3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review
Abstract
1. Introduction
2. 3D Printing Techniques
2.1. Fused Deposition Modeling (FDM) or Free Form Fabrication (FFF)
2.2. Laminated Object Manufacturing
2.3. Selective Deposition Laminations
2.4. Ultrasonic Additive Manufacturing
2.5. Digital Light Processing
2.6. Direct Ink Writing
2.7. Liquid Deposition Modeling
2.8. Extrusion-Based Bioprinting
2.9. Stereolithography (SLA)
2.10. Selective Laser Sintering (SLS) and Selective Laser Melting (SLM)
2.11. Inkjet or Binder Jet Printing
2.12. PolyJet Printing
3. Bioink
3.1. Viscosity
3.2. Shear Thinning
3.3. Yield Stress and Viscoelastisity
4. Polysaccharides
4.1. Cellulose
4.2. Chitosan
4.3. Alginate
4.4. Pectin
4.5. Hyaluronic Acid
4.6. Starch
4.7. Glucan and Its Derivatives
4.8. Agarose and Its Derivatives
4.9. Dextran
4.10. Xanthan Gum
4.11. Gellan Gum
4.12. Konjac Gum
4.13. Guar Gum (GG)
4.14. Pullulan
5. Stimuli-Responsive Polysaccharide-Based Hydrogels
5.1. pH-Responsive Polysaccharide Hydrogels
5.2. Redox-Responsive Polysaccharide Hydrogels
5.3. Photo-Responsive Polysaccharide Hydrogels
6. Dual Responsive Hydrogels
6.1. pH- and Temperature-Responsive Polysaccharide Hydrogels
6.2. pH- and ROS-Responsive Polysaccharide Hydrogels
6.3. pH- and Electro-Responsive Polysaccharide Hydrogels
7. 3D Printing Hydrogels for Tissue Engineering and Drug Delivery
7.1. Alginate and Its Derivates for Biomedical Use
7.2. Biomedical Applications of Chitosan Derivatives
7.3. Cellulose Applications in the Medical and Biomedical Fields
8. Limitations
9. Conclusions and Future Prospective
Funding
Conflicts of Interest
List of Abbreviations
| FDA | Food and Drug Administration |
| AM | Additive manufacturing |
| SLS | Selective laser sintering |
| SLA | Stereolithography |
| FDM | Fused deposition modeling |
| ROS | Reactive oxygen species |
| DIC | Digital image correlation |
| GAG | Glycosaminoglycans |
| LCST | Lower critical solution temperature |
| UCST | Upper critical solution temperature |
| CS | Chitosan |
| CAD | Computer-aided design |
| HME | Hot melt extrusion |
| API | Active pharmaceutical ingredients |
| PAM | Pressure-assisted microsyringe |
| NMMO | N-methylmorpholine-N-oxide monohydrate |
| MBA | N, N′-methylene bisacrylamide |
| HA | Hyaluronic acid |
| ADA-GEL-PPy | Gelatin-content oxidized alginate-gelatin polypyrrole |
| L-PRF | Lyophilized platelet-rich fibrin |
| HAP | Hydroxyapatite |
| CN+HAMA | Cellulose nanofibers and hyaluronic acid methacrylate |
| PCL | Polycaprolactone |
| PDA | Polydopamine |
| CFZ | Cefazolin |
| RFP | Rifampicin |
| CNFs | Cellulose nanofibers |
| PHB/PCL | Poly-hydroxybutyrate/poly(ε-caprolactone) |
| OKGM/PEI | Oxidized konjac glucomannan/polyethyleneimine |
| CNTs | Carbon nanotubes |
| TFNA | Tetrahedral framework nucleic acid |
| SMSCs | Synovial mesenchymal stem cells |
| AC | Articular cartilage |
| pNPP | p-nitrophenyl phosphate |
| VEGF | Vascular Endothelial Growth Factor |
| OHA | Oxidized hyaluronate |
| GC | Glycol chitosan |
| ADH | Adipic acid dihydrazide |
| hBMSCs | Human Bone Marrow Mesenchymal Stem Cell |
| MHBC | Methacrylated Hydroxylbutyl Chitosan |
| GO | Graphene oxide |
| GEL | Gelatin |
| ALG | Alginate |
| NFC | Fibrillated cellulose |
| CMC | Carboxymethyl cellulose |
| TS | Tensile strength |
| CS | Compressive strength |
| FS | Flexural strength |
| CLPs | Colloidal lignin particles |
| DCM | Dichloromethane |
| 2-Bu | 2-butoxy ethanol |
| DBP | Dibutyl phthalate |
| PLLA | Poly(L-lactide) |
| CHWs | Chitin whiskers |
| DIW | Direct ink writing |
| CP | CHWs/PLLA |
| TOCNFs | (2,2,6,6-tetramethylpiperidine-1-oxylradi-cal)-mediated oxidized cellulose nano-fibrils |
References
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D Printing to Innovate Biopolymer Materials for Demanding Applications: A Review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Ortega Arevalo, M.D.P.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent Trends in Natural Polysaccharide Based Bioinks for Multiscale 3D Printing in Tissue Regeneration: A Review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef] [PubMed]
- Shokrani, A.; Shokrani, H.; Munir, M.T.; Kucinska-Lipka, J.; Khodadadi Yazdi, M.; Saeb, M.R. Monitoring Osteoarthritis: A Simple Mathematical Model. Biomed. Eng. Adv. 2022, 4, 100050. [Google Scholar] [CrossRef]
- Forgacs, G.; Jakab, K.; Neagu, A.; Mironov, V. U.S. Patent Application No. 14/477,148, 14 August 2015.
- Shokrani, H.; Shokrani, A.; Saeb, M.R. Methods for Biomaterials Printing: A Short Review and Perspective. Methods 2022, 206, 1–7. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials Based on Marine Resources for 3D Bioprinting Applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-Based Inks for 3D Printing and Bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-Based Biomaterials for Advanced Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2020, 326, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Shokri, Z.; Seidi, F.; Saeb, M.; Jin, Y.; Li, C.; Xiao, H. Elucidating the Impact of Enzymatic Modifications on the Structure, Properties, and Applications of Cellulose, Chitosan, Starch and Their Derivatives: A Review. Mater. Today Chem. 2022, 24, 100780. [Google Scholar] [CrossRef]
- Besford, Q.A.; Cavalieri, F.; Caruso, F. Glycogen as a Building Block for Advanced Biological Materials. Adv. Mater. 2020, 32, e1904625. [Google Scholar] [CrossRef]
- Khosravi, A.; Fereidoon, A.; Khorasani, M.M.; Naderi, G.; Ganjali, M.R.; Zarrintaj, P.; Saeb, M.R.; Gutiérrez, T.J. Soft and Hard Sections from Cellulose-Reinforced Poly(Lactic Acid)-Based Food Packaging Films: A Critical Review. Food Packag. Shelf Life 2020, 23, 100429. [Google Scholar] [CrossRef]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-Based Blends for Biomedical Applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Jana, S. Functional Polysaccharides for Biomedical Applications. Funct. Polysaccharides Biomed. Appl. 2019, 2019, 1–526. [Google Scholar] [CrossRef]
- Shokri, Z.; Seidi, F.; Karami, S.; Li, C.; Saeb, M.R.; Xiao, H. Laccase Immobilization onto Natural Polysaccharides for Biosensing and Biodegradation. Carbohydr. Polym. 2021, 262, 117963. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Shokrani, H.; Shokrani, A.; Jabbour, K.; Abida, O.; Mousavi Khadem, S.S.; Habibzadeh, S.; Sonawane, S.H.; Saeb, M.R.; Bonilla-Petriciolet, A.; et al. Recent Advances in Aqueous Virus Removal Technologies. Chemosphere 2022, 305, 135441. [Google Scholar] [CrossRef]
- Midhun Dominic, C.D.; Raj, V.; Neenu, K.V.; Begum, P.M.S.; Formela, K.; Saeb, M.R.; Prabhu, D.D.; Poornima Vijayan, P.; Ajithkumar, T.G.; Parameswaranpillai, J. Chlorine-Free Extraction and Structural Characterization of Cellulose Nanofibers from Waste Husk of Millet (Pennisetum glaucum). Int. J. Biol. Macromol. 2022, 206, 92–104. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.; Urbanska, A.; Saeb, M.; Venugopal, J.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2020, 117, 100721. [Google Scholar] [CrossRef]
