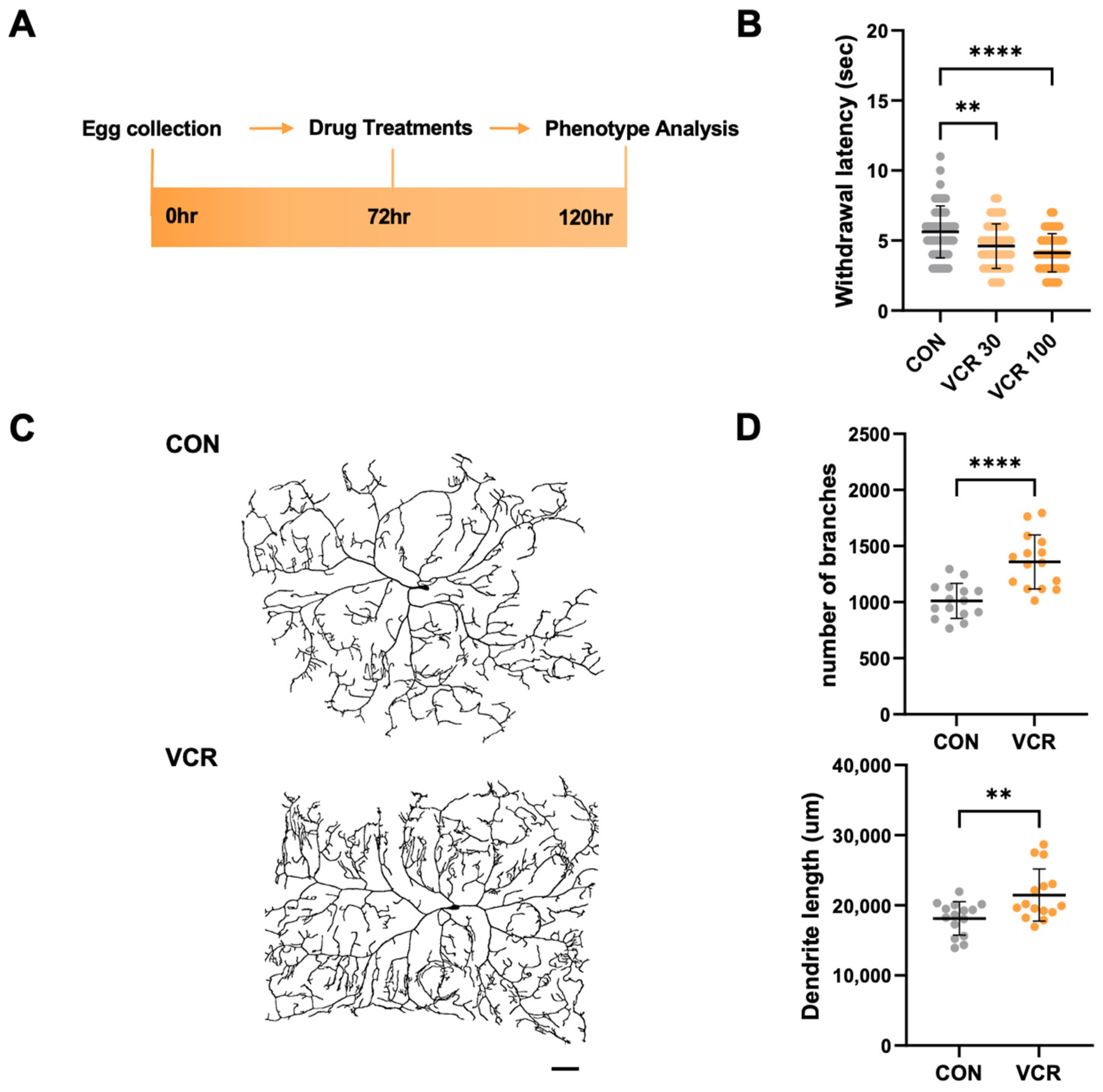
3.1. Vincristine Feeding to Drosophila Larvae Induces a Heat-Sensitive Phenotype with Alterations in Dendrites
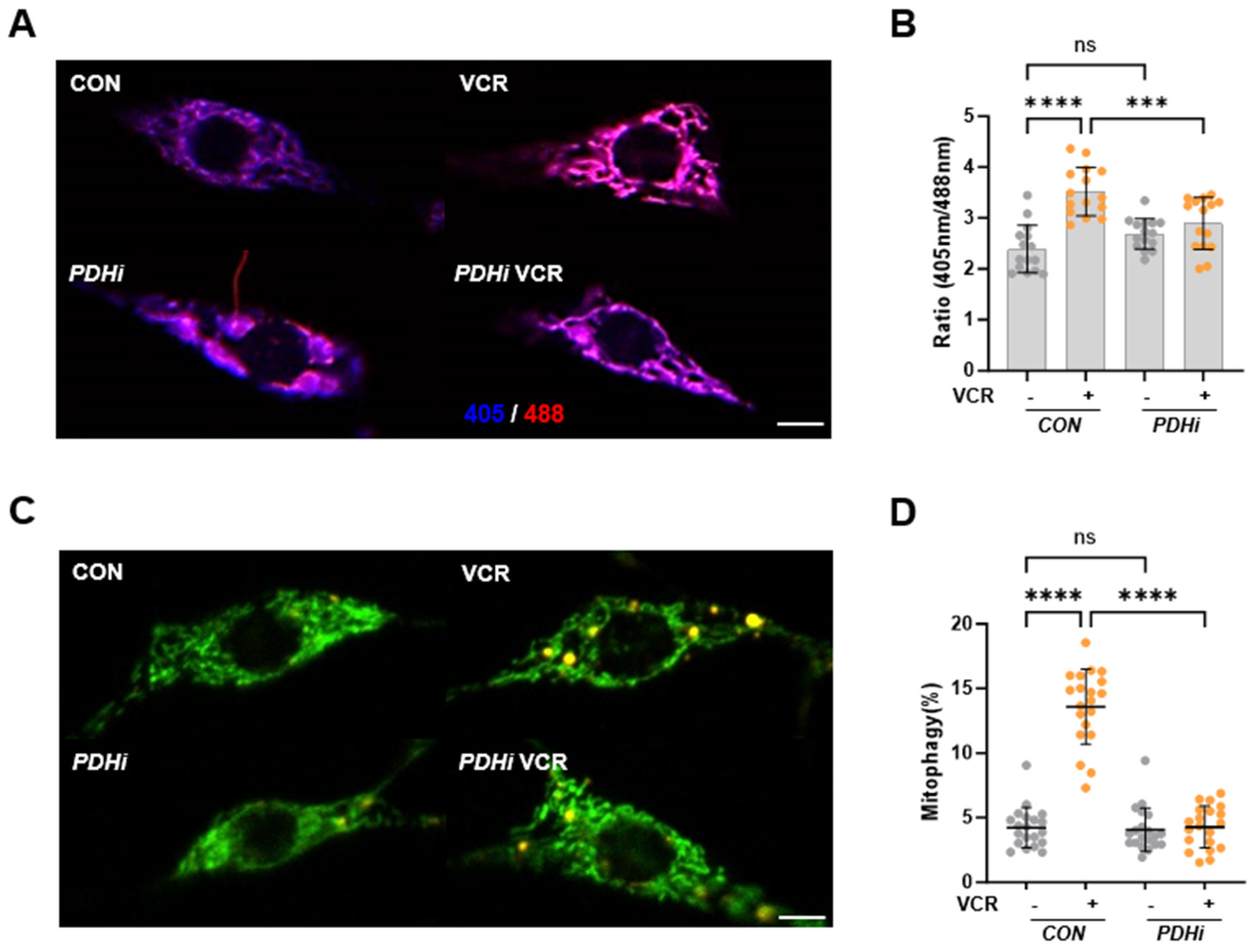
3.2. Vincristine Causes Mitochondrial Damage in C4da Neurons, Which Is Rescued by PDH Inhibition
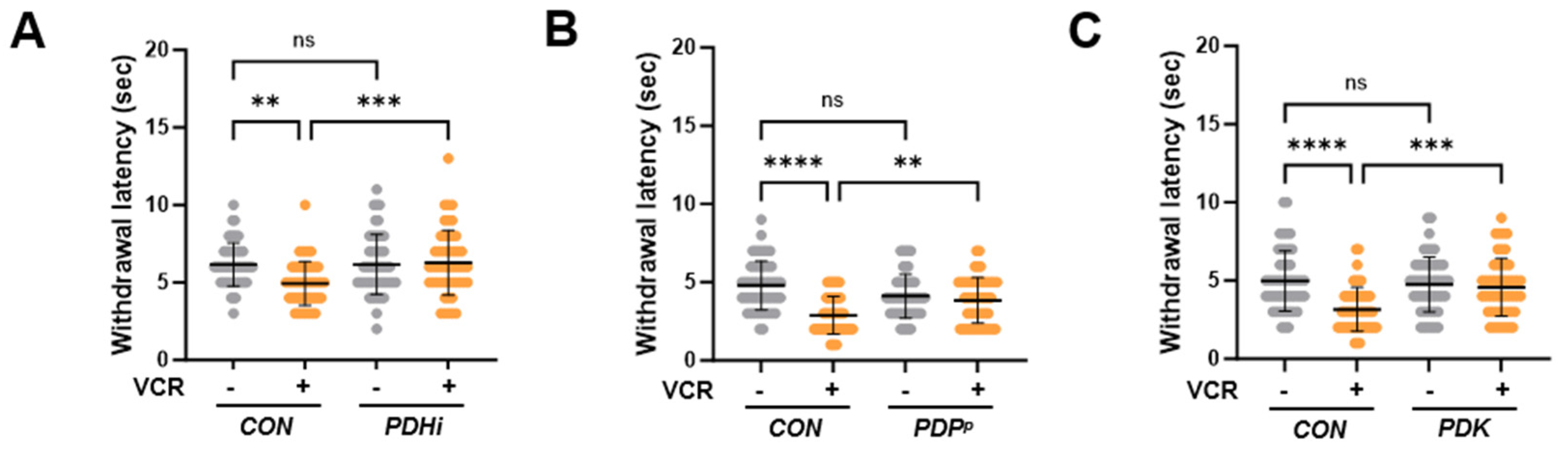
3.3. PDH Inhibition Ameliorates Thermal Hypersensitivity upon Vincristine Treatment
3.4. PDH Inhibition Rescues C4da Neuron Alterations During Vincristine Treatment
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Hausheer, F.H.; Schilsky, R.L.; Bain, S.; Berghorn, E.J.; Lieberman, F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin. Oncol. 2006, 33, 15–49. [Google Scholar] [CrossRef] [PubMed]
- Gidding, C.E.; Kellie, S.J.; Kamps, W.A.; de Graaf, S.S. Vincristine revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Slusher, B.S.; Wozniak, K.M.; Farah, M.H.; Smiyun, G.; Wilson, L.; Feinstein, S.; Jordan, M.A. Structural Basis for Induction of Peripheral Neuropathy by Microtubule-Targeting Cancer Drugs. Cancer Res. 2016, 76, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S. Vincristine- and bortezomib-induced neuropathies-from bedside to bench and back. Exp. Neurol. 2021, 336, 113519. [Google Scholar] [CrossRef]
- Verstappen, C.C.; Koeppen, S.; Heimans, J.J.; Huijgens, P.C.; Scheulen, M.E.; Strumberg, D.; Kiburg, B.; Postma, T.J. Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology 2005, 64, 1076–1077. [Google Scholar] [CrossRef]
- Casey, E.B.; Jellife, A.M.; Le Quesne, P.M.; Millett, Y.L. Vincristine neuropathy. Clinical and electrophysiological observations. Brain 1973, 96, 69–86. [Google Scholar] [CrossRef]
