Abstract
Objectives: The objective of this study was to identify novel autoantibodies specific for open-angle glaucoma (OAG), including normal-tension glaucoma (NTG) and primary open-angle glaucoma (POAG), using proteome-wide autoantibody screening and to determine their utility for diagnosis. Methods: We conducted proteome-wide autoantibody screening by wet protein arrays. Autoantibody reactivity in the plasma of OAG patients (50 NTG and 69 POAG patients) was quantitatively analyzed and compared to that of controls (35 cataract patients). The area under the curve (AUC) of the receiver operating characteristic (ROC) and multivariate analyses were used to determine diagnostic potential in patients with OAG. Results: Based on differences in autoantibody titers and positivity rates, four autoantibodies against ETNK1, VMAC, NEXN, and SUN1 were selected as potential biomarkers to discriminate OAG and cataract. In discrimination between POAG and cataract, the AUCs of ETNK1 and VMAC were calculated to be 0.820 (95%CI: 0.733–0.907) and 0.889 (95%CI: 0.818–0.959), respectively. Furthermore, the combination of these four antibodies demonstrated diagnostic potential for OAG with an AUC of 0.828 (95%CI: 0.757–0.898) by multivariate analysis. Conclusions: Four new glaucoma-associated autoantibodies were identified in this study. The differences in autoantibody patterns in the plasma between glaucoma and cataract patients support their potential utility as biomarkers for glaucoma screening.
1. Introduction
Glaucoma is characterized by irreversible and progressive damage to retinal ganglion cells (RGCs) [1] and is the leading cause of blindness in adults [2]. Open-angle glaucoma (OAG), the most common form of glaucoma, includes primary open-angle glaucoma (POAG), in which intraocular pressure (IOP) is 21 mmHg or higher, and normal-tension glaucoma (NTG), in which IOP is within the normal range. NTG is common in the Japanese population, accounting for more than 90% of glaucoma cases in Japan [3], and accounts for up to 83% of OAG in China [4]. IOP-lowering therapy is the only treatment for glaucoma for which there is clinical evidence [2] and is also effective in NTG [5]. Early detection and treatment are essential in the management of glaucoma, but many patients with OAG are unaware of symptoms and do not seek medical attention until the late stages of illness [2] at a time when 25–35% of RGCs have already been lost [6]. Thus, it is important to develop objective and simple screening methods for early detection of OAG.
OAG is a multifactorial disease, and its pathogenesis is not yet fully elucidated. Elevated IOP is the greatest risk factor for the onset and progression of glaucoma. However, there are significant numbers of patients with visual field deterioration despite well-controlled IOP, suggesting that other risk factors exist for OAG progression [7]. Indeed, previous studies suggest that autoimmune mechanisms are involved in the pathogenesis of glaucoma [8,9,10]. Consistent with this, autoantibodies against heat shock proteins (HSPs) [11], γ-enolase [12], myelin basic protein (MBP) [13], and other antigens [14] have been detected in OAG.
In this study, we analyzed plasma autoantibodies using the comprehensive wet protein array (CWPA) [15,16,17,18], a type of proteome-wide analysis. We also performed multivariate receiver operating characteristic (ROC) analyses of multiple autoantibodies in combination with age, sex, and IOP to detect OAG.
2. Materials and Methods
2.1. Subjects
This study used a retrospective case–control design and was approved by the Ethics Committee of Tohoku University (2022-1-831). Patients who attended the Department of Ophthalmology of Tohoku University Hospital from March 2015 to May 2018 were included in this study after providing full written informed consent in accordance with the Declaration of Helsinki on Medical Research Involving Human Subjects. Blood tests and ophthalmologic examinations were performed. Patient information, symptoms, medications, and laboratory findings at the closest point in time to the date of blood tests were collected retrospectively from electronic medical record information.
All patients were evaluated for visual acuity, IOP, central corneal thickness (CCT), and axial length (AL), and underwent fundus photography and optical coherence tomography (OCT). The mean deviation (MD) was measured with the Humphrey Field Analyzer (HFA; Carl Zeiss Meditec, Dublin, CA, USA) using the Swedish Interactive Threshold Algorithm (SITA)-standard strategy of the 24-2 program.
Glaucoma was defined by the presence of glaucomatous optic nerve papillary changes (diffuse, focal thinning of the optic nerve limbus) and corresponding glaucomatous visual field defects. Glaucomatous optic nerve papillary changes were determined by fundus photographs and OCT. Glaucomatous visual field defects were defined by the Anderson–Patella diagnostic criteria [19] using Humphrey visual field measures:
- (1)
- Three or more points with p < 5%, except for the most peripheral inspection points in the pattern deviation probability plot that are adjacent to each other with p < 1%.
- (2)
- Pattern standard deviation with a probability of less than 5%.
- (3)
- Glaucoma hemifield test indicating that the field is outside normal limits.
The OAG group included patients with mild or early (MD ≥ −6 dB; 11.8%) and progressive (MD < −6 dB; 88.2%) glaucomatous visual field loss [20]. NTG was defined as IOP ≤ 21 mmHg before treatment initiation, and POAG was defined as IOP > 21 mmHg before treatment initiation. The control group consisted of age- and sex-matched cataract patients without other ocular diseases, including glaucoma. IOP, fundus photographs, and OCT imaging tests were referenced to exclude ocular diseases other than glaucoma and cataract. Considering the average age of the OAG group, most patients already had cataracts [21,22]. Therefore, patients seen at our hospital who only had cataracts were used as the control group. The control subjects had cataracts classified as grade 2 or lower according to the Emery Little classification. OAG cases and controls were excluded if they self-reported a history of autoimmune diseases, cancers, or internal ocular surgery within the past year as these conditions could affect blood autoantibodies. Other systemic diseases were not excluded; subjects with glaucoma types other than NTG and POAG were excluded. Statistical methods were not used to predetermine the sample size as this was an exploratory analysis.
2.2. Wet Proteome Analysis
A proteome-wide autoantibody screening method was used as previously outlined [16,17,18]. All blood samples were allowed to stand for 30 min, centrifuged at 4 °C and 2000× g for 25 min, and then plasma was separated and frozen at −80 °C. The plasma was then stored at −80 °C.
2.2.1. Protein Expression In Vitro
A total of 19,446 human proteins for antigens were synthesized from HuPEX (Human Proteome Expression Resource) entry clones of the entire human proteome cDNA library [15]. pEW-5FG with a SP6 promoter and an N-terminal FLAG-GST tag was used as the destination vector and protein synthesis was performed using the wheat germ cell-free translation system (CellFree Sciences Co., Ltd., Matsuyama, Japan) [15].
2.2.2. Step 1. Measurement of Plasma Autoantibodies by Comprehensive Wet Protein Array (CWPA) in Initial Screening
Autoantibody measurements were performed with a previously reported CWPA [16,17,18] that contains 19,446 human proteins. A glutathione (GSH)-modified glass plate (S339009, Matsunami Glass Ind., Ltd., Kishiwada, Japan) was used for plating. Human proteins were spotted onto the plate using a HORNET-NX multi-dispense system equipped with a 1536-pin head (Fujifilm Wako Pure Chemical Co., Osaka, Japan) and bound via their GST-tags. As a preliminary study, CWPA analysis was performed using mixed plasma from each of 10 NTG, 10 POAG, and 10 cataract cases to measure autoantibodies. Then, 3:1000 diluted plasma was added to the CWPA and incubated at room temperature for 1 h. The blocking buffer described by Fukuda et al. [16] was prepared as follows: 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 200 mM NaCl, 1 mM DTT, 0.3% (w/v) skim milk, 5 mM GSH, 25%(v/v) glycerol, and 0.08% Triton X-100. After washing, goat anti-human IgG (H + L) Alexa Flour® 647 conjugate (A21445, Thermo Fisher Scientific, Waltham, MA, USA) diluted 1:1000 was added and incubated at room temperature for 1 h. After washing again, the CWPA was air-dried and fluorescence images were measured with a fluorescence imager (Typhoon FLA 9500, Cytiva, Marlborough, MA, USA). Fluorescent images were used for quantitative analysis of autoantibodies (Supplementary Figure S1).
2.2.3. Step 2. Plasma Autoantibody Assay with Custom-Designed WPA
The next step was quantification of autoantibodies in patient plasma of each disease type by custom-designed WPA. A GSH-modified glass plate (SDM0011, Matsunami Glass Industry Co., Ltd., Kishiwada, Japan) was used for custom-designed WPA. GST-tag fused human proteins were spotted using a 1536-channel independent cylinder system (EDR-384SX; Biotec, Tokyo, Japan). The target antigen of the autoantibody detected by CWPA in Step 1, previously reported glaucoma-related proteins, proteins expressed in the optic nerve, and astrocytes were spotted on custom-designed WPA. The reaction conditions and fluorescence-image-capturing method for custom-designed WPA are the same as for CWPA.
2.2.4. Protein Array Data Analysis
For quantification of autoantibodies, the level of each autoantibody was calculated based on the fluorescence values obtained from the reaction of plasma with the protein spot [16]. The level of each autoantibody was calculated as follows.
Fautoantigen: fluorescence intensity of autoantigen (human protein) spots reacted with plasma;
Fnegative controls: average fluorescence intensity of negative control (negative control for protein synthesis) spots (mock) reacting with plasma;
Fpositive control: average fluorescent intensity of positive control (human immunoglobulin to which the anti-human IgG used to detect autoantibodies binds directly).