- Badylak, S.F.; Tullius, R.; Kokini, K.; Shelbourne, K.D.; Klootwyk, T.; Voytik, S.L.; Kraine, M.R.; Simmons, C. The Use of Xenogeneic Small Intestinal Submucosa as a Biomaterial for Achille’s Tendon Repair in a Dog Model. J. Biomed. Mater. Res. 1995, 29, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.-C.; et al. Three-Dimensional Bioprinted Hepatorganoids Prolong Survival of Mice with Liver Failure. Gut 2021, 70, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Garshasbi, H.R.; Naghib, S.M. Smart Stimuli-Responsive Alginate Nanogels for Drug Delivery Systems and Cancer Therapy: A Review. Curr. Pharm. Des. 2023, 29, 3546–3562. [Google Scholar] [CrossRef]
- Garshasbi, H.; Salehi, S.; Naghib, S.M.; Ghorbanzadeh, S.; Zhang, W. Stimuli-Responsive Injectable Chitosan-Based Hydrogels for Controlled Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 10, 1126774. [Google Scholar] [CrossRef]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.-S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef]
- Loo, Y.; Lakshmanan, A.; Ni, M.; Toh, L.L.; Wang, S.; Hauser, C.A.E. Peptide Bioink: Self-Assembling Nanofibrous Scaffolds for Three-Dimensional Organotypic Cultures. Nano Lett. 2015, 15, 6919–6925. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D Printed Drug Delivery and Testing Systems—A Passing Fad or the Future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]
- Ursan, I.D.; Chiu, L.; Pierce, A. Three-Dimensional Drug Printing: A Structured Review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Elshaer, A.; Sareh, P.; Elsayed, M.; Hassanin, H. Additive Manufacturing Technologies for Drug Delivery Applications. Int. J. Pharm. 2020, 580, 119245. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Zhou, L.; Jianzhong, F. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D Printed Medicines: A New Branch of Digital Healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping Drug Development Using 3D Printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef]
- Boehm, R.; Miller, P.; Daniels, J.; Stafslien, S.; Narayan, J. Inkjet Printing for Pharmaceutical Applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-Based 3D Inkjet Printing of an Oral Pharmaceutical Dosage Form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet Printing of Drug Substances and Use of Porous Substrates-towards Individualized Dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Choi, H.; Moon, S.; Kim, S.; Bajracharya, R.; Min, J.; Han, H.-K. Pharmaceutical Applications of 3D Printing Technology: Current Understanding and Future Perspectives. J. Pharm. Investig. 2018, 49, 575–585. [Google Scholar] [CrossRef]
- Dimitrov, D.; Schreve, K.; de Beer, N. Advances in Three Dimensional Printing—State of the Art and Future Perspectives. Rapid Prototyp. J. 2006, 12, 136–147. [Google Scholar] [CrossRef]
- Schmidt, M.; Pohle, D.; Rechtenwald, T. Selective Laser Sintering of PEEK. CIRP Ann. 2007, 56, 205–208. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-Filament 3D Printing (3DP) for Fabrication of Tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Madzarevic, M.; Medarevic, D.; Vulovic, A.; Sustersic, T.; Djuris, J.; Filipovic, N.; Ibric, S. Optimization and Prediction of Ibuprofen Release from 3D DLP Printlets Using Artificial Neural Networks. Pharmaceutics 2019, 11, 544. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Chimate, C.; Koc, B. Pressure Assisted Multi-Syringe Single Nozzle Deposition System for Manufacturing of Heterogeneous Tissue Scaffolds. Int. J. Adv. Manuf. Technol. 2014, 75, 317–330. [Google Scholar] [CrossRef]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality Considerations on the Pharmaceutical Applications of Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2021, 592, 119901. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the New Asset for the Production of Tailored Medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef]
- Chennakesava, P.; Narayan, Y.S. Fused Deposition Modeling in Rapid Prototyping Technology: Selection and Application; Cooper, K.G., Ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 1345–1350. ISBN 9789384743123. [Google Scholar]
- Walker, J.L.; Santoro, M. Processing and Production of Bioresorbable Polymer Scaffolds for Tissue Engineering. In Bioresorbable Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–203. [Google Scholar]
- Bähr, F.; Westkämper, E. Correlations between Influencing Parameters and Quality Properties of Components Produced by Fused Deposition Modeling. Procedia CIRP 2018, 72, 1214–1219. [Google Scholar] [CrossRef]
- Garzon-Hernandez, S.; Arias, A.; Garcia-Gonzalez, D. A Continuum Constitutive Model for FDM 3D Printed Thermoplastics. Compos. Part B Eng. 2020, 201, 108373. [Google Scholar] [CrossRef]
- Grigora, M.-E.; Terzopoulou, Z.; Tsongas, K.; Klonos, P.; Kalafatakis, N.; Bikiaris, D.N.; Kyritsis, A.; Tzetzis, D. Influence of Reactive Chain Extension on the Properties of 3D Printed Poly(Lactic Acid) Constructs. Polymers 2021, 13, 1381. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, X. High-Quality 3D Printing of Ethylene Vinyl Acetate with Direct Pellet-Based FDM for Medical Applications: Mechanical Analysis, Energy Absorption and Recovery Evaluation. J. Mech. Behav. Biomed. Mater. 2023, 148, 106231. [Google Scholar] [CrossRef]
- Poomathi, N.; Singh, S.; Prakash, C.; Subramanian, A.; Sahay, R.; Cinappan, A.; Ramakrishna, S. 3D Printing in Tissue Engineering: A State of the Art Review of Technologies and Biomaterials. Rapid Prototyp. J. 2020, 26, 1313–1334. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C 2020, 109, 110578. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Kumari, S.; Mondal, P.; Chatterjee, K. Digital Light Processing-Based 3D Bioprinting of κ-Carrageenan Hydrogels for Engineering Cell-Loaded Tissue Scaffolds. Carbohydr. Polym. 2022, 290, 119508. [Google Scholar] [CrossRef] [PubMed]
- Greant, C.; Van Durme, B.; Van Damme, L.; Brancart, J.; Van Hoorick, J.; Van Vlierberghe, S. Digital Light Processing of Poly(ε-Caprolactone)-Based Resins into Porous Shape Memory Scaffolds. Eur. Polym. J. 2023, 195, 112225. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B Rev. 2016, 23, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kachit, M.; Kopp, A.; Adrien, J.; Maire, E.; Boulnat, X. Direct-Ink Writing and Compression Behavior by in Situ Micro-Tomography of Architectured 316L Scaffolds with a Two-Scale Porosity. J. Mater. Res. Technol. 2022, 20, 1341–1351. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Yu, S.; Wu, W.; Zhang, L.; Liu, Q.; Ren, L. Direct Ink Writing of Porous Fe Scaffolds for Bone Implants: Pore Size Evolution and Effect on Degradation and Mechanical Properties. J. Mater. Res. Technol. 2023, 25, 4901–4912. [Google Scholar] [CrossRef]