- Hirvonen, H.E.; Salmi, T.T.; Heinonen, E.; Antila, K.J.; Valimaki, I.A. Vincristine treatment of acute lymphoblastic leukemia induces transient autonomic cardioneuropathy. Cancer 1989, 64, 801–805. [Google Scholar] [CrossRef]
- Roca, E.; Bruera, E.; Politi, P.M.; Barugel, M.; Cedaro, L.; Carraro, S.; Chacon, R.D. Vinca alkaloid-induced cardiovascular autonomic neuropathy. Cancer Treat. Rep. 1985, 69, 149–151. [Google Scholar]
- Neely, G.G.; Hess, A.; Costigan, M.; Keene, A.C.; Goulas, S.; Langeslag, M.; Griffin, R.S.; Belfer, I.; Dai, F.; Smith, S.B.; et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell 2010, 143, 628–638. [Google Scholar] [CrossRef]
- Hwang, R.Y.; Zhong, L.; Xu, Y.; Johnson, T.; Zhang, F.; Deisseroth, K.; Tracey, W.D. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Tiburi, M.; Reguly, M.L.; Schwartsmann, G.; Cunha, K.S.; Lehmann, M.; Rodrigues de Andrade, H.H. Comparative genotoxic effect of vincristine, vinblastine, and vinorelbine in somatic cells of Drosophila melanogaster. Mutat. Res. 2002, 519, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.; Clements, J.; Zoeller, P.; Phillips, M. Absence of a mutagenic effect after feeding 4 anti-cancer drugs to Drosophila melanogaster. Mutat. Res. 1983, 120, 121–125. [Google Scholar] [CrossRef]
- Chalifoux, O.; Faerman, B.; Mailloux, R.J. Mitochondrial hydrogen peroxide production by pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase in oxidative eustress and oxidative distress. J. Biol. Chem. 2023, 299, 105399. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Kim, H.; Han, J.E.; Kim, S.; Kang, K.H.; Kim, D.; Kim, J.M.; Koh, H. Pyruvate Dehydrogenase Kinase Protects Dopaminergic Neurons from Oxidative Stress in Drosophila DJ-1 Null Mutants. Mol. Cells 2022, 45, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Um, J.H.; Yoon, J.H.; Kim, H.; Lee, D.Y.; Lee, Y.J.; Jee, H.J.; Kim, Y.M.; Jang, J.S.; Jang, Y.G.; et al. Assessment of mitophagy in mt-Keima Drosophila revealed an essential role of the PINK1-Parkin pathway in mitophagy induction in vivo. FASEB J. 2019, 33, 9742–9751. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Yoon, J.H.; Um, J.H.; Jeong, D.J.; Shin, D.J.; Hong, Y.B.; Kim, J.K.; Kim, D.H.; Kim, C.; Chung, C.G.; et al. PINK1 alleviates thermal hypersensitivity in a paclitaxel-induced Drosophila model of peripheral neuropathy. PLoS ONE 2020, 15, e0239126. [Google Scholar] [CrossRef]
- Bhattacharya, M.R.; Gerdts, J.; Naylor, S.A.; Royse, E.X.; Ebstein, S.Y.; Sasaki, Y.; Milbrandt, J.; DiAntonio, A. A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 5054–5061. [Google Scholar] [CrossRef]
- Brazill, J.M.; Cruz, B.; Zhu, Y.; Zhai, R.G. Nmnat mitigates sensory dysfunction in a Drosophila model of paclitaxel-induced peripheral neuropathy. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef]
- Markstein, M.; Dettorre, S.; Cho, J.; Neumuller, R.A.; Craig-Muller, S.; Perrimon, N. Systematic screen of chemotherapeutics in Drosophila stem cell tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 4530–4535. [Google Scholar] [CrossRef]
- Han, C.; Jan, L.Y.; Jan, Y.N. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc. Natl. Acad. Sci. USA 2011, 108, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Wang, L.; Song, X.Y. Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction. Biomed. Pharmacother. 2020, 125, 110003. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.C.; Barata, A.G.; Grosshans, J.; Teleman, A.A.; Dick, T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011, 14, 819–829. [Google Scholar] [CrossRef]