To detect the binding of each antibody to CWPA and custom-designed WPA, positive spots were determined by the average + 3 standard deviation of the negative control spots as a threshold. As a result, for convenience, an AU value > 10 was defined as positive, and the positivity rate for each sample was calculated as follows.
Positivity rate (PR) = number of positive samples/number of samples in disease group × 100
2.3. Statistical Analysis
All analyses were performed using R version 4.1.2. In addition, t-tests and Fisher’s exact test were used to compare the backgrounds of the subjects. A comparison of autoantibody titers in each form was carried out using Dunnett’s multiple comparisons test, and the association of each autoantibody in OAG with age, sex, and ophthalmic clinical information (MD, IOP, AL, CCT) was evaluated using Spearman’s correlation coefficient. The association of each autoantibody was also evaluated using Spearman’s correlation coefficient. For ophthalmologic examination items, the visual field severity eye was employed in OAG, while the right eye was employed in the control cataract group. To evaluate the diagnostic power of each autoantibody in each disease type, ROC curves were drawn using sensitivity and specificity calculated from cross-tabulations, and optimal cutoff and the area under the curve (AUC) values were obtained.
It should be noted that this criterion for positivity by optimal cutoff value differs from the criterion that defines an AU value > 10 as positive. This was carried out in order to detect binding of each antibody to CWPA and custom-designed WPA. A comparison of the positivity rates in each disease group was performed by Fisher’s multiple testing (with Bonferroni correction to account for multiple comparisons).
To evaluate the diagnostic power of multiple autoantibodies, we used all glaucoma autoantibodies corrected for age, sex, and IOP to draw ROC curves and obtain AUC values by logistic regression analysis. Model 1 consisted of age, sex, and IOP variables. Model 2 consisted of age, sex, IOP, and all four autoantibody variables. The AUC for each model was tested with the Delong test. For all analyses (except Fisher’s multiple testing for comparing multiple antibodies), p values were considered statistically significant, when the values were lower than 0.05 (two-tailed).
3. Results
Initial screening identified 171 antigens, 149 of which tested positive only for OAG and 22 of which tested positive only for cataracts. These 171 antigens, plus 25 antigens previously associated with glaucoma and 24 antigens expressed in the optic nerve and astrocytes, leading to a total of 220 antigens, were loaded onto custom-designed WPA plates (Supplementary Table S1) and analyzed for plasma autoantibodies in cataract, NTG, and POAG. In total, 35 cataract, 50 NTG, and 69 POAG samples were available for study, and 99 antigens were detectable from these samples. Table 1 shows the clinical characteristics of the patients from whom these specimens were obtained. Age, sex, IOP, and CCT showed no significant differences. Among the OAG group, IOP was 12.67 ± 2.54 mmHg in the NTG group and 15.89 ± 4.99 mmHg in the POAG group (p < 0.001). MD was −17.50 ± 7.98 dB in the NTG group and −19.27 ± 8.93 dB in the POAG group (no significant difference between the two groups, p = 0.268). Most OAG patients (88%) were in an advanced stage, with few cases (12%) better than −6 dB. AL showed a significant difference between the cataract and OAG groups (p = 0.042).

Table 1.
Characteristics of each study population.
3.1. Extraction of Autoantibodies
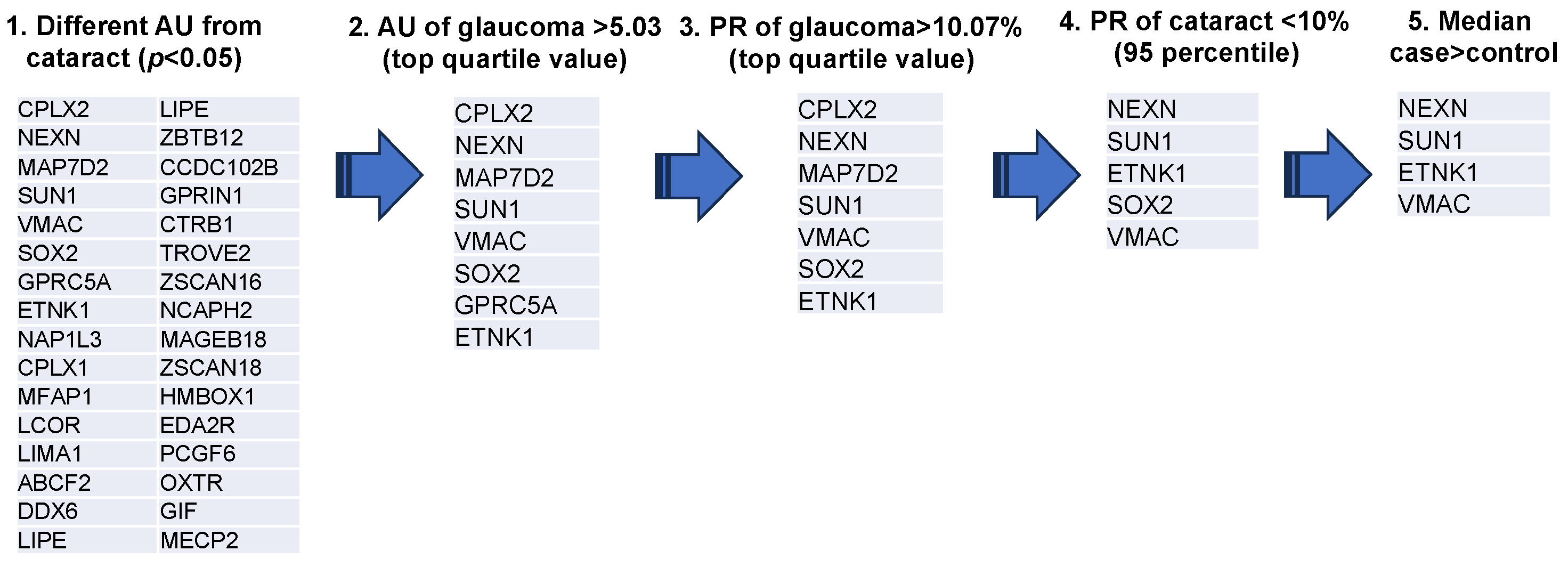
A representative example of the custom-designed WPA results is shown in Figure 1. From these results, the AUs (antibody titer in arbitrary units) and PR were calculated for each disease for the 99 autoantigens detected in the custom-designed WPA (Supplementary Table S2). We next extracted 32 glaucoma-related autoantibodies that showed statistically significant differences in titers between the cataract and glaucoma groups (Figure 2). Among them, we selected the top quartile of antibody titers in the glaucoma group (AU > 5.03) and the top quartile of positivity rates (PR > 10.07). Two antibodies, showing high titers and positivity rates for cataracts (in the top 95% of the samples), were excluded. Finally, SOX2, which has a higher median in the cataract group than the glaucoma group, was excluded. As a result, ethanolamine kinase 1 (ETNK1), vimentin-type intermediate filament-associated coiled-coil protein (VMAC), nexilin (NEXN), and Sad1 and UNC84 Domain Containing 1 (SUN1) were identified as glaucoma-associated autoantibodies.
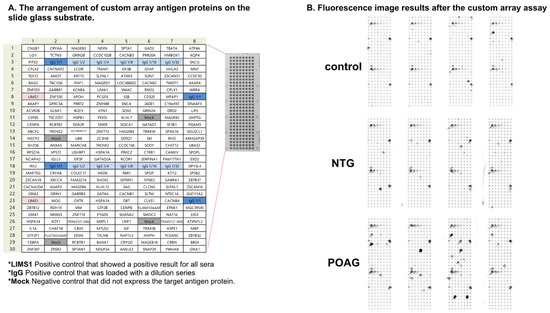
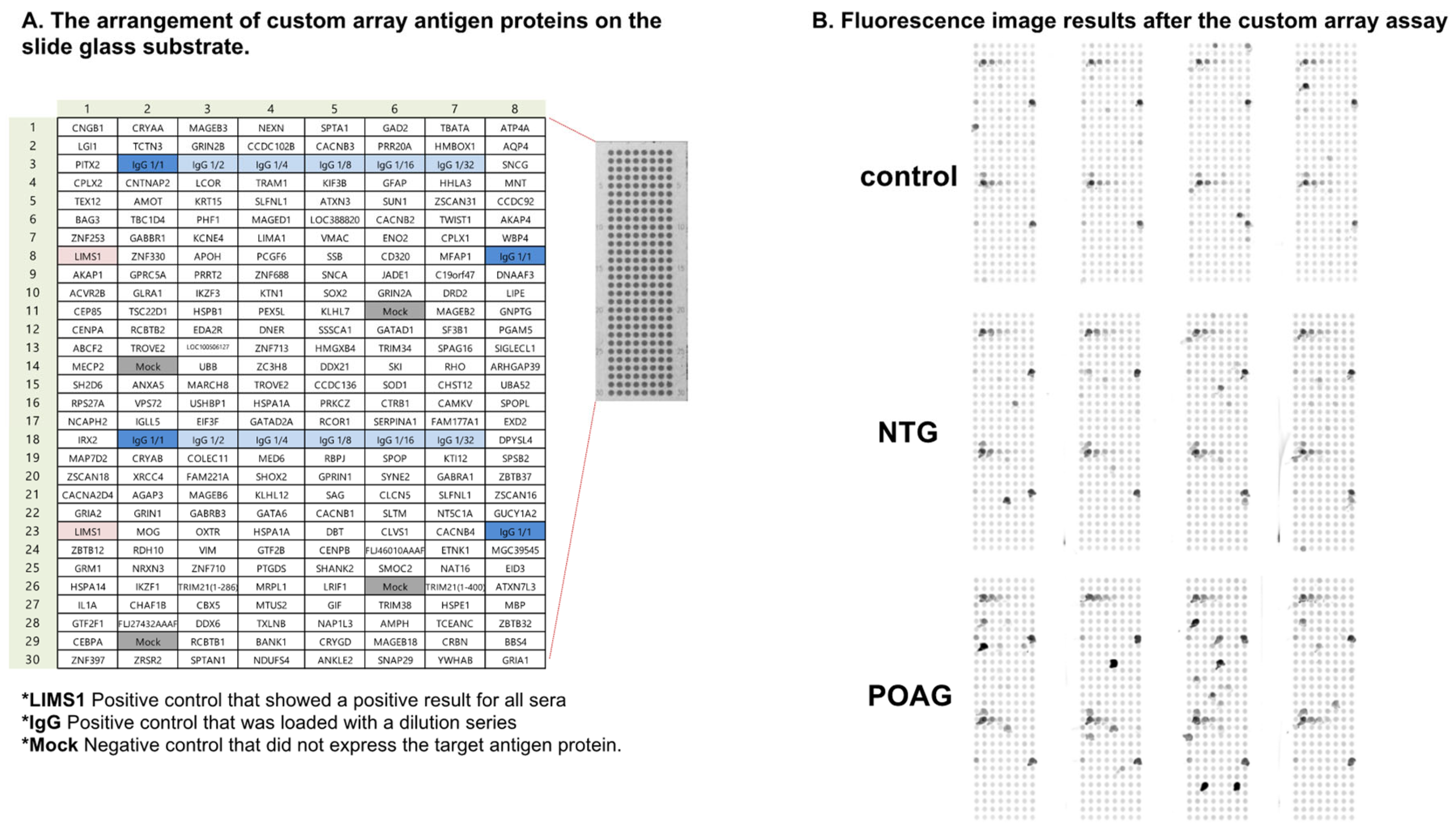
Figure 1.