- He, Y.; Xie, M.; Gao, Q.; Fu, J. Why Choose 3D Bioprinting? Part I: A Brief Introduction of 3D Bioprinting for the Beginners. Bio-Design Manuf. 2019, 2, 221–224. [Google Scholar] [CrossRef]
- Angelats Lobo, D.; Ginestra, P. Cell Bioprinting: The 3D-BioplotterTM Case. Materials 2019, 12, 4005. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Wulle, F.; Gorke, O.; Schmidt, S.; Nistler, M.; Tovar, G.E.; Riedel, O.; Verl, A.; Weber, A.; Southan, A. Multi-axis 3D printing of gelatin methacryloyl hydrogels on a non-planar surface obtained from magnetic resonance imaging. Addit. Manuf. 2022, 50, 102566. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Vlierberghe, S.V.; Gu, L.; Ovsianikov, A. Bioink Properties before, during and after 3D Bioprinting. Biofabrication 2016, 8, 32002. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural Hydrogels R&D Process: Technical and Regulatory Aspects for Industrial Implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, e1701161. [Google Scholar] [CrossRef]
- Leonards, H.; Engelhardt, S.; Hoffmann, A.; Pongratz, L.; Schriever, S.; Bläsius, J.; Wehner, M.; Gillner, A. Advantages and Drawbacks of Thiol-Ene Based Resins for 3D-Printing. In Proceedings of the Laser 3D Manufacturing II, San Francisco, CA, USA, 7–12 February 2015. [Google Scholar]
- Schmidleithner, C.; Kalaskar, D.M. Stereolithography. In 3D Printing; Cvetković, D., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-Dimensional Printing for Medical Applications. Int. J. Bioprinting 2020, 6. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Chichkov, B. Capillary-like Formations of Endothelial Cells in Defined Patterns Generated by Laser Bioprinting. Micromachines 2021, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with Polymers: Challenges among Expanding Options and Opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; Osman, N.A.A. A Review on Powder-Based Additive Manufacturing for Tissue Engineering: Selective Laser Sintering and Inkjet 3D Printing. Sci. Technol. Adv. Mater. 2015, 16, 33502. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding Mechanisms in Selective Laser Sintering and Selective Laser Melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective Laser Sintering (SLS) 3D Printing of Medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A Review on Selective Laser Sintering/Melting (SLS/SLM) of Aluminium Alloy Powders: Processing, Microstructure, and Properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Tan, W.S.; Chua, C.K.; Chong, T.H.; Fane, A.G.; Jia, A. 3D Printing by Selective Laser Sintering of Polypropylene Feed Channel Spacers for Spiral Wound Membrane Modules for the Water Industry. Virtual Phys. Prototyp. 2016, 11, 151–158. [Google Scholar] [CrossRef]
- Yi, X.; Tan, Z.-J.; Yu, W.-J.; Li, J.; Li, B.-J.; Huang, B.-Y.; Liao, J. Three Dimensional Printing of Carbon/Carbon Composites by Selective Laser Sintering. Carbon N. Y. 2016, 96, 603–607. [Google Scholar] [CrossRef]
- Xia, M.; Gu, D.; Yu, G.; Dai, D.; Chen, H.; Shi, Q. Selective Laser Melting 3D Printing of Ni-Based Superalloy: Understanding Thermodynamic Mechanisms. Sci. Bull. 2016, 61, 1013–1022. [Google Scholar] [CrossRef]
- Chen, L.Y.; Huang, J.C.; Lin, C.H.; Pan, C.T.; Chen, S.Y.; Yang, T.L.; Lin, D.Y.; Lin, H.K.; Jang, J.S.C. Anisotropic Response of Ti-6Al-4V Alloy Fabricated by 3D Printing Selective Laser Melting. Mater. Sci. Eng. A 2017, 682, 389–395. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Yao, J.; Cheng, X.; Li, X. Bio-Functional and Anti-Corrosive 3D Printing 316L Stainless Steel Fabricated by Selective Laser Melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, D. A Study on the Residual Stress during Selective Laser Melting (SLM) of Metallic Powder. Int. J. Adv. Manuf. Technol. 2016, 87, 647–656. [Google Scholar] [CrossRef]
- Salmi, A.; Atzeni, E.; Iuliano, L.; Galati, M. Experimental Analysis of Residual Stresses on AlSi10Mg Parts Produced by Means of Selective Laser Melting (SLM). Procedia CIRP 2017, 62, 458–463. [Google Scholar] [CrossRef]
- Protasov, C.E.; Safronov, V.A.; Kotoban, D.V.; Gusarov, A. V Experimental Study of Residual Stresses in Metal Parts Obtained by Selective Laser Melting. Phys. Procedia 2016, 83, 825–832. [Google Scholar] [CrossRef]
- Zhou, Z.; Lennon, A.; Buchanan, F.; McCarthy, H.O.; Dunne, N. Binder Jetting Additive Manufacturing of Hydroxyapatite Powders: Effects of Adhesives on Geometrical Accuracy and Green Compressive Strength. Addit. Manuf. 2020, 36, 101645. [Google Scholar] [CrossRef]
- Salaoru, I.; Zhou, Z.; Morris, P.; Gibbons, G.J. Inkjet Printing of Polyvinyl Alcohol Multilayers for Additive Manufacturing Applications. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Bogala, M.R. Three-Dimensional (3D) Printing of Hydroxyapatite-Based Scaffolds: A Review. Bioprinting 2022, 28, e00244. [Google Scholar] [CrossRef]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef]
- Mironov, V. Printing Technology to Produce Living Tissue. Expert Opin. Biol. Ther. 2003, 3, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Thayer, P.; Martinez, H.; Gatenholm, E.; Khademhosseini, A. A Perspective on the Physical, Mechanical and Biological Specifications of Bioinks and the Development of Functional Tissues in 3D Bioprinting. Bioprinting 2018, 9, 19–36. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Feng, C.; Shi, H.; Zhao, G.; Bian, Y. Rheological Behavior, 3D Printability and the Formation of Scaffolds with Cellulose Nanocrystals/Gelatin Hydrogels. J. Mater. Sci. 2020, 55, 15709–15725. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing Bioink Shape Fidelity to Aid Material Development in 3D Bioprinting. Biofabrication 2017, 10, 14102. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Khoda, B. Rheological Analysis of Bio-Ink for 3D Bio-Printing Processes. J. Manuf. Process. 2022, 76, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Caballero, S.S.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D Printing of Chitosan-Calcium Phosphate Inks: Rheology, Interactions and Characterization. J. Mater. Sci. Mater. Med. 2018, 30, 6. [Google Scholar] [CrossRef]
- Heid, S.; Boccaccini, A.R. Advancing Bioinks for 3D Bioprinting Using Reactive Fillers: A Review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Ahlfeld, T.; Lode, A.; Gelinsky, M. A Versatile Method for Combining Different Biopolymers in a Core/Shell Fashion by 3D Plotting to Achieve Mechanically Robust Constructs. Biofabrication 2016, 8, 45001. [Google Scholar] [CrossRef]