- Sun, N.; Yun, J.; Liu, J.; Malide, D.; Liu, C.; Rovira, I.I.; Holmstrom, K.M.; Fergusson, M.M.; Yoo, Y.H.; Combs, C.A.; et al. Measuring In Vivo Mitophagy. Mol. Cell 2015, 60, 685–696. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.H.; Min, B.K.; Ha, C.M.; Thoudam, T.; Park, B.Y.; Lee, I.K. Role of the Pyruvate Dehydrogenase Complex in Metabolic Remodeling: Differential Pyruvate Dehydrogenase Complex Functions in Metabolism. Diabetes Metab. J. 2018, 42, 270–281. [Google Scholar] [CrossRef]
- Im, S.H.; Galko, M.J. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev. Dyn. 2012, 241, 16–26. [Google Scholar] [CrossRef]
- Parrish, J.Z.; Xu, P.; Kim, C.C.; Jan, L.Y.; Jan, Y.N. The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in drosophila sensory neurons. Neuron 2009, 63, 788–802. [Google Scholar] [CrossRef]
- Cheng, A.; Hou, Y.; Mattson, M.P. Mitochondria and neuroplasticity. ASN Neuro 2010, 2, e00045. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sung, H.H.; Huang, Y.C.; Cheng, Y.J.; Yeh, H.F.; Pi, H.; Giniger, E.; Chien, C.T. Fringe-positive Golgi outposts unite temporal Furin 2 convertase activity and spatial Delta signal to promote dendritic branch retraction. Cell Rep. 2022, 40, 111372. [Google Scholar] [CrossRef]
- Tari, C.; Fournier, N.; Briand, C.; Ducet, G.; Crevat, A. Action of vinca alkaloides on calcium movements through mitochondrial membrane. Pharmacol. Res. Commun. 1986, 18, 519–528. [Google Scholar] [CrossRef]
- Chine, V.B.; Au, N.P.B.; Ma, C.H.E. Therapeutic benefits of maintaining mitochondrial integrity and calcium homeostasis by forced expression of Hsp27 in chemotherapy-induced peripheral neuropathy. Neurobiol. Dis. 2019, 130, 104492. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Dey, A.; Woods, J.K.; Feany, M.B. Elevated Oxidative Stress and DNA Damage in Cortical Neurons of Chemotherapy Patients. J. Neuropathol. Exp. Neurol. 2021, 80, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Deza, J.; Slavutsky, A.L.; Nebiyou, M.; Le Pichon, C.E. Local production of reactive oxygen species drives vincristine-induced axon degeneration. Cell Death Dis. 2023, 14, 807. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.K.; Otero, L.J.; LeGris, M.; Brown, R.M. Pyruvate dehydrogenase deficiency. J. Med. Genet. 1994, 31, 875–879. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Kim, S.; Han, J.E.; Kang, K.-h.; Koh, H. PDH Inhibition in Drosophila Ameliorates Sensory Dysfunction Induced by Vincristine Treatment in the Chemotherapy-Induced Peripheral Neuropathy Models. Biomedicines 2025, 13, 783. https://doi.org/10.3390/biomedicines13040783
Song H, Kim S, Han JE, Kang K-h, Koh H. PDH Inhibition in Drosophila Ameliorates Sensory Dysfunction Induced by Vincristine Treatment in the Chemotherapy-Induced Peripheral Neuropathy Models. Biomedicines. 2025; 13(4):783. https://doi.org/10.3390/biomedicines13040783
Chicago/Turabian StyleSong, Harim, Sohee Kim, Ji Eun Han, Kyong-hwa Kang, and Hyongjong Koh. 2025. "PDH Inhibition in Drosophila Ameliorates Sensory Dysfunction Induced by Vincristine Treatment in the Chemotherapy-Induced Peripheral Neuropathy Models" Biomedicines 13, no. 4: 783. https://doi.org/10.3390/biomedicines13040783
APA StyleSong, H., Kim, S., Han, J. E., Kang, K.-h., & Koh, H. (2025). PDH Inhibition in Drosophila Ameliorates Sensory Dysfunction Induced by Vincristine Treatment in the Chemotherapy-Induced Peripheral Neuropathy Models. Biomedicines, 13(4), 783. https://doi.org/10.3390/biomedicines13040783