The custom-designed WPA. (A). The panel depicts the arrangement of custom array antigen proteins on the slide glass substrate. *LIMS1 was a positive control that showed positive results for all sera. *IgG was also a positive control that was loaded with a dilution series. *Mock was a negative control that did not express the target antigen protein. (B). Fluorescence image results after the custom array assay are shown.
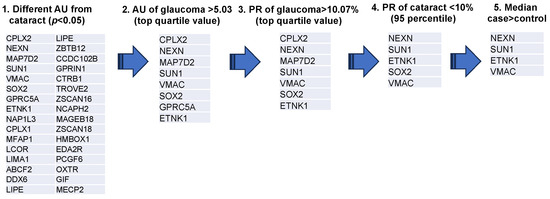
Figure 2.
Extraction process of glaucoma associated autoantibodies. The diagram depicts the procedure for selection of glaucoma-associated autoantibodies. Step 1 was to extract 32 antigens for which there was a statistically significant difference in AUs between cataract and glaucoma groups (OAG, NTG, and POAG). Step 2 screened eight antigens with antibody titers in the top quartile. Step 3 screened seven antigens with positive titers in the top quartile for AU. Step 4 excluded two antibodies that fell into the top 95% of positivity rate for cataracts. Step 5 excluded SOX2, which showed higher median values in the cataract group compared to the glaucoma group. Antigen proteins targeted by autoantibodies were described by the gene symbol from which they were derived.
3.2. Comparison of Autoantibody Titers
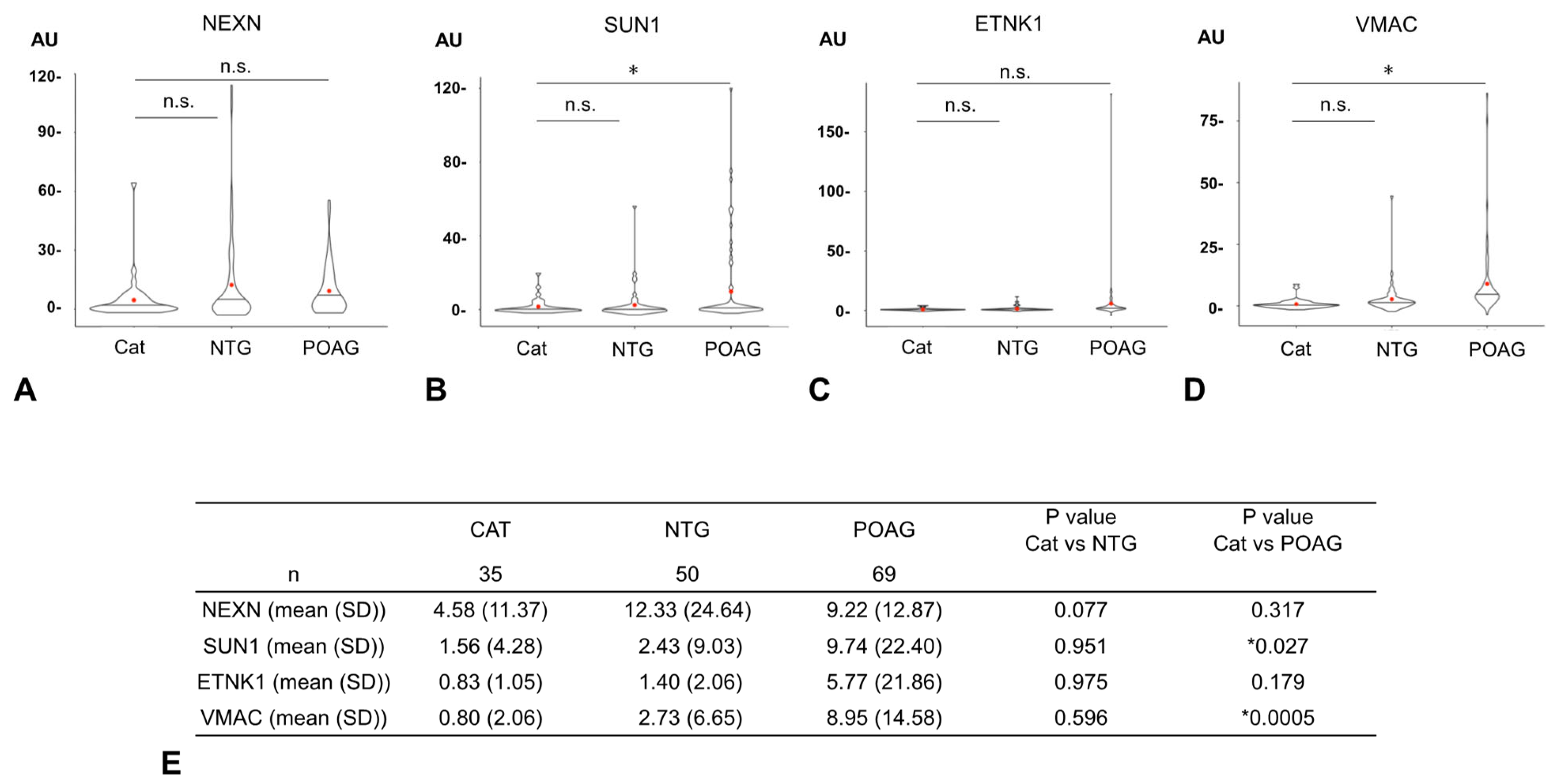
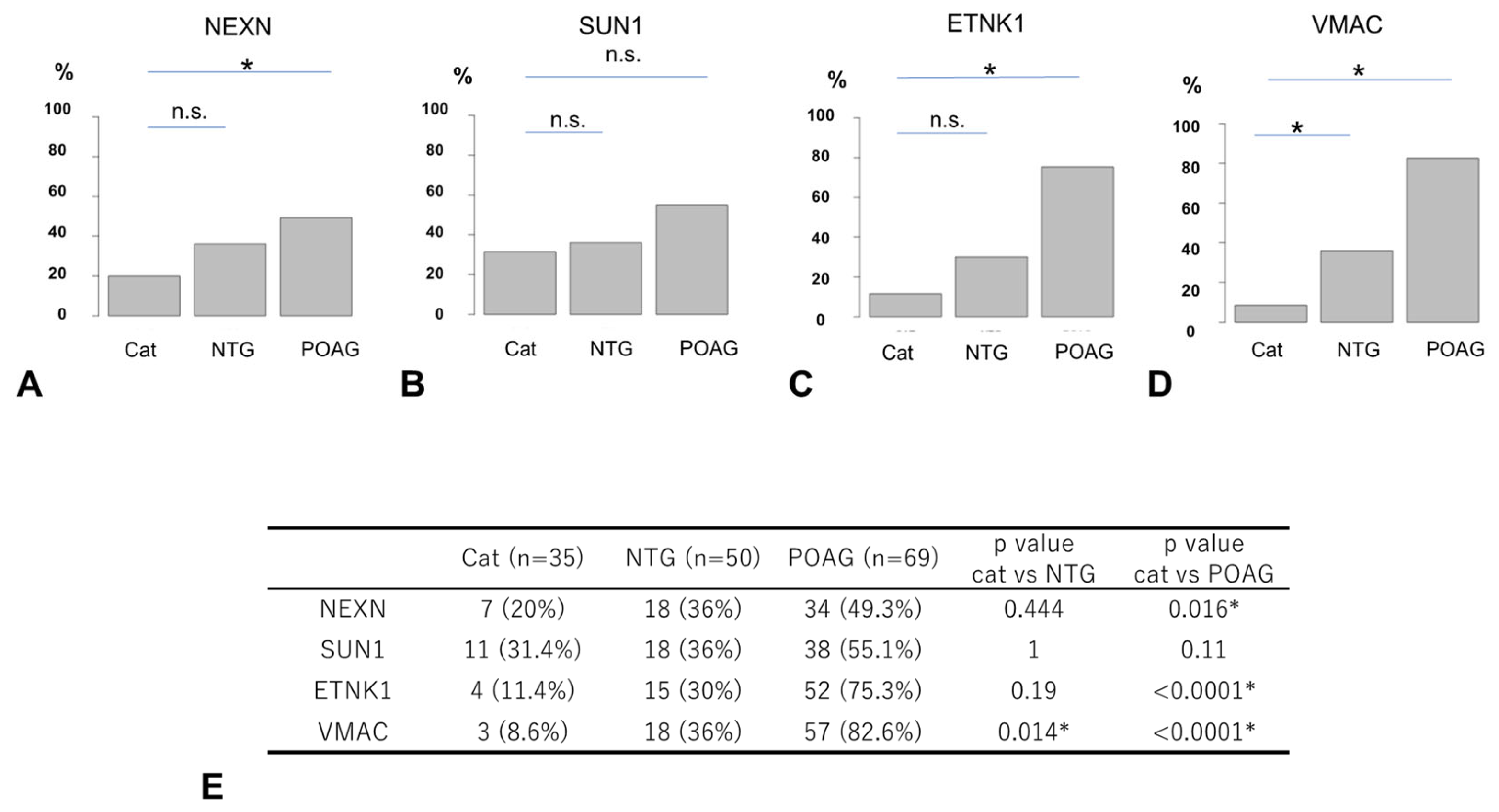
The AUs of all four glaucoma-related autoantibodies were compared for the 35 cataract, 50 NTG, and 69 POAG samples (Figure 3). For SUN1 and VMAC, the AUs were significantly higher in the POAG group than in the cataract group, but no significant difference was detected between the cataract and NTG groups. For other autoantibodies (NEXN, ETNK1), there were no significant differences in AUs between the cataract and NTG groups or between the cataract and POAG groups.