- Muskan; Gupta, D.; Negi, N.P. 3D Bioprinting: Printing the Future and Recent Advances. Bioprinting 2022, 27, e00211. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Advanced Cellulose Nanocrystals (CNC) and Cellulose Nanofibrils (CNF) Aerogels: Bottom-up Assembly Perspective for Production of Adsorbents. Int. J. Biol. Macromol. 2022, 222, 1–29. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced Rheological Behaviors of Alginate Hydrogels with Carrageenan for Extrusion-Based Bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield Stress Determines Bioprintability of Hydrogels Based on Gelatin-Methacryloyl and Gellan Gum for Cartilage Bioprinting. Biofabrication 2016, 8, 35003. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to Assess Printability of Bioinks for Extrusion-Based Bioprinting and Evaluation of Rheological Properties Governing Bioprintability. Biofabrication 2017, 9, 44107. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Duan, L.; Cui, J.; Li, G.; Jiang, H.; Pan, H. Influence of Compaction Temperature on the Mechanical Properties and Micro Morphology of Cu/CNTs Composites Prepared by Electromagnetic Impacting. Powder Technol. 2022, 396, 433–443. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing Cellulose Hydrogels from Non-Woody Biomass. Carbohydr. Polym. 2021, 264, 118036. [Google Scholar] [CrossRef]
- Zheng, Q.; Shang, M.; Li, X.; Jiang, L.; Chen, L.; Long, J.; Jiao, A.; Ji, H.; Jin, Z.; Qiu, C. Advances in Intelligent Response and Nano-Enhanced Polysaccharide-Based Hydrogels: Material Properties, Response Types, Action Mechanisms, Applications. Food Hydrocoll. 2024, 146, 109190. [Google Scholar] [CrossRef]
- Hou, D.-F.; Yuan, P.-P.; Feng, Z.-W.; An, M.; Li, P.-Y.; Liu, C.; Yang, M.-B. Sustainable Conversion Regenerated Cellulose into Cellulose Oleate by Sonochemistry. Front. Chem. Sci. Eng. 2023, 17, 1096–1108. [Google Scholar] [CrossRef]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Nurul Fitri Marzaman, A.; Sartini; Mudjahid, M.; Puspita Roska, T.; Sam, A.; Permana, A.D. Development of Chloramphenicol Whey Protein-Based Microparticles Incorporated into Thermoresponsive in Situ Hydrogels for Improved Wound Healing Treatment. Int. J. Pharm. 2022, 628, 122323. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, D.; Ji, Z.; Yang, J.; Wang, M.; Wang, Z.; Tao, S.; Wang, X.; Xu, M. Cellulose-Reinforced Poly(Ionic Liquids) Composite Hydrogel for Infected Wounds Therapy and Real-Time Reliable Bioelectronic. Chem. Eng. J. 2023, 476, 146816. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in Preparation and Properties of Chitosan-Based Hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-Based Hydrogel Wound Dressing: From Mechanism to Applications, a Review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, C.-D.; Tang, G.-P.; Li, J.-S.; Li, J.; Yang, W.-T.; Xu, F.-J. Functionalization of Chitosan via Atom Transfer Radical Polymerization for Gene Delivery. Adv. Funct. Mater. 2010, 20, 3106–3116. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wei, Y.; Tao, L. Chitosan-Based Self-Healing Hydrogel for Bioapplications. Chin. Chem. Lett. 2017, 28, 2053–2057. [Google Scholar] [CrossRef]
- Bonilla, F.; Chouljenko, A.; Lin, A.; Young, B.; Goribidanur, T.; Blake, J.; Bechtel, P.; Sathivel, S. Chitosan and Water-Soluble Chitosan Effects on Refrigerated Catfish Fillet Quality. Food Biosci. 2019, 31, 100426. [Google Scholar] [CrossRef]
- Zhang, M.; Wan, T.; Fan, P.; Shi, K.; Chen, X.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y. Photopolymerizable Chitosan Hydrogels with Improved Strength and 3D Printability. Int. J. Biol. Macromol. 2021, 193, 109–116. [Google Scholar] [CrossRef]
- Mu, B.; Wu, Q.; Xu, L.; Yang, Y. A Sustainable Approach to Synchronous Improvement of Wet-Stability and Toughness of Chitosan Films. Food Hydrocoll. 2022, 123, 107138. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-Based Materials: Preparation, Modification and Application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Li, D.-H.; Liu, L.; Tian, K.-L.; Liu, J.-C.; Fan, X.-Q. Synthesis, Biodegradability and Cytotoxicity of Water-Soluble Isobutylchitosan. Carbohydr. Polym. 2007, 67, 40–45. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Attjioui, M.; Ferreira, A.P.G.; Moerschbacher, B.M.; Cavalheiro, É.T.G. New Series of Metal Complexes by Amphiphilic Biopolymeric Schiff Bases from Modified Chitosans: Preparation, Characterization and Effect of Molecular Weight on Its Biological Applications. Int. J. Biol. Macromol. 2020, 145, 417–428. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. Antifungal Activity of Double Schiff Bases of Chitosan Derivatives Bearing Active Halogeno-Benzenes. Int. J. Biol. Macromol. 2021, 179, 292–298. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Ahmed, F. Cellulosic Fibres Modified by Chitosan and Synthesized Ecofriendly Carboxymethyl Chitosan from Prawn Shell Waste. J. Text. Inst. 2019, 111, 49–59. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Buatong, J.; Saetang, J.; Benjakul, S. Chitooligosaccharide and Its Derivatives: Potential Candidates as Food Additives and Bioactive Components. Foods 2023, 12, 3854. [Google Scholar] [CrossRef]
- Abedin, R.M.A.; Abd Elwaly, D.R.M.; Abd El-Salam, A.E. Production, Statistical Evaluation and Characterization of Chitosanase from Fusarium Oxysporum D18. Ann. Microbiol. 2023, 73, 27. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Cui, S.; Jing, X.; Feng, Y.; Coseri, S. Rapid Self-Healing Carboxymethyl Chitosan/Hyaluronic Acid Hydrogels with Injectable Ability for Drug Delivery. Carbohydr. Polym. 2024, 328, 121707. [Google Scholar] [CrossRef]
- Li, Y.; Qu, X.; Wang, Q.; Li, S.; Zhang, Q.; Zhang, X. Tannic Acid and Carboxymethyl Chitosan-Based Multi-Functional Double-Layered Hydrogel with PH-Stimulated Response Behavior for Smart Real-Time Infection Monitoring and Wound Treatment. Int. J. Biol. Macromol. 2024, 261, 129042. [Google Scholar] [CrossRef]
- Jing, X.; Sun, Y.; Ma, X.; Hu, H. Marine Polysaccharides: Green and Recyclable Resources as Wound Dressings. Mater. Chem. Front. 2021, 5, 5595–5616. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A Review on Phytoconstituents of Marine Brown Algae. Futur. J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-Drug Delivery System Based on the Hydrogels of Alginate and Sodium Carboxymethyl Cellulose for Colorectal Cancer Treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Zhao, X.; Gao, J. Investigation on Ionical Cross-Linking of Alginate by Monovalent Cations to Fabrication Alginate Gel for Biomedical Application. React. Funct. Polym. 2022, 183, 105484. [Google Scholar] [CrossRef]
- Al-Hawat, M.-L.; Cherifi, K.; Tricou, L.-P.; Lamontagne, S.; Tran, M.; Ngu, A.; Manrique, G.; Guirguis, N.; Machuca-Parra, A.; Matoori, S. Fluorescent PH-sensing Bandage for Point-of-care Wound Diagnostics. Aggregate 2023, 5, e472. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Xiang, G.; Jiang, T.; Zhao, X. Photodynamic Alginate Zn-MOF Thermosensitive Hydrogel for Accelerated Healing of Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 22830–22842. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-Responsive Smart Hydrogel Releasing Immune Adjuvant Synchronized with Repeated Chemotherapy or Radiotherapy to Boost Antitumor Immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Aljohani, W.; Li, W.; Ullah, M.; Zhang, X.; Yang, G. Application of Sodium Alginate Hydrogel. IOSR J. Biotechnol. Biochem. 2017, 03, 19–31. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial Effects of Hyaluronic Acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Zhang, T.; Zhao, J.; Cui, S.; Chen, W. Absorption, Metabolism, and Functions of Hyaluronic Acid and Its Therapeutic Prospects in Combination with Microorganisms: A Review. Carbohydr. Polym. 2023, 299, 120153. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, J. Preparation and Applications of Hyaluronic Acid and Its Derivatives. Int. J. Biol. Macromol. 2019, 125, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Wang, H.; Huang, H.; Liao, L.; Wang, Y.; Wang, M.; Du, G.; Kang, Z. Enzymatic Production of Low-Molecular-Weight Hyaluronan and Its Oligosaccharides: A Review and Prospects. J. Agric. Food Chem. 2022, 70, 14129–14139. [Google Scholar] [CrossRef]
- Xiong, Y.-H.; Zhang, L.; Xiu, Z.; Yu, B.; Duan, S.; Xu, F.-J. Derma-like Antibacterial Polysaccharide Gel Dressings for Wound Care. Acta Biomater. 2022, 148, 119–132. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and Application of Hyaluronic Acid in Tumor Targeting Drug Delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic Acid-Based Biopharmaceutical Delivery and Tumor-Targeted Drug Delivery System. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Liu, P.; Hu, Y.; Chen, Y.; Fang, Y.; Sun, G.; Huang, H.; Wu, J. Hyaluronic Acid-Based Glucose-Responsive Antioxidant Hydrogel Platform for Enhanced Diabetic Wound Repair. Acta Biomater. 2022, 147, 147–157. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Gulfam, M.; Jo, S.-H.; Gal, Y.-S.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Multi-Stimuli Responsive Hydrogels Derived from Hyaluronic Acid for Cancer Therapy Application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, X.; Chen, Q.; Zhu, Y.; Shi, Z.; Deng, X.; Wang, C.; Chen, H. Injectable Responsive Hydrogel Delivery Platform: Enabling High Tissue Penetration and Sonogenetic-Like Potentiating Anti-Tumor Immunotherapy. Adv. Funct. Mater. 2024, 34, 2313723. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Wu, G.; Zhu, Z.; Zheng, H.; Sun, X.; Heng, Y.; Pan, S.; Xiu, H.; Zhang, J.; et al. Hyaluronic Acid-Based Reactive Oxygen Species-Responsive Multifunctional Injectable Hydrogel Platform Accelerating Diabetic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2302626. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, e2102545. [Google Scholar] [CrossRef]
- Gotoh, S.; Onaya, J.; Abe, M.; Miyazaki, K.; Hamai, A.; Horie, K.; Tokuyasu, K. Effects of the Molecular Weight of Hyaluronic Acid and Its Action Mechanisms on Experimental Joint Pain in Rats. Ann. Rheum. Dis. 1993, 52, 817–822. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A Review of Natural Polysaccharides for Drug Delivery Applications: Special Focus on Cellulose, Starch and Glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and Its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Matta Junior, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels Based on Ozonated Cassava Starch: Effect of Ozone Processing and Gelatinization Conditions on Enhancing 3D-Printing Applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef]
- Noè, C.; Tonda-Turo, C.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Light Processable Starch Hydrogels. Polymers 2020, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, T.; Wu, L.; Han, Q.; Chen, S.; Kong, Y.; Li, G.; Ma, L.; Wu, H.; Zhao, Y.; et al. Fabrication and Characterization of 3D-Printed Gellan Gum/Starch Composite Scaffold for Schwann Cells Growth. Nanotechnol. Rev. 2021, 10, 50–61. [Google Scholar] [CrossRef]
- Kumar, D.; Narwal, S.; Venkatesh, K.; Verma, R.; Singh, G. Grain Beta-Glucan as Selection Criteria for Wort Beta-Glucan in Malt Barley Improvement. J. Cereal Sci. 2022, 107, 103519. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Marasca, E.; Boulos, S.; Nyström, L. Bile Acid-Retention by Native and Modified Oat and Barley β-Glucan. Carbohydr. Polym. 2020, 236, 116034. [Google Scholar] [CrossRef]
- Bai JunYing, B.J.; Ren YiKai, R.Y.; Li Yan, L.Y.; Fan MingCong, F.M.; Qian HaiFeng, Q.H.; Wang Li, W.L.; Wu GangCheng, W.G.; Zhang Hui, Z.H.; Qi XiGuang, Q.X.; Xu MeiJuan, X.M.; et al. Physiological Functionalities and Mechanisms of β-Glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar]
- He, Y.; Yang, W.; Zhang, C.; Yang, M.; Yu, Y.; Zhao, H.; Guan, F.; Yao, M. ROS/PH Dual Responsive PRP-Loaded Multifunctional Chitosan Hydrogels with Controlled Release of Growth Factors for Skin Wound Healing. Int. J. Biol. Macromol. 2024, 258, 128962. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, S.; Li, M.; Chen, J.; Pei, D.; Tang, Z.; Guo, B. Bacteria-Responsive Programmed Self-Activating Antibacterial Hydrogel to Remodel Regeneration Microenvironment for Infected Wound Healing. Natl. Sci. Rev. 2024, 11, nwae044. [Google Scholar] [CrossRef]
- Xiang, S.; Guilbaud-Chéreau, C.; Hoschtettler, P.; Stefan, L.; Bianco, A.; Ménard-Moyon, C. Preparation and Optimization of Agarose or Polyacrylamide/Amino Acid-Based Double Network Hydrogels for Photocontrolled Drug Release. Int. J. Biol. Macromol. 2024, 255, 127919. [Google Scholar] [CrossRef]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of Microbial Polysaccharide Hydrogels for Tissue Engineering and Regenerative Medicine—A Review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for Vascular and Vascularized Tissue Biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan Gum: Production, Recovery, and Properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan Gum Derivatives: Review of Synthesis, Properties and Diverse Applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Shin, S.Y.; Cha, J.M.; Bae, H. Optimization of Polysaccharide Hydrocolloid for the Development of Bioink with High Printability/Biocompatibility for Coextrusion 3D Bioprinting. Polymers 2021, 13, 1773. [Google Scholar] [CrossRef]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.H.; Sriram, G.; Cao, T. 3D Bioprinting and Microscale Organization of Vascularized Tissue Constructs Using Collagen-Based Bioink. Biotechnol. Bioeng. 2021, 118, 3150–3163. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan Gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; in het Panhuis, M. Tissue Engineering with Gellan Gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef]
- Qiu, B.; Bessler, N.; Figler, K.; Buchholz, M.B.; Rios, A.C.; Malda, J.; Levato, R.; Caiazzo, M. Bioprinting Neural Systems to Model Central Nervous System Diseases. Adv. Funct. Mater. 2020, 30, 1910250. [Google Scholar] [CrossRef]