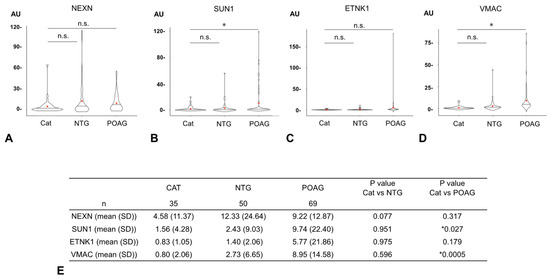
Figure 3.
Comparison of autoantibody titers between cataract and glaucoma. The graphs show violin plot analyses comparing the distribution of antibody titers (AUs) of each glaucoma-related autoantibody by disease type ((A). NEXN, (B). SUN1, (C). ETNK, (D). VMAC). The antibody titers of autoantibodies between the cataract (CAT), NTG, and POAG groups were compared by Dunnett’s multiple comparison test (* p < 0.05) and are summarized in table (E). The n.s. indicates not significant. The red dot represents the average value. For SUN1 and VMAC, autoantibody titers (AUs) were significantly higher in the POAG group than in the cataract group, while there were no significant differences between the cataract and NTG groups. For other autoantibodies (NEXN, ETNK), there were no significant differences in AUs between the cataract and NTG groups or between the cataract and POAG groups.
3.3. Examination of the Ability of Each Autoantibody to Discriminate Between the Cataract and Glaucoma Groups
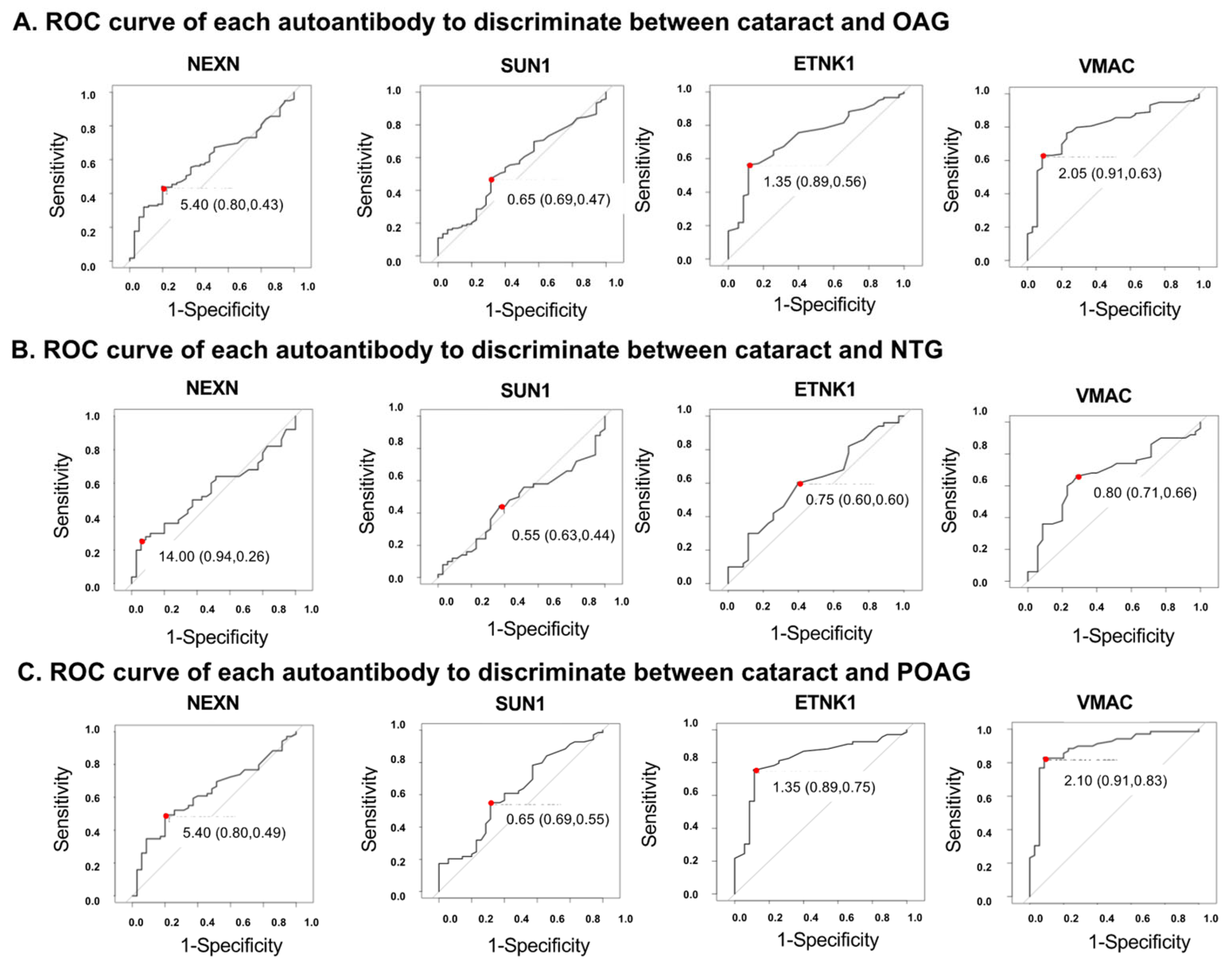
An ROC analysis was performed for each autoantibody to discriminate the glaucoma groups from the cataract (control) group (Figure 4, Supplementary Table S3). To determine the optimal cutoff values, a Youden Index, defined as sensitivity-(1-specificity), was calculated. The AU with the largest Youden Index was used as the optimal cutoff value (Supplementary Figure S2). For example, in the ROC analysis of the discrimination between cataract and POAG groups using ETNK1 (Supplementary Table S3), the autoantibody titer with the maximum Youden Index is 1.35. This is the optimal cutoff value to separate cataract and POAG. In this case, sensitivity is 0.886, specificity is 0.754, the Youden Index is 0.64.