- Wu, D.; Yu, Y.; Tan, J.; Huang, L.; Luo, B.; Lu, L.; Zhou, C. 3D Bioprinting of Gellan Gum and Poly (Ethylene Glycol) Diacrylate Based Hydrogels to Produce Human-Scale Constructs with High-Fidelity. Mater. Des. 2018, 160, 486–495. [Google Scholar] [CrossRef]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-Layer Ultraviolet Assisted Extrusion-Based (UAE) Bioprinting of Hydrogel Constructs with High Aspect Ratio for Soft Tissue Engineering Applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.T.S.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum–Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Q.; Guo, Z.; Liu, Y.; Gao, W.; Pu, Y.; He, B. Konjac Glucomannan Hydrogel Dressing and Its Combination with Chinese Medicine for the Wound Treatment. New J. Chem. 2022, 46, 23077–23087. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Ning, D.; Dai, L.; Liu, K.; Ni, Y.; Chen, L.; Huang, L. Ultraflexible Self-Healing Guar Gum-Glycerol Hydrogel with Injectable, Antifreeze, and Strain-Sensitive Properties. ACS Biomater. Sci. Eng. 2018, 4, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Indurkar, A.; Bangde, P.; Gore, M.; Reddy, P.; Jain, R.; Dandekar, P. Optimization of Guar Gum-Gelatin Bioink for 3D Printing of Mammalian Cells. Bioprinting 2020, 20, e00101. [Google Scholar] [CrossRef]
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Shen, J. Sustainable, Flexible and Biocompatible Hydrogels Derived from Microbial Polysaccharides with Tailorable Structures for Tissue Engineering. Carbohydr. Polym. 2020, 237, 116160. [Google Scholar] [CrossRef]
- Della Giustina, G.; Gandin, A.; Brigo, L.; Panciera, T.; Giulitti, S.; Sgarbossa, P.; D’Alessandro, D.; Trombi, L.; Danti, S.; Brusatin, G. Polysaccharide Hydrogels for Multiscale 3D Printing of Pullulan Scaffolds. Mater. Des. 2019, 165, 107566. [Google Scholar] [CrossRef]
- Asadi, K.; Samiraninezhad, N.; Akbarizadeh, A.R.; Amini, A.; Gholami, A. Stimuli-Responsive Hydrogel Based on Natural Polymers for Breast Cancer. Front. Chem. 2024, 12, 1325204. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-Healing/PH-Responsive/Inherently Antibacterial Polysaccharide-Based Hydrogel for a Photothermal Strengthened Wound Dressing. Colloids Surf. B. Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef]
- Psarrou, M.; Mitraki, A.; Vamvakaki, M.; Kokotidou, C. Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers 2023, 15, 986. [Google Scholar] [CrossRef]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.-Q. An Intrinsically Bioactive Hydrogel with On-Demand Drug Release Behaviors for Diabetic Wound Healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl Chitosan-Based Hydrogels Containing Fibroblast Growth Factors for Triggering Diabetic Wound Healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Y.; Shen, W.; Dou, J.; Liu, R.; Jin, Q.; Fang, S. PH-Responsive Injectable Polysaccharide Hydrogels with Self-Healing, Enhanced Mechanical Properties Based on POSS. React. Funct. Polym. 2021, 158, 104773. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and Hemocompatible PH-Responsive Hydrogel for Skin Wound Healing Application: In Vitro Drug Release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Haldar, J. Biocompatible Injectable Hydrogel with Potent Wound Healing and Antibacterial Properties. Mol. Pharm. 2017, 14, 1218–1230. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Yao, Z.; Fang, Q.; Feng, L.; Guo, R.; Cheng, B. Preparation of Antimicrobial Hyaluronic Acid/Quaternized Chitosan Hydrogels for the Promotion of Seawater-Immersion Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Guan, S.; Li, Y.; Cheng, C.; Gao, X.; Gu, X.; Han, X.; Ye, H. Manufacture of PH- and HAase-Responsive Hydrogels with on-Demand and Continuous Antibacterial Activity for Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2020, 164, 2418–2431. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment via PH-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. PH-Responsive Hydrogel Loaded with Insulin as a Bioactive Dressing for Enhancing Diabetic Wound Healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Gao, Z.; Golland, B.; Tronci, G.; Thornton, P.D. A Redox-Responsive Hyaluronic Acid-Based Hydrogel for Chronic Wound Management. J. Mater. Chem. B 2019, 7, 7494–7501. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.-J. Photo-Responsive Supramolecular Hyaluronic Acid Hydrogels for Accelerated Wound Healing. J. Control. Release Off. J. Control. Release Soc. 2020, 323, 24–35. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik e.V 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and PH-Responsive Tannin-Containing Hydroxypropyl Chitin Hydrogel with Long-Lasting Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-Responsive Injectable Hydrogels Encapsulating Drug-Loaded Micelles for on-Demand Antimicrobial Activity and Accelerated Wound Healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable Antibacterial Conductive Hydrogels with Dual Response to an Electric Field and PH for Localized “Smart” Drug Release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically Conductive and 3D-Printable Oxidized Alginate-Gelatin Polypyrrole:PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D Printed Hydrogel/PCL Core/Shell Fiber Scaffolds with NIR-Triggered Drug Release for Cancer Therapy and Wound Healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.T. 3D-Printed Bioactive and Biodegradable Hydrogel Scaffolds of Alginate/Gelatin/Cellulose Nanocrystals for Tissue Engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Chen, B.; Luo, Y. Magnetically-Driven Drug and Cell on Demand Release System Using 3D Printed Alginate Based Hollow Fiber Scaffolds. Int. J. Biol. Macromol. 2021, 168, 38–45. [Google Scholar] [CrossRef]
- Olate-Moya, F.; Arens, L.; Wilhelm, M.; Mateos-Timoneda, M.A.; Engel, E.; Palza, H. Chondroinductive Alginate-Based Hydrogels Having Graphene Oxide for 3D Printed Scaffold Fabrication. ACS Appl. Mater. Interfaces 2020, 12, 4343–4357. [Google Scholar] [CrossRef]
- Heo, D.N.; Alioglu, M.A.; Wu, Y.; Ozbolat, V.; Ayan, B.; Dey, M.; Kang, Y.; Ozbolat, I.T. 3D Bioprinting of Carbohydrazide-Modified Gelatin into Microparticle-Suspended Oxidized Alginate for the Fabrication of Complex-Shaped Tissue Constructs. ACS Appl. Mater. Interfaces 2020, 12, 20295–20306. [Google Scholar] [CrossRef]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef]
- Gutierrez, E.; Burdiles, P.A.; Quero, F.; Palma, P.; Olate-Moya, F.; Palza, H. 3D Printing of Antimicrobial Alginate/Bacterial-Cellulose Composite Hydrogels by Incorporating Copper Nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Beneventi, D.; Dufresne, A. Biomimetic Mineralization of Three-Dimensional Printed Alginate/TEMPO-Oxidized Cellulose Nanofibril Scaffolds for Bone Tissue Engineering. Biomacromolecules 2018, 19, 4442–4452. [Google Scholar] [CrossRef]