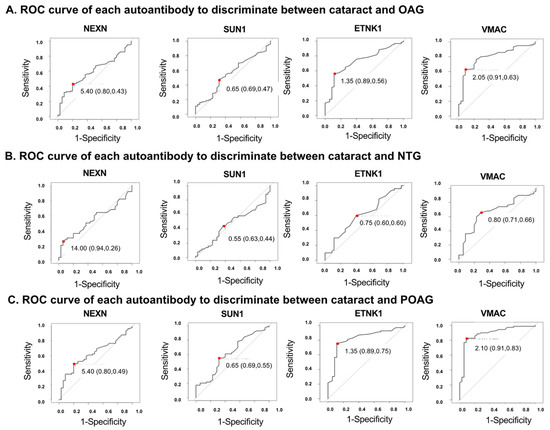
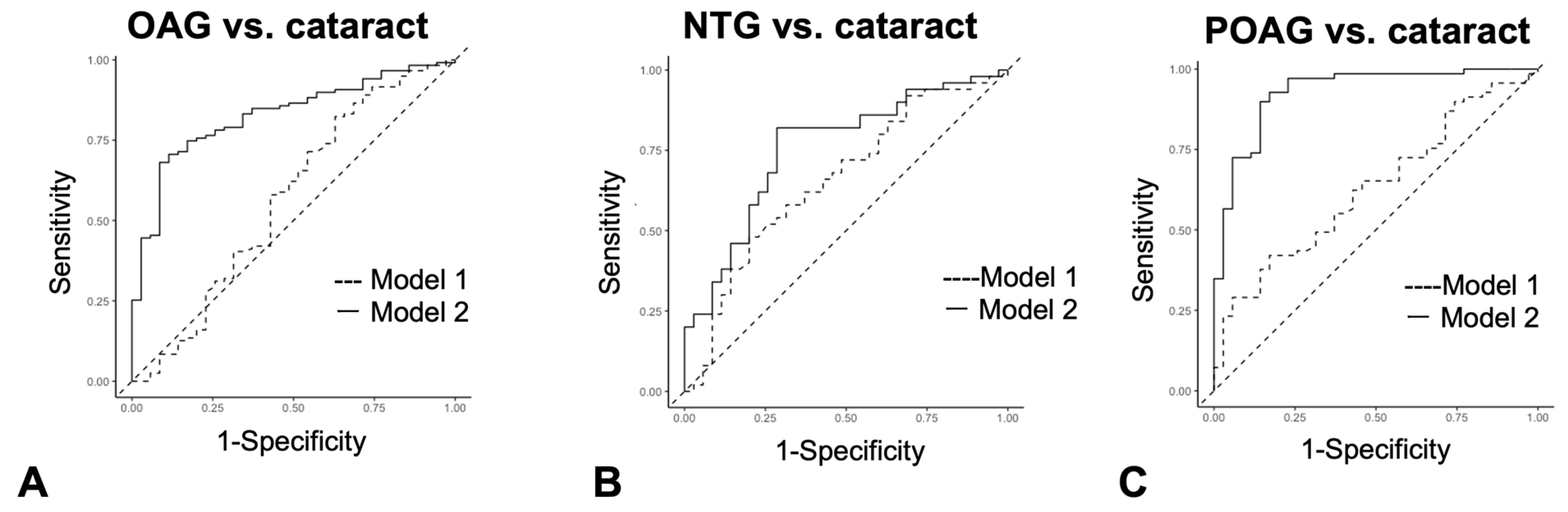
Figure 4.
ROC curves for the ability of each autoantibody to discriminate between cataracts and OAG. Optimal cutoff values are indicated by red dots. Optimal cutoff value, sensitivity, and specificity, shown in parentheses, are inserted in each graph. (A). ROC curve for each autoantibody when discriminating OAG from cataract. Optimal cutoff value, sensitivity, and specificity was 5.40 (0.80, 0.43) in NEXN, 0.65 (0.69, 0.47) in SUN1, 1.35 (0.89, 0.56) in ETNK1, 2.05 (0.91, 0.63) in VMAC. (B). ROC curve for each autoantibody when discriminating NTG from cataract. Optimal cutoff value, sensitivity, and specificity was 14.00 (0.94, 0.26) in NEXN, 0.55 (0.63, 0.44) in SUN1, 0.75 (0.60, 0.60) in ETNK1, 0.80 (0.71, 0.66) in VMAC. (C). ROC curve for each autoantibody when discriminating POAG from cataract. Optimal cutoff value, sensitivity, and specificity was 5.40 (0.80, 0.49) in NEXN, 0.65 (0.69, 0.55) in SUN1, 1.35 (0.89, 0.75) in ETNK1, 2.10 (0.91, 0.83) in VMAC.
In discriminating between the OAG and cataract (control) groups (Figure 4A, Supplementary Table S3), autoantibodies against ETNK1 and VMAC were highest among the four autoantibodies, with AUCs of 0.728 (95%CI: 0.639–0.817) and 0.796 (95%CI: 0.717–0.876), respectively. VMAC showed the highest AUC (0.669 [95%CI: 0.615–0.832]) to make a distinction between NTG and cataract (Figure 4B, Supplementary Table S3). In discrimination between the POAG and cataract groups (Figure 4C, Supplementary Table S3), the AUCs of ETNK1 and VMAC were calculated to be 0.820 (95%CI: 0.733–0.907) and 0.889 (95%CI: 0.818–0.959), respectively.
3.4. Comparison of Autoantibody Positivity Rates
The comparison of the positivity rate (PR) by disease type for each autoantibody is shown in Figure 5, where positive and negative results were determined based on the optimal cutoff value for discriminating between the OAG and cataract groups (Figure 4, Supplementary Table S3). The PR of antibodies to NEXN and ETNK1 was significantly higher in POAG compared to cataract, while there was no significant difference between NTG and cataract (Figure 5). For antibodies to SUN1, there was no significant difference in the PR between the different types of glaucoma and cataract. The PR of antibodies to VMAC was significantly higher in the NTG and POAG groups compared to the cataract group.
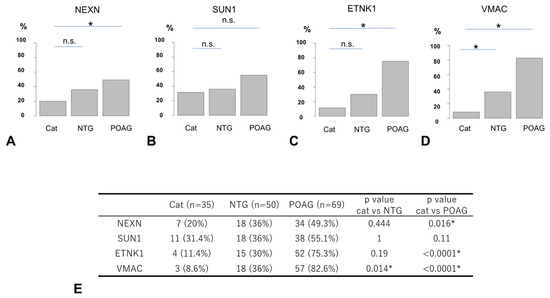
Figure 5.
Comparison of positivity rates between cataract and glaucoma. Percentage positive for each autoantibody using the optimal cutoff value for OAG as a threshold ((A). NEXN, (B). SUN1, (C). ETNK1, (D). VMAC). The positivity rate (PR) of autoantibodies between the cataract, NTG, and POAG groups is summarized in table (E) and compared by Fisher’s exact test and Bonferroni correction (* p < 0.05). The n.s. indicates not significant. NEXN and ETNK1 showed significant differences in the PR between cataracts and POAG, but not between cataracts and NTG. VMAC showed significant differences in the PR between cataracts and POAG and between cataracts and NTG. SUN1 showed no significant difference in the PR between cataract and POAG and between cataract and NTG.
3.5. Overlap of Autoantibodies
Examination of overlap of autoantibody in NTG and POAG revealed that in NTG, autoantibodies showed overlap in 48.8% of cases. On the other hand, POAG had clearly more cases of autoantibody overlap than NTG with 88.2% (Supplementary Figure S3). Among the overlapping antibodies, those containing NEXN were the most common in NTG (65.0%) and those containing VMAC were the most common in POAG (91.5%).
3.6. Relationship Between Autoantibodies and Clinical Findings
Spearman’s rank correlation coefficient test showed that autoantibodies against ETNK1, VMAC, NEXN, or SUN1 were not significantly correlated with each other (Supplementary Table S4). In all glaucoma types, the four glaucoma-associated autoantibodies other than NEXN did not correlate significantly with any of the clinical factors: MD, IOP, IOL AL, CCT, or age (Table 2). The NEXN autoantibody correlated with age only in POAG, but did not correlate significantly with MD, IOP, AL, or CCT. To explore the usefulness of each antibody in the detection of early-stage glaucoma, we compared the positivity rates between early-stage and more-progressed-stage glaucoma. No significant differences between these two stages were observed for any of the antibodies (Supplementary Figure S4).

Table 2.
Spearman’s rank correlation of each autoantibody with age, IOP, and ophthalmic clinical parameters.
3.7. Logistic Regression Analysis to Predict Glaucoma Using a Set of Glaucoma-Associated Autoantibodies
Logistic regression analysis was used to determine if a series of autoantibody combinations were useful as diagnostic tests in discriminating OAG, NTG, and POAG from cataracts (Figure 6A–C, Supplementary Table S5). The cutoff value in logistic regression analysis is a probability and is different from antibody titer. As a result, in the comparison between cataract and OAG (Figure 6A) and cataract and POAG (Figure 6C), the AUC of Model 2, which added autoantibodies to age, sex, and IOP, was significantly greater than that of Model 1, which consisted of conventional health examination items, namely age, sex, and IOP variables. On the other hand, between cataract and NTG, the alpha level between Model 1 and Model 2 was 0.05, and the AUC difference did not reach statistical significance (Figure 6B).
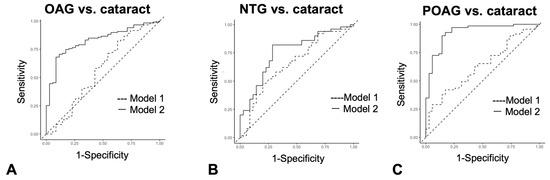
Figure 6.
Discrimination between cataract and glaucoma by the ROC logistic regression model. (A–C). ROC logistic regression model for glaucoma discrimination. Model 1 consists of age, sex, and IOP variables. Model 2 consists of variables for age, sex, IOP and all four autoantibodies. (A). ROC logistic regression analysis when discriminating OAG from cataract. (B). ROC logistic regression analysis when discriminating NTG from cataract. (C). ROC logistic regression analysis when discriminating POAG from cataract.