- Valentin, T.M.; Landauer, A.K.; Morales, L.C.; DuBois, E.M.; Shukla, S.; Liu, M.; Stephens Valentin, L.H.; Franck, C.; Chen, P.Y.; Wong, I.Y. Alginate-Graphene Oxide Hydrogels with Enhanced Ionic Tunability and Chemomechanical Stability for Light-Directed 3D Printing. Carbon N. Y. 2019, 143, 447–456. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.K.; Son, K.H.; Lee, J.W. PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy. Gels 2022, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, H.; Yao, J.; Yang, L.; Zhang, X.; Li, L.; Wang, J. Scaffold Based on Lyophilized Platelet-Rich Fibrin OPEN ACCESS Bone Scaffold Based on Lyophilized Platelet-Rich Fibrin. Biomed. Mater. 2023, 18, 025022. [Google Scholar]
- Li, P.; Fu, L.; Liao, Z.; Peng, Y.; Ning, C.; Gao, C.; Zhang, D.; Sui, X.; Lin, Y.; Liu, S.; et al. Chitosan Hydrogel/3D-Printed Poly(ε-Caprolactone) Hybrid Scaffold Containing Synovial Mesenchymal Stem Cells for Cartilage Regeneration Based on Tetrahedral Framework Nucleic Acid Recruitment. Biomaterials 2021, 278, 121131. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Pinto Ramos, D.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, C.; Kim, H.S.; Moon, C.; Lee, K.Y. 3D Printing of Dynamic Tissue Scaffold by Combining Self-Healing Hydrogel and Self-Healing Ferrogel. Colloids Surfaces B Biointerfaces 2021, 208, 112108. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Wu, W.; Zhang, A.; Lu, B.; Zhang, T.; Kong, M. Dual Cure (Thermal/Photo) Composite Hydrogel Derived from Chitosan/Collagen for in Situ 3D Bioprinting. Int. J. Biol. Macromol. 2021, 182, 689–700. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Prakash, C.; Ramakrishna, S.; Lamberti, L.; Pruncu, C.I. 3D Printed Biodegradable Composites: An Insight into Mechanical Properties of PLA/Chitosan Scaffold. Polym. Test. 2020, 89, 106722. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, L.; Tang, S.; Wen, W.; Lu, L.; Liu, M.; Zhou, C.; Luo, B. Fabrication and Evaluation of a Chitin Whisker/Poly(l-Lactide) Composite Scaffold by the Direct Trisolvent-Ink Writing Method for Bone Tissue Engineering. Nanoscale 2020, 12, 18225–18239. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in Vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Seok, E.; Kim, C.; Choi, Y.; Yong, K. 3D Printing of Self-Healing Ferrogel Prepared from Glycol Chitosan, Oxidized Hyaluronate, and Iron Oxide Nanoparticles. Carbohydr. Polym. 2020, 245, 116496. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-Triggered Drug Release from 3D-Printed Magnetic Chitosan Microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an in-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liang, M.; Guo, Z.; Wang, A.; Cai, G.; Yuan, T.; Mi, S.; Sun, W. A Study on Dual-Response Composite Hydrogels Based on Oriented Nanocellulose. Int. J. Bioprinting 2022, 8, 126–139. [Google Scholar] [CrossRef]
- Yue, C.; Li, M.; Liu, Y.; Fang, Y.; Song, Y.; Xu, M.; Li, J. Three-Dimensional Printing of Cellulose Nanofibers Reinforced PHB/PCL/Fe3O4 Magneto-Responsive Shape Memory Polymer Composites with Excellent Mechanical Properties. Addit. Manuf. 2021, 46, 102146. [Google Scholar] [CrossRef]
- Mohan, T.; Dobaj Štiglic, A.; Beaumont, M.; Konnerth, J.; Gürer, F.; Makuc, D.; Maver, U.; Gradišnik, L.; Plavec, J.; Kargl, R.; et al. Generic Method for Designing Self-Standing and Dual Porous 3D Bioscaffolds from Cellulosic Nanomaterials for Tissue Engineering Applications. ACS Appl. Bio Mater. 2020, 3, 1197–1209. [Google Scholar] [CrossRef]
- Ji, S.; Abaci, A.; Morrison, T.; Gramlich, W.M.; Guvendiren, M. Bioprinting Novel Bioinks from UV-Responsive Norbornene-Functionalized Carboxymethyl Cellulose Macromers. Bioprinting 2020, 18, e00083. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Teck, C.; Fernandez, J.G. International Journal of Biological Macromolecules Stimuli-Responsive Injectable Cellulose Thixogel for Cell Encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Zhang, L.; Cao, X. 4D Printing of Robust Hydrogels Consisted of Agarose Nanofibers and Polyacrylamide. ACS Macro Lett. 2018, 7, 442–446. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Dias, C.I.; Mano, J.F.; Alves, N.M. PH-Responsive Biomineralization onto Chitosan Grafted Biodegradable Substrates. J. Mater. Chem. 2008, 18, 2493–2499. [Google Scholar] [CrossRef]
- Li, D.-Q.; Wang, S.-Y.; Meng, Y.-J.; Guo, Z.-W.; Cheng, M.-M.; Li, J. Fabrication of Self-Healing Pectin/Chitosan Hybrid Hydrogel via Diels-Alder Reactions for Drug Delivery with High Swelling Property, PH-Responsiveness, and Cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of Tracheobronchomalacia with 3D-Printed Personalized Medical Devices in Pediatric Patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef] [PubMed]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical Properties and Shape Memory Effect of 3D-Printed PLA-Based Porous Scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Lee, V.K.; Dai, G. Printing of Three-Dimensional Tissue Analogs for Regenerative Medicine. Ann. Biomed. Eng. 2017, 45, 115–131. [Google Scholar] [CrossRef]
- Harb, S.C.; Rodriguez, L.L.; Vukicevic, M.; Kapadia, S.R.; Little, S.H. Three-Dimensional Printing Applications in Percutaneous Structural Heart Interventions. Circ. Cardiovasc. Imaging 2019, 12, e009014. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef]
- Amirpour, M.; Battley, M. Study of manufacturing defects on compressive deformation of 3D-printed polymeric lattices. Int. J. Adv. Manuf. Technol. 2022, 122, 2561–2576. [Google Scholar] [CrossRef]
- Smith, D.M.; Kapoor, Y.; Klinzing, G.R.; Procopio, A.T. Pharmaceutical 3D printing: Design and qualification of a single step print and fill capsule. Int. J. Pharm. 2018, 544, 21–30. [Google Scholar] [CrossRef] [PubMed]
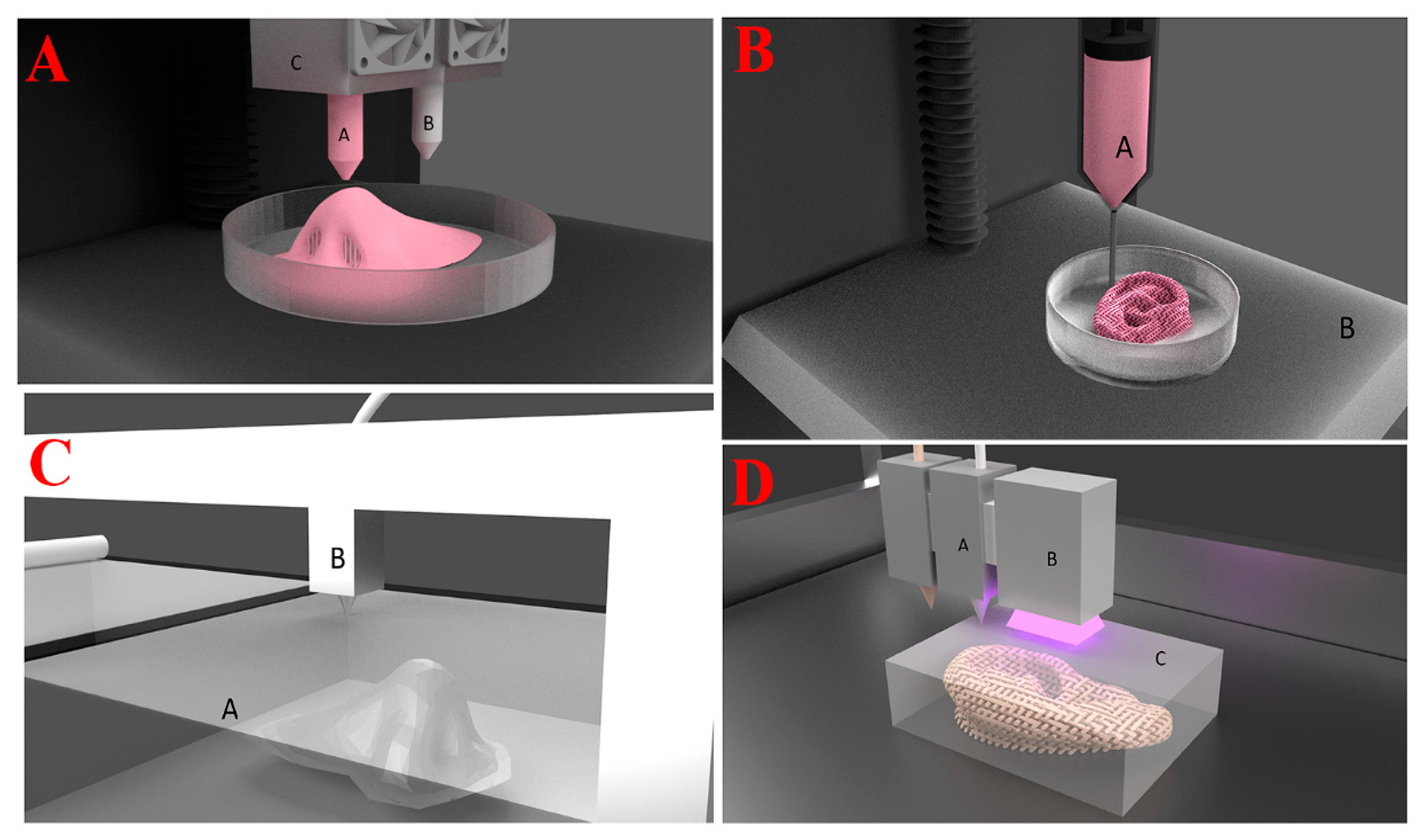

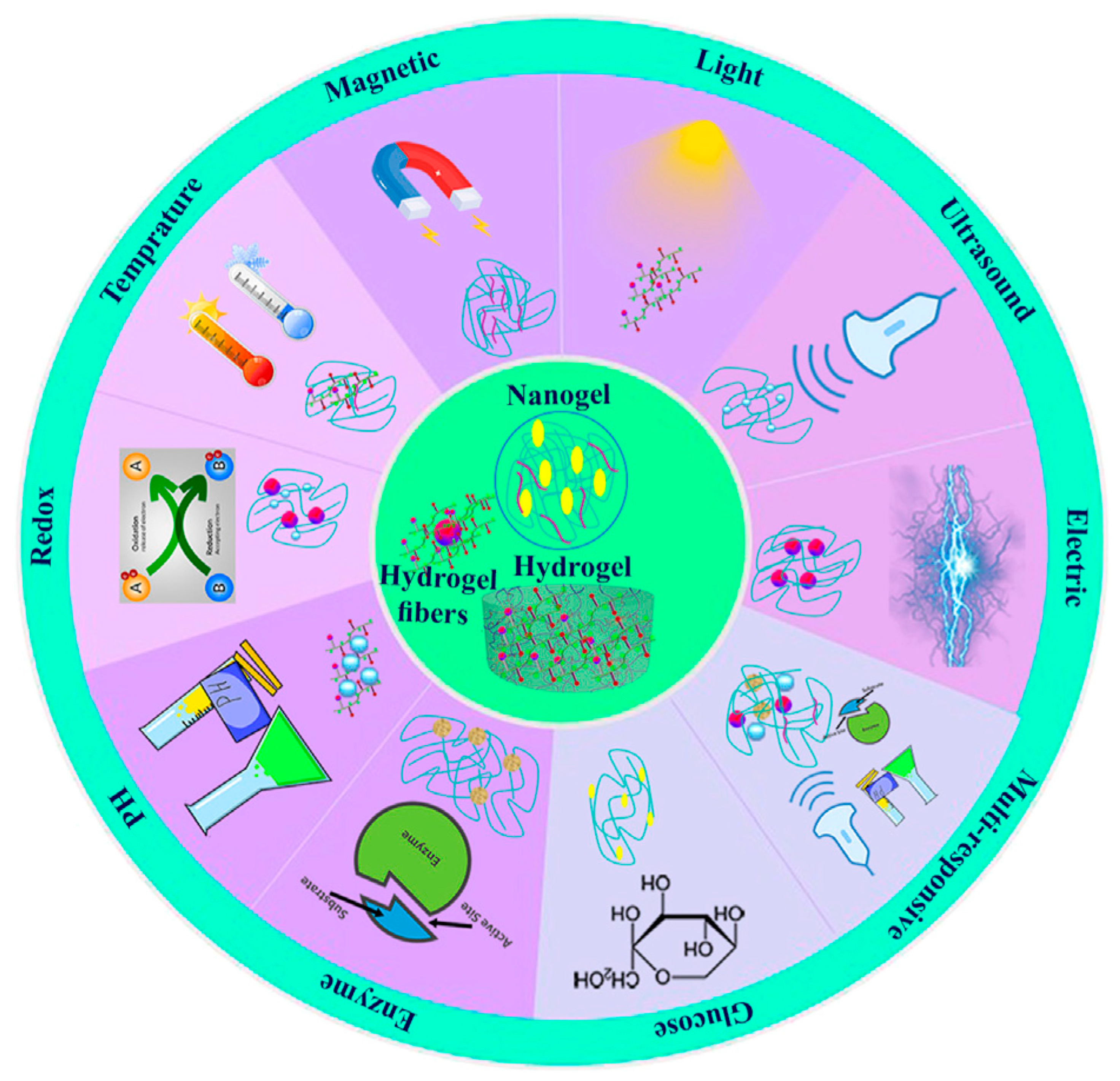
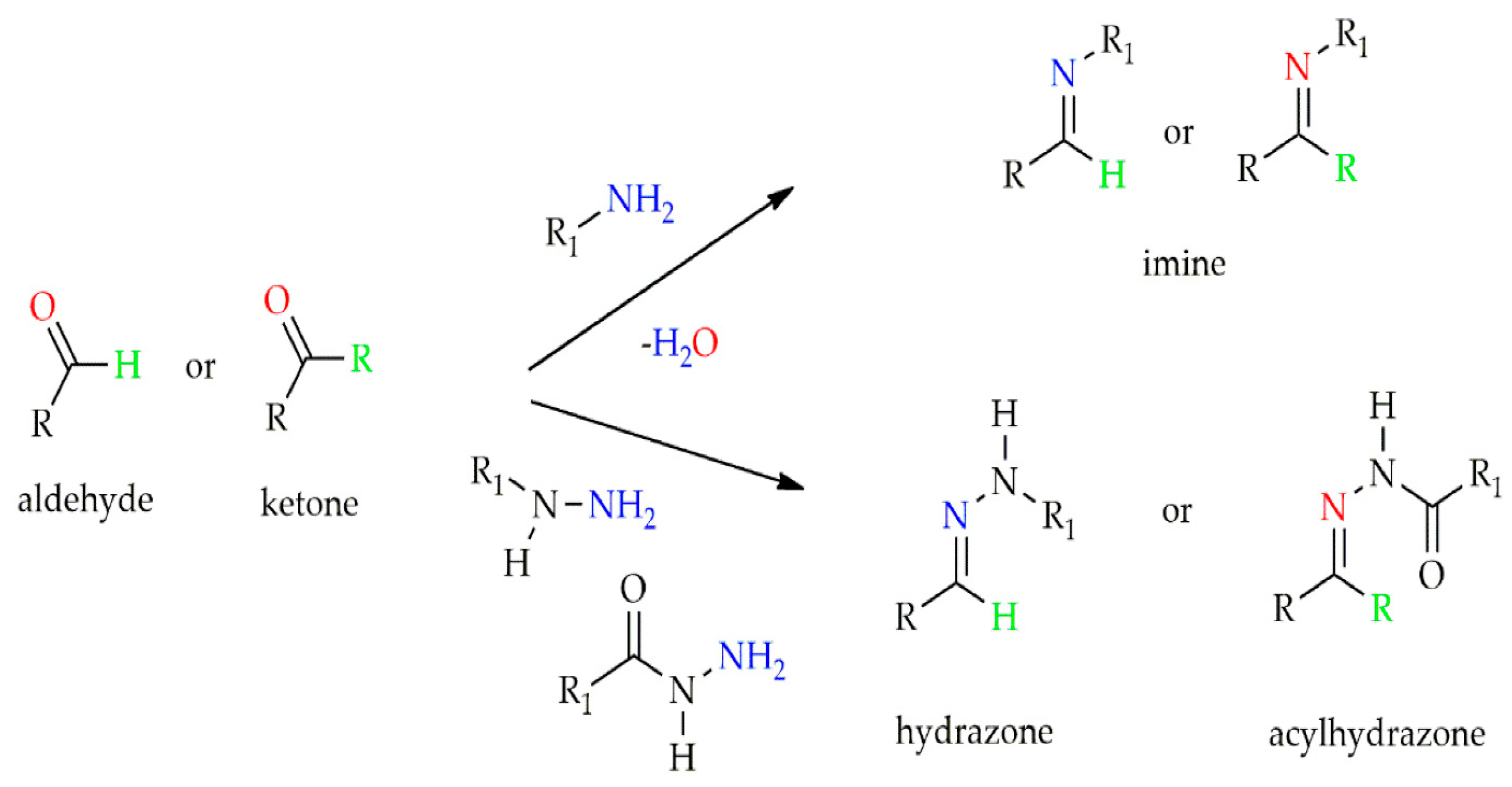








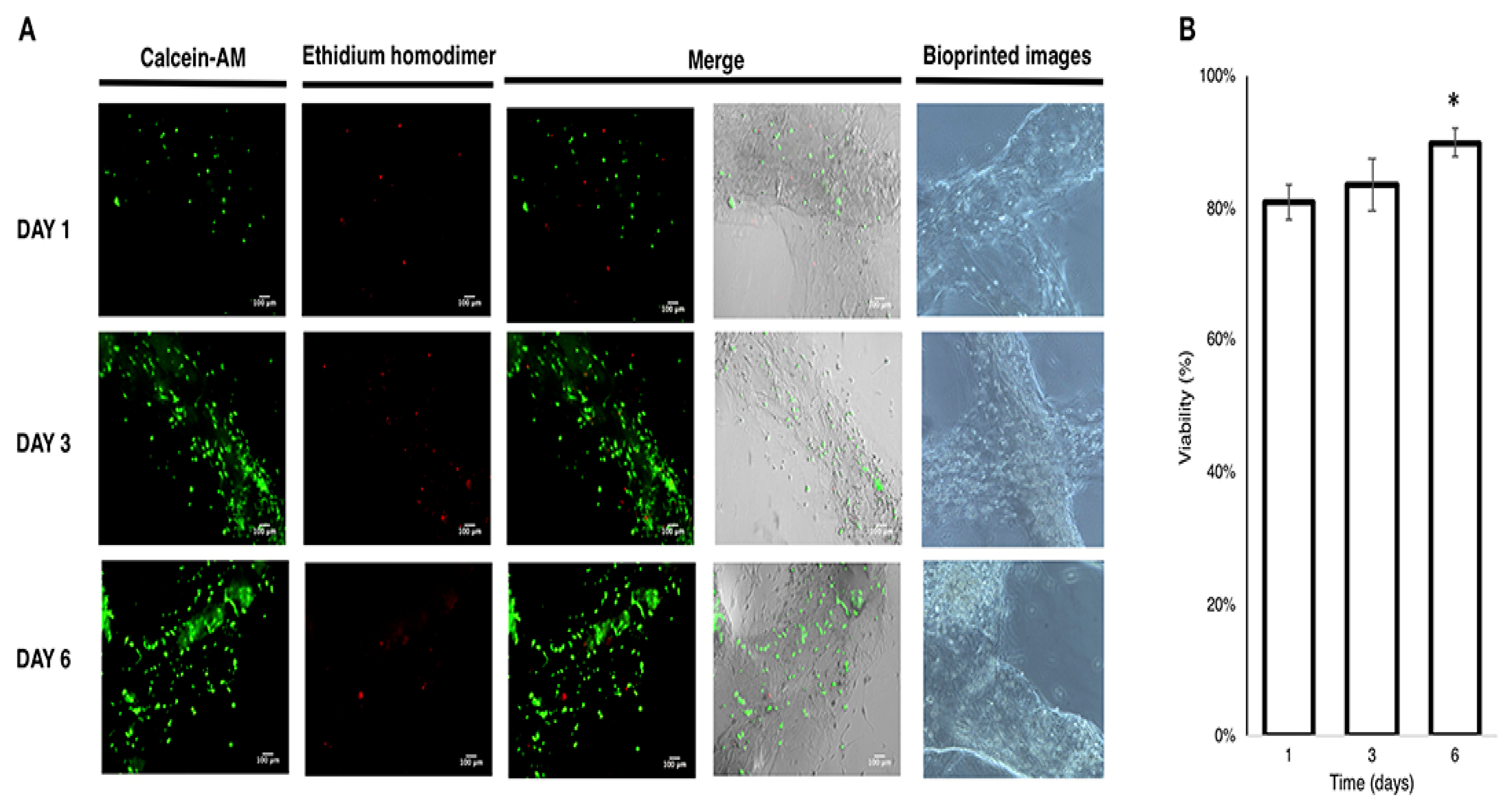

| 3D Printing Technologies | Mechanism of Action | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Inkjet printing | Thin layer of active pharmaceutical ingredient with excipients on a solid platform is selectively bonded by sprayed formulations or binders in microdots | High resolution and precision, efficient, economical, high-speed manufacturing, multi-material printing | Nozzle clogging, low friability and hardness | [41,42,43,44] |
| Continuous inkjet | Pressurized continuous stream of droplets (50–80 μm) directed by electrostatic plates to solidify | High drop velocity covering longer distances, no nozzle clogging, faster output | High degree of wasted ink due to recirculation, limited availability of solvent-based inks | [41,42,43,44] |