4. Discussion
In this study, a cohort of cataract, NTG, and POAG patients was examined by CWPA for plasma autoantibodies, and four new autoantibodies, ETNK1, VMAC, NEXN, and SUN1, were detected as glaucoma-related. For each autoantibody, an ROC curve analysis was performed to examine the ability to discriminate glaucoma patients from cataract patients, and optimal cutoff values were calculated to determine the positivity rate by disease type. Furthermore, we performed multivariate analyses using all four autoantibodies to clarify the diagnostic usefulness of combining these autoantibodies compared to analyses using individual autoantibodies.
In recent years, various methods of hematological proteome-wide analysis have been developed to quantify proteins in a comprehensive manner. The WPA method used in this study has several advantages over conventional human protein array or ELISA methods. First, the CWPA plate carries 19,446 proteins for antigens that were comprehensively synthesized from a human cDNA library, and the range of self-antigens that can be loaded is comparable to the level of the entire proteome [15,16,17]. Second, the CWPA has an extremely narrow average protein loading range (Max/Min) of 47.3, making it less prone to mass bias in the antigen–antibody reaction due to protein loading. Third, the entire process is designed to maintain the protein’s three-dimensional conformation by handling the arrays under wet conditions, allowing for more accurate analysis of specific antigen–antibody binding [16,17,18]. In this study, we were unable to confirm previously reported glaucoma-associated autoantibodies such as HSPs [11], γ-enolase [12] and MBP [13]. Their original detection was mainly based on results of ELISA, which does not preserve a three-dimensional protein structure. Additionally, the sample size may influence the present results. Therefore, further investigation in a larger population is needed in the future.
Antibodies are primarily a defense system acquired to protect the body from foreign bacteria and viruses. However, antibodies are generated not only against foreign antigens, but also against proteins that are produced in excess or released outside the cells in diseases. In autoimmune diseases, autoantibodies can be both disease markers and causes of disease. In the present study, the four novel autoantibodies were not significantly correlated with the severity of visual field changes (MD value) (Table 2), indicating that these four autoantibodies are not likely to be markers of glaucoma severity.
Overlapping autoantibodies were detected in only 48.8% of patients in NTG, whereas overlap was 88.2% in POAG (Supplementary Figure S3). This finding suggests that autoimmune-positive NTG can be discriminated from autoimmune-negative NTG and each of the four autoantibodies may define a new independent entity. Also, POAG can be divided into autoantibody-related POAG (APOAG) and non-APOAG (Supplementary Figure S5).
The antigens of the four autoantibodies associated with glaucoma are all proteins implicated in the pathogenesis of glaucoma. Some of these proteins are also related to myopia, a risk factor of glaucoma, especially NTG.
NEXN is an actin-binding protein that regulates actin filament polymerization. Chronically elevated IOP induces polymerization of actin filaments in trabecular cells [23]. Although the detailed mechanism linking increased IOP and actin remodeling has not been fully elucidated, NEXN may protect trabecular cells or RGCs from the mechanical stress caused by increased IOP by promoting actin polymerization. If NEXN is released from trabecular cells or RGCs by elevated IOP in an early stage of OAG, NEXN may contribute to the pathogenesis of glaucoma in a previously uncharacterized manner.
ETNK1 is involved in the phosphorylation of ethanolamine (Et) to phosphoethanolamine (P-Et), which plays a key role in the major metabolic pathway that synthesizes phosphatidylethanolamine (PE) and phosphatidylcholine (PC), the two most abundant phospholipids in mammalian cell membranes. PE is required for optimal mitochondrial respiratory activity and ubiquinone function [24], and mitochondrial dysfunction is associated with glaucomatous neurodegeneration [25]. Furthermore, aging may reduce mitochondrial function and affect the survival of RGCs in glaucoma [26]. Thus, ENTK1 may be strongly involved in the pathophysiology of glaucoma via multiple actions, including effects on mitochondrial function.
VMAC is indirectly associated with vimentin-type intermediate filaments via vimentin-binding proteins [27]. The presence of autoantibodies to vimentin has been previously reported in patients with NTG. Since elevated IOP increases vimentin synthesis in astrocytes [28], VMAC may be associated with glaucomatous damage caused by elevated IOP. Furthermore, myopia is a known risk factor for glaucoma [29]. Myopia is characterized by prolonged ocular AL and extracellular matrix (ECM) remodeling of the sclera. Because myopia is associated with differentiation of myofibroblasts that are characterized with vimentin [30], it is plausible that VMAC contributes to the pathogenesis of glaucoma via myopia.
SUN1 is a nuclear membrane protein with an Unc84 (SUN) domain. Ueda et al. [31]. examined the effect of SUN protein deletion on maturation of the actin cytoskeleton and newly generated focal adhesions (nascent focal adhesions, FAs). SUN1 is essential for actin formation, generation of intercellular traction, and maturation of FAs [32]. SUN1 is also a key component of actin and FA maturation. Thus, SUN1 may be involved in the pathogenesis of glaucoma via actin and FAs. In addition, actin, the molecular target of SUN1, is closely related to the induction of myopia through ocular AL elongation [33,34].
In POAG, damage to the cell membrane caused by elevated intraocular pressure may have resulted in these proteins involved with the cytoskeleton and mitochondria flowing out of the cell, triggering an immune mechanism and producing autoantibodies. NTG, on the other hand, is pathologically affected by a variety of non-intraocular pressure-dependent factors, such as blood flow, neurotrophic factors, or oxidative stress [35,36,37]. As a result, there is less damage to the cell membrane due to IOP, less extravasation of these intracellular proteins, and possibly less antibody production as a result.
The present analysis has several limitations. First, the sample size is limited. Statistical methods were not used to predetermine the sample size, as this was an exploratory analysis. Considering the number of possible autoantibodies with CWPA and the sample size, there may be other autoantibodies that were not detected. This also may lead to overestimation and a decrease in the diagnostic power of these autoantibodies in OAG patients. Further investigation in a larger population is needed in the future. Second, there may be selection bias based on clinical ascertainment of the study population. The current results are based on patients from the same clinical center, and there is a risk of overfitting. To enhance generalizability, we plan to examine external validity in other cohorts. Third, although patients with autoimmune diseases or cancers that could affect the biomarkers under study were excluded, other confounding factors could have affected plasma biomarker values.
5. Conclusions
In the present study, four new autoantibodies have been identified in association with NTG and POAG by the application of a newly developed proteome-wide analysis. A combination of these autoantibodies appears to be a suitable biomarker for the diagnosis of OAG, even in an early disease stage. Autoantibody testing could help to promote early OAG detection and prevent severe vision loss by timelier onset of treatment.
6. Patents
Tohoku University, Proteobridge, Santen Pharmaceutical Co., Ltd., Diagnosis of glaucoma, P2023-129215A, Japan Patent Office, JPO, 2023.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13030718/s1, Table S1: Antigen proteins on custom arrays. References [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] are cited in the supplementary materials.; Table S2: Antibody titers and positivity rates by disease type for 99 autoantibodies that could be detected by custom arrays; Table S3: ROC analysis of each disease type; Table S4: Spearman’s rank correlation between autoantibodies in OAG; Table S5: ROC logistic analysis between glaucoma and cataract; Figure S1: Representative image of proteins bound on the CWPA; Figure S2: Relationship between optimal cutoff value by Youden Index, sensitivity, and specificity; Figure S3: Autoantibody overlap in NTG (A) and POAG (B); Figure S4: Comparison of positivity rates between early stage and progress stage of glaucoma. Figure S5. Diagnostic criteria for glaucoma.
Author Contributions
Involved in the design of and conducting this study (N.T., M.I., K.S., T.K., C.S. and T.N. (Toru Nakazawa)); conducting the WPA and creating the dataset (E.F., K.Y., C.O. and N.G.); preparation, collection, management, analysis, and interpretation of the data (N.T., M.I., K.S., H.K., T.N. (Takahiro Ninomiya), A.H., C.T., A.O., Y.I., C.F.Z. and T.N. (Toru Nakazawa)); and approval of the manuscript (A.H., Y.I., C.F.Z. and T.N. (Toru Nakazawa)). N.T. and M.I. wrote the main manuscript text. N.T. prepared all tables and all figures, and all authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the JST COI-NEXT JPMJPF2201, the JSPS KAKENHI Grants-in-Aid for Scientific Research C to MI (22K09827), the JSPS KAKENHI Grants-in-Aid for Scientific Research C to HK (23K09037), the Japan Agency for Medical Research and Development (AMED) (grant numbers JP17bm0404017 and JP18hm0102029), and by Santen Pharmaceutical Co., Ltd. (RD20190108).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Tohoku University (2022-1-831).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Restrictions apply to the datasets. The datasets presented in this article are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to the corresponding author(s).
Acknowledgments
The authors thank Junko Sato, Kanako Sakai, Rieko Kamii, and Mayumi Suda for technical assistance.
Conflicts of Interest
The authors Naoko Takada, Makoto Ishikawa, Hiroshi Kunikata, Takahiro. Ninomiya, Akiko Hanyuda, Eriko. Fukuda, Kei Yamaguchi, Chihiro Ono, Chihiro Tsusu, Akiko Osanai, Naoki Goshima, Yukitoshi Izumi, and Toru Nakazawa declare no conflicts of interest. The author Kota Sato’s labor costs are partially paid by Santen Pharmaceutical Co. The author Charles F. Zorumski serves on the Scientific Advisory Board of Sage Therapeutics and has equity in the company. Sage Therapeutics was not involved in this work. The authors Tomoko Kirihara and Chie Shintani are employed by Santen Pharmaceutical Co., Ltd.
References
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef]
- Matoba, R.; Morimoto, N.; Kawasaki, R.; Fujiwara, M.; Kanenaga, K.; Yamashita, H.; Sakamoto, T.; Morizane, Y. A nationwide survey of newly certified visually impaired individuals in Japan for the fiscal year 2019: Impact of the revision of criteria for visual impairment certification. Jpn. J. Ophthalmol. 2023, 67, 346–352. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef]
- Zhao, J.; Solano, M.M.; Oldenburg, C.E.; Liu, T.; Wang, Y.; Wang, N.; Lin, S.C. Prevalence of Normal-Tension Glaucoma in the Chinese Population: A Systematic Review and Meta-Analysis. Am. J. Ophthalmol. 2019, 199, 101–110. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Wang, L.H.; Huang, C.H.; Lin, I.C. Advances in Neuroprotection in glaucoma: Pharmacological strategies and emerging technologies. Pharmaceuticals 2024, 17, 1261. [Google Scholar] [CrossRef]
- Becerra, C.M.C.; Funk, R.O.; Kohli, D.; Hodge, D.O.; Roddy, G.W. Evaluating the association between autoimmune disease and normal tension glaucoma: A retrospective case-control study. BMC Ophthalmol. 2025, 25, 73. [Google Scholar] [CrossRef]
- Liang, S. Role of T cell-induced autoimmune response in the pathogenesis of glaucoma. Int. Ophthalmol. 2024, 44, 241. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X. T Cell-Mediated Autoimmunity in Glaucoma Neurodegeneration. Front. Immunol. 2021, 12, 803485. [Google Scholar] [CrossRef]
- Tezel, G.; Seigel, G.M.; Wax, M.B. Autoantibodies to small heat shock proteins in glaucoma. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2277–2287. [Google Scholar]
- Maruyama, I.; Ikeda, Y.; Nakazawa, M.; Ohguro, H. Clinical roles of serum autoantibody against neuron-specific enolase in glaucoma patients. Tohoku J. Exp. Med. 2002, 197, 125–132. [Google Scholar] [CrossRef]
- Shin, Y.J.; Kim, E.; Han, B.K.; Yi, K. Serum biomarkers for the diagnosis of glaucoma. Diagnostics 2020, 11, 20. [Google Scholar] [CrossRef]
- Auler, N.; Tonner, H.; Pfeiffer, N.; Gru, F.H. Antibody and Protein Profiles in Glaucoma: Screening of Biomarkers and Identification of Signaling Pathways. Biology 2021, 10, 1296. [Google Scholar] [CrossRef]
- Goshima, N.; Kawamura, Y.; Fukumoto, A.; Miura, A.; Honma, R.; Satoh, R.; Wakamatsu, A.; Yamamoto, J.; Kimura, K.; Nishikawa, T.; et al. Human protein factory for converting the transcriptome into an in vitro-expressed proteome. Nat. Methods 2008, 5, 1011–1017. [Google Scholar] [CrossRef]
- Fukuda, E.; Tanaka, H.; Yamaguchi, K.; Takasaka, M.; Kawamura, Y.; Okuda, H.; Isotani, A.; Ikawa, M.; Shapiro, V.S.; Tsuchida, J.; et al. Identification and characterization of the antigen recognized by the germ cell mAb TRA98 using a human comprehensive wet protein array. Genes Cells 2021, 26, 180–189. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Yoshizaki, A.; Yamaguchi, K.; Fukuda, E.; Okumura, T.; Ogawa, K.; Ono, C.; Norimatsu, Y.; Kotani, H.; Hisamoto, T.; et al. Autoantibody landscape revealed by wet protein array: Sum of autoantibody levels reflects disease status. Front. Immunol. 2022, 13, 893086. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Kotani, H.; Yamaguchi, K.; Okumura, T.; Fukuda, E.; Kono, M.; Hisamoto, T.; Kawanabe, R.; Norimatsu, Y.; Kuzumi, A.; et al. Significance of anti-transcobalamin receptor antibodies in cutaneous arteritis revealed by proteome-wide autoantibody screening. J. Autoimmun. 2023, 135, 102995. [Google Scholar] [CrossRef]
- Anderson, D.R.; Chauhan, B.; Johnson, C.; Katz, J.; Patella, V.M.; Drance, S.M. Criteria for progression of glaucoma in clinical management and in outcome studies. J. Ophthalmol. 2000, 130, 827–829. [Google Scholar] [CrossRef]
- Takahashi, N.; Omodaka, K.; Nakazawa, A.; Kikawa, T.; Ninomiya, T.; Kiyota, N.; Tsuda, S.; Himori, N.; Akiba, M.; Nakazawa, T. Correlation Between Enlargement of Retinal Nerve Fiber Defect Angle in En Face Imaging and Visual Field Progression. Transl. Vis. Sci. Technol. 2022, 11, 8. [Google Scholar] [CrossRef]
- Prokofyeva, E.; Wegener, A.; Zrenner, E. Cataract prevalence and prevention in Europe: A literature review. Acta Ophthalmol. 2013, 91, 395–405. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Yang, F.; Xu, B.; Tang, Y.; Lu, Y. Prevalence rates of cataract and cataract surgery in elderly Chinese people living in suburban Shanghai: The Pujiang Cataract Cohort Study. Br. J. Ophthalmol. 2023, 107, 683–689. [Google Scholar] [CrossRef]
- Lakk, M.; Krizaj, D. TRPV4-Rho signaling drives cytoskeletal and focal adhesion remodeling in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 2021, 320, C1013–C1030. [Google Scholar] [CrossRef]
- Steenbergen, R.; Nanowski, T.S.; Beigneux, A.; Kulinski, A.; Young, S.G.; Vance, J.E. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J. Biol. Chem. 2005, 280, 40032–40040. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Xie, Z.; Chen, S.Y.; Zhang, X. Mitochondrial dysfunction in glaucomatous degeneration. Int. J. Ophthalmol. 2023, 16, 811–823. [Google Scholar] [CrossRef]
- Chaphalkar, R.M.; Kodati, B.; Maddineni, P.; He, S.; Brooks, C.D.; Stankowska, D.L.; Yang, S.; Zode, G.; Krishnamoorthy, R.R. A Reduction in Mitophagy Is Associated with Glaucomatous Neurodegeneration in Rodent Models of Glaucoma. Int. J. Mol. Sci. 2024, 25, 13040. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Irie, K.; Kurihara, H.; Sakai, T.; Takai, Y. Vmac: A novel protein associated with vimentin-type intermediate filament in podocytes of rat kidney. Biochem. Biophys. Res. Commun. 2004, 315, 1120–1125. [Google Scholar] [CrossRef]
- Rutigliani, C.; Tribble, J.R.; Hagström, A.; Lardner, E.; Jóhannesson, G.; Stålhammar, G.; Williams, P.A. Widespread retina and optic nerve neuroinflammation in enucleated eyes from glaucoma patients. Acta Neuropathol. Commun. 2022, 10, 118. [Google Scholar] [CrossRef]
- Jiravarnsirikul, A.; Belghith, A.; Rezapour, J.; Bowd, C.; Moghimi, S.; Jonas, J.B.; Christopher, M.; Fazio, M.A.; Yang, H.; Burgoyne, C.F. Evaluating glaucoma in myopic eyes: Challenges and opportunities. Surv. Ophthalmol. 2024, 70, 563–582. [Google Scholar] [CrossRef]
- Jiang, L.; Koh, J.H.Z.; Seah, S.H.Y.; Dan, Y.S.; Wang, Z.; Chan, X.; Zhou, L.; Barathi, V.A.; Hoang, Q.V. Key role for inflammation-related signaling in the pathogenesis of myopia based on evidence from proteomics analysis. Sci. Rep. 2024, 14, 23486. [Google Scholar] [CrossRef]
- Ueda, N.; Maekawa, M.; Matsui, T.S.; Deguchi, S.; Takata, T.; Katahira, J.; Higashiyama, S.; Hieda, M. Inner Nuclear Membrane Protein, SUN1, is Required for Cytoskeletal Force Generation and Focal Adhesion Maturation. Front. Cell Dev. Biol. 2022, 10, 885859. [Google Scholar] [CrossRef]
- Badrinarayanan, L.; Nagarajan, H.; Rishi, P.; Rishi, E.; George, R.J.; Chitipothu, S. Whole-genome sequencing unravels novel genetic determinants and regulatory pathways associated with triamcinolone acetonide-induced ocular hypertension. Mol. Genet. Genom. 2023, 298, 13–26. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, D.; Zhou, Q.; Zhao, F.; He, M.; Yang, Z.; Su, Y.; Zhai, Y.; Yan, J.; Zhang, G.; et al. Scleral HIF-1alpha is a prominent regulatory candidate for genetic and environmental interactions in human myopia pathogenesis. EBioMedicine 2020, 57, 102878. [Google Scholar] [CrossRef]
- Bao, B.; Liu, J.; Li, T.; Yang, Z.; Wang, G.; Xin, J.; Bi, H.; Guo, D. Elevated retinal fibrosis in experimental myopia is involved in the activation of the PI3K/AKT/ERK signaling pathway. Arch. Biochem. Biophys. 2023, 743, 109663. [Google Scholar] [CrossRef]
- Kiyota, N.; Shiga, Y.; Omodaka, K.; Pak, K.; Nakazawa, T. Time-Course Changes in Optic Nerve Head Blood Flow and Retinal Nerve Fiber Layer Thickness in Eyes with Open-angle Glaucoma. Ophthalmology 2021, 128, 663–671. [Google Scholar] [CrossRef]