| Drop on demand | Droplets (10–50 μm) through multiple nozzles—thermal or piezoelectric heads | Faster solidification, economical | Susceptible to nozzle clogging resulting in inaccurate jetting and dropping | [41,42,44,45] |
| Binder jetting | Polymer powder or other solid particles with liquid binder | Ability to produce porous constructs, multi-material printing, no support needed | Limited selection of materials, low structural integrity | [41,42,44,45] |
| Powder deposition technique | Sprayed drops from print heads are deposited as layer and laser beam sinters the powder layer to form solid structures | Average resolution and speed, no support needed, recyclable feed materials | Particle size of powder binding materials is critical, change in mechanical properties | [46] |
| Selective laser sintering | Melting and fusion of high-melting-point thermoplastic polymers and low-melting-point binding powder materials | High resolution (30 μm) and precision, porous structures, faster fabrication, no post-curing required | Low efficiency, expensive, significant wastage of powder materials, limited availability of active pharmaceutical ingredients and excipients suitable for the process | [47] |
| Fused deposition modelling | Materials extruded through a nozzle or orifice under controlled conditions to deposit in layer-by-layer fashion to form 3D object | Inexpensive, compact equipment, diverse, readily available, ecofriendly and non-contaminating raw materials, ability to create complex, innovative and customized dosage forms | Requirement of solvent, heat and cross-linking agents, difficulty to recycle printing materials, risk of drug and excipient degradation, slow printing speed, delamination due to temperature fluctuations | [41,48] |
| Injection molding | Molds created by auto-computer-aided design software in stereolithography file format and sliced into G-code | Creation of drug delivery systems with specific geometric shape and dimension, scalable, continuous manufacturing technique, no solvent requirement, mechanical anisotropy of structures is minimal | Limited design, relatively expensive technique | [5,41] |
| Stereolithography | Digital mirroring device utilizing laser beam to initiate photochemical reaction to transform liquid monomer into solid object | High accuracy and resolution, complex and customized drug delivery systems with desired release pattern, minimum drug decomposition, compact equipment, suitable for personalized dosage form development in clinical setting, minimum mechanical anisotropy | Potential toxicity, low drug loading, rinsing and post curing process is necessary, limited availability of biocompatible photopolymerizable polymers | [49,50] |
| Digital light processing | Laser beam projected through digital mirror device | High resolution, high-speed manufacturing | Toxicity, needs support | [49] |
| Continuous liquid interface production | Projecting ultraviolet light through oxygen permeable membrane | Fastest manufacturing speed, high precision | Probable toxicity, expensive | [49] |
| Semisolid extrusion system | Semisolid materials extruded through pressure-assisted microsyringe by compressed air | High-speed process, operation can be carried out at room temperature, suitable for thermolabile drugs, high drug loading, cost effective for bulk production | Specific rheological characteristics required for starting material, pseudoplastic and cross-linking polymers preferred, chance of nozzle clogging, resolution limited by nozzle size, drying step is necessary, low resolution, slow production speed, low mechanical strength and durability | [38,51] |
| Designation Additive Manufacturing Process | Technologies | Medical Use | Pros | Cons |
|---|---|---|---|---|
| Vat photo-polymerization | Stereolithography (SLA) Digital light processing (DLP) | Bone, dental models, dental implant guides, hearing aids | High resolution and accuracy Complex parts Decent surface finish Flexible printing setup | Lacking in strength and durability They are still affected by UV light after printing Not for heavy use |
| Material jetting | MultiJet modelling (MJM) | Medical models, dental casts, dental implant guides | High accuracy Low waste of materials Multiple material parts and colors in one process | Requires support material Only polymers and waxes are supported |
| Binder jetting | Powder bed and inkjet head 3D printing (PDIH) Plaster-based 3D printing (PP) | Color models, especially color coding of anatomy | Range of colors Multiple materials supported Faster Different binder powder combinations | Not always suitable for structural parts Cleaning the 3D-printing result takes time and increases the time required for the procedure |
| Material extrusion | Fused deposition modeling (FDM) Fused filament fabrication (FFF) | Medical instruments and devices, rapid prototyping exoskeletons | Inexpensive process Widespread ABS plastic supported | Dependence of quality on the nozzle radius Low accuracy Low speed Contact pressure needed to increase quality |
| Powder bed fusion | Selective laser sintering (SLS) Direct metal laser sintering (DMLS) Selective heat sintering (SHS) Selective laser melting (SLM) Electron beam melting (EBM) | Models that require a lattice, medical devices such as implants, and fixations | Inexpensive Small technology Extensive range of material options | Low speed Limited sizes Dependence on powder grain size |
| Materials | Printing Type | Response to Stimuli/Biomedical Application | References |
|---|---|---|---|
| Agarose/acrylamide | Situ polymerizing | Temperature/human ear or nose printing | [255] |
| Alginate glycerin hydrogel | Microfluidic coaxial extrusion | PH/skin dressing | [256] |
| Chitosan | Plasma polymerization | PH/surface modification | [257] |
| Chitosan/methacrylated alginate | Extrusion bioprinter | Voltage/vascular stents | [258] |
| Hyaluronic acid/polycaprolactone | Laser sinter | Tension/tracheobronchial splint | [259] |
| Hyaluronic acid/polylactide | Fused deposition modeling | Temperature/orthopedic implant | [260] |
| Sodium alginate/agarose/N, N′-methylene bis (acrylamide) | Laser-machining and screen printing | Temperature/patch | [261] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. https://doi.org/10.3390/biomedicines13030731
Aghajani M, Garshasbi HR, Naghib SM, Mozafari MR. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines. 2025; 13(3):731. https://doi.org/10.3390/biomedicines13030731
Chicago/Turabian StyleAghajani, Mohammad, Hamid Reza Garshasbi, Seyed Morteza Naghib, and M. R. Mozafari. 2025. "3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review" Biomedicines 13, no. 3: 731. https://doi.org/10.3390/biomedicines13030731
APA StyleAghajani, M., Garshasbi, H. R., Naghib, S. M., & Mozafari, M. R. (2025). 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines, 13(3), 731. https://doi.org/10.3390/biomedicines13030731