- Sato, K.; Takada, N.; Fujioka, A.; Himori, N.; Yokoyama, Y.; Tsuda, S.; Omodaka, K.; Kirihara, T.; Ishikawa, M.; Kunikata, H.; et al. Reduced Plasma BDNF Levels in Normal Tension Glaucoma Compared to Open Angle Glaucoma. J. Glaucoma 2023, 32, 734–737. [Google Scholar] [CrossRef]
- Sato, K.; Saigusa, D.; Kokubun, T.; Fujioka, A.; Feng, Q.; Saito, R.; Uruno, A.; Matsukawa, N.; Ohno-Oishi, M.; Kunikata, H.; et al. Reduced glutathione level in the aqueous humor of patients with primary open-angle glaucoma and normal-tension glaucoma. PJ Aging 2023, 9, 28. [Google Scholar] [CrossRef]
- Von Thun Und Hohenstein-Blaul, N.; Kunst, S.; Pfeiffer, N.; Grus, F.H. Biomarkers for glaucoma: From the lab to the clinic. Eye 2017, 31, 225–231. [Google Scholar] [CrossRef]
- Joachim, S.C.; Bruns, K.; Lackner, K.J.; Pfeiffer, N.; Grus, F.H. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr. Eye Res. 2007, 32, 501–509. [Google Scholar] [CrossRef]
- Tsai, T.; Grotegut, P.; Reinehr, S.; Joachim, S.C. Role of Heat Shock Proteins in Glaucoma. Int. J. Mol. Sci. 2019, 20, 5160. [Google Scholar] [CrossRef]
- Vanags, D.; Williams, B.; Johnson, B.; Hall, S.; Nash, P.; Taylor, A.; Weiss, J.; Feeney, D. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: A double-blind randomised trial. Lancet 2006, 368, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Gupta, V.B.; Chick, J.M.; Greco, T.M.; Wu, Y.; Chitranshi, N.; Wall, R.V.; Hone, E.; Deng, L.; Dheer, Y.; et al. Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci. Rep. 2017, 7, 12685. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Wilding, C.; Funke, S.; Pfeiffer, N.; Grus, F.H. Protective effect of 14-3-3 antibodies on stressed neuroretinal cells via the mitochondrial apoptosis pathway. BMC Ophthalmol. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.E.; Davis, B.M.; Guo, L.; Normando, E.M.; Cordeiro, M.F. Annexins in Glaucoma. Int. J. Mol. Sci. 2018, 19, 1218. [Google Scholar] [CrossRef]
- Joachim, S.C.; Reichelt, J.; Berneiser, S.; Pfeiffer, N.; Grus, F.H. Sera of glaucoma patients show autoantibodies against myelin basic protein and complex autoantibody profiles against human optic nerve antigens. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 573–580. [Google Scholar] [CrossRef]
- Beutgen, V.M.; Perumal, N.; Pfeiffer, N.; Grus, F.H. Autoantibody biomarker discovery in primary open angle glaucoma using serological proteome analysis (SERPA). Front. Immunol. 2019, 10, 381. [Google Scholar] [CrossRef]
- Maruyama, I.; Ohguro, H.; Ikeda, Y. Retinal ganglion cells recognized by serum autoantibody against gamma-enolase found in glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1657–1665. [Google Scholar]
- Boehm, N.; Wolters, D.; Thiel, U.; Lossbrand, U.; Wietgel, N.; Pfeiffer, N.; Grus, F.H. New insights into autoantibody profiles from immune privileged sites in the eye: A glaucoma study. Brain Behav. Immun. 2012, 26, 96–102. [Google Scholar] [CrossRef]
- Latalska, M.; Gerkowicz, M.; Kosior-Jarecka, E.; Kozioł-Montewka, M.; Pietraś-Trzpiel, M. Antibodies to beta-2 glycoprotein I in serum and aqueous humor of patients with glaucoma and their influence on the static perimetry. Klin. Oczna 2004, 106, 160–161. [Google Scholar]
- Surgucheva, I.; McMahan, B.; Ahmed, F.; Tomarev, S.; Wax, M.B.; Surguchov, A. Synucleins in glaucoma: Implication of gamma-synuclein in glaucomatous alterations in the optic nerve. J. Neurosci. Res. 2002, 68, 97–106. [Google Scholar] [CrossRef]
- Saccà, S.C.; Centofanti, M.; Izzotti, A. New proteins as vascular biomarkers in primary open angle glaucomatous aqueous humor. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4242–4253. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Barrett, D.A.; Li, Z.; Pestronk, A.; Wax, M.B. Anti-rhodopsin antibodies in sera from patients with normal-pressure glaucoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1968–1975. [Google Scholar] [PubMed]
- Grus, F.H.; Joachim, S.C.; Bruns, K.; Lackner, K.J.; Pfeiffer, N.; Wax, M.B. Serum autoantibodies to alpha-fodrin are present in glaucoma patients from Germany and the United States. Investig. Opthalmol Vis. Sci. 2006, 47, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, A.; Sheng, M.H.; Constantine-Paton, M. Eye opening induces a rapid dendritic localization of PSD-95 in central visual neurons. Proc. Natl. Acad. Sci. USA 2003, 100, 1334–1339. [Google Scholar] [CrossRef]
- García-Barcina, J.M.; Matute, C. AMPA-selective glutamate receptor subunits in glial cells of adult bovine white matter. Brain Res. Mol. Brain Res. 1998, 53, 270–276. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Zhang, D.; Ozkaynak, E.; Wang, X.; Kasper, E.M.; Leguern, E.; Baulac, S.; Anderson, M.P. Epilepsy gene LGI1 regulates postnatal developmental remodeling of retinogeniculate synapses. J. Neurosci. 2012, 32, 903–910. [Google Scholar] [CrossRef]
- Lennon, V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B.G. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Kezuka, T.; Ishikawa, H. Diagnosis and treatment of anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis. Jpn. J. Ophthalmol. 2018, 62, 101–108. [Google Scholar] [CrossRef]
- Yasin, A.; Dudeck, L.; Redick, D.W.; Khodeiry, M.M.; Lam, B.L.; Jiang, H. Severe vision loss and optic disc edema associated with GAD-65 antibody positive Miller Fisher syndrome. J. Neuroophthalmol. 2022, 44, e40–e44. [Google Scholar] [CrossRef]
- Huntsman, M.M.; Jones, E.G. Expression of alpha3, beta3 and gamma1 GABA(A) receptor subunit messenger RNAs in visual cortex and lateral geniculate nucleus of normal and monocularly deprived monkeys. Neuroscience 1998, 87, 385–400. [Google Scholar] [CrossRef]
- Moldavan, M.G.; Allen, C.N. GABAB receptor-mediated frequency-dependent and circadian changes in synaptic plasticity modulate retinal input to the suprachiasmatic nucleus. J. Physiol. 2013, 591, 2475–24390. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yang, H.; Zhu, F.; Wang, Q.; Shan, W. Clinical character of CASPR2 autoimmune encephalitis: A multiple center retrospective study. Front. Immunol. 2021, 12, 652864. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, M.; Xu, S.; Yang, L.; Yang, P.; Song, Y.; Zhu, H.; Wang, Y.; Sun, Y.; Yan, C.; et al. Mirror image pain mediated by D2 receptor regulation of astrocytic Cx43 phosphorylation and channel opening. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166657. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Rios, J.C.; Lu, Y.; Garcia-Fresco, G.P.; Ching, W.; Martin, M.S.; Li, J.; Einheber, S.; Chesler, M.; Rosenbluth, J.; et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 2001, 30, 369–383. [Google Scholar] [CrossRef]
- Keeley, P.W.; Reese, B.E. DNER and NFIA are expressed by developing and mature AII amacrine cells in the mouse retina. J. Comp. Neurol. 2018, 526, 467–479. [Google Scholar] [CrossRef]
- Murali, S.S.; Napier, I.A.; Mohammadi, S.A.; Alewood, P.F.; Lewis, R.J.; Christie, M.J. High-voltage-activated calcium current subtypes in mouse DRG neurons adapt in a subpopulation-specific manner after nerve injury. J. Neurophysiol. 2015, 113, 1511–1519. [Google Scholar] [CrossRef]
- Butt, A.M.; Vanzulli, I.; Papanikolaou, M.; De La Rocha, I.C.; Hawkins, V.E. Metabotropic glutamate receptors protect oligodendrocytes from acute ischemia in mouse optic nerve. Neurochem. Res. 2017, 42, 2468–2478. [Google Scholar] [CrossRef]
- Simmonds, M.A. Depolarizing responses to glycine, beta-alanine and muscimol in isolated optic nerve and cuneate nucleus. Br. J. Pharmacol. 1983, 79, 799–806. [Google Scholar] [CrossRef]
- Pittock, S.J.; Lucchinetti, C.F.; Parisi, J.E.; Benarroch, E.E.; Mokri, B.; Stephan, C.L.; Kim, K.; Kilimann, M.W.; Lennon, V.A. Amphiphysin autoimmunity: Paraneoplastic accompaniments. Ann. Neurol. 2005, 58, 96–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).