Gut Microbial Targets in Inflammatory Bowel Disease: Current Position and Future Developments
Abstract
1. Introduction
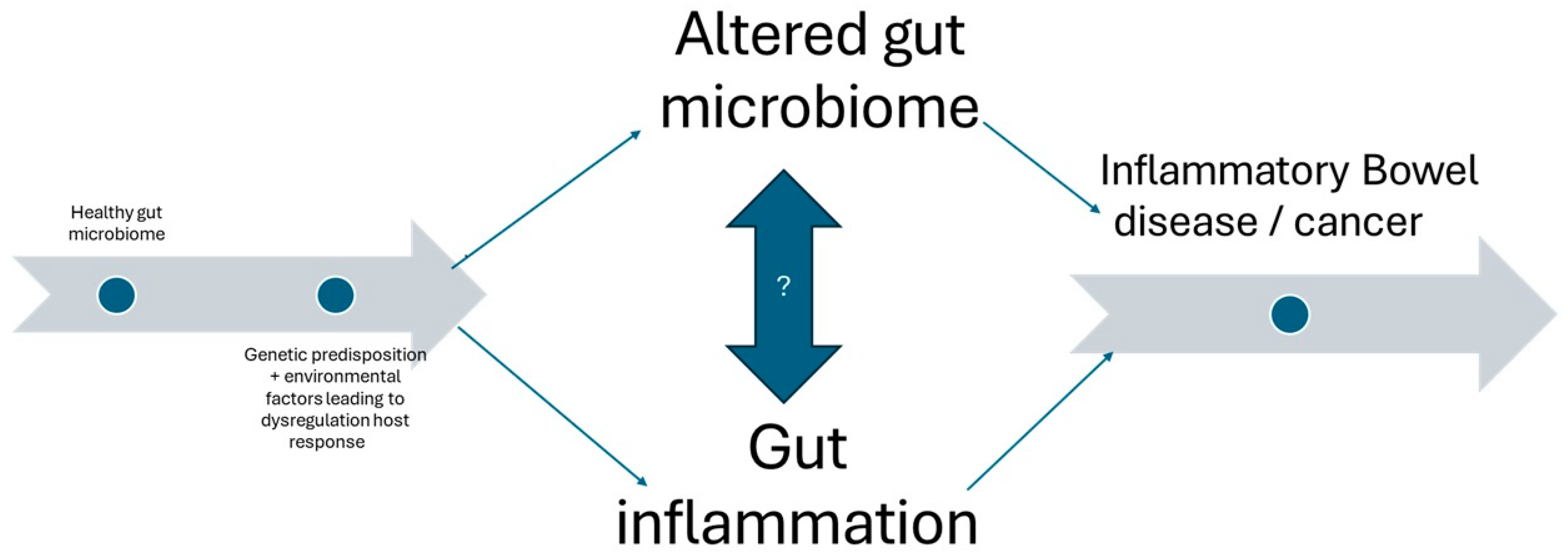
2. Overview of the Gut Microbiome and Its Implications in IBD
Altered Tryptophan Metabolism in IBD as a Biomarker
3. Microbiota and the Role of Short-Chain Fatty Acids (SCFAs) in IBD
4. Prebiotics and Probiotics in IBD Treatment
5. Faecal Microbiota Transplantation
5.1. Microbiota Restoration Therapy (MRT)
5.2. Modification of Host–Microbe Interactions
6. Nanoparticle Gene Delivery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| UC | Ulcerative colitis |
| SCFAs | Short-chain fatty acids |
| FMT | Faecal microbiota transplantation |
| MRT | Microbiota restoration therapy |
| mRNA | Messenger ribonucleic acid |
| mmRNA | Modified messenger ribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. eBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, E969–E983. [Google Scholar] [CrossRef]
- Boppana, K.; E Almansouri, N.; Bakkannavar, S.; Faheem, Y.; Jaiswal, A.; Shergill, K.; Nath, T.S. Alterations in Gut Microbiota as Early Biomarkers for Predicting Inflammatory Bowel Disease Onset and Progression: A Systematic Review. Cureus 2024, 16, e58080. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.; Liu, L.; Zhao, H.; Wang, J.; Huang, W.; Peng, W.; Chen, Y.; et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2022, 8, 818902. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Hang, S.; Yuan, X.; Qian, H.; Zhou, M.; Olovo, C.V.; Zhang, X.; Mao, F. The diagnostic and prognostic potential of gut bacteria in inflammatory bowel disease. Gut Microbes 2023, 15, 2176118. [Google Scholar] [CrossRef]
- Foppa, C.; Rizkala, T.; Repici, A.; Hassan, C.; Spinelli, A. Microbiota and IBD: Current knowledge and future perspectives. Dig. Liver Dis. 2024, 56, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Goudarzi, M.; Singh, N.; Tong, M.; McHardy, I.H.; Ruegger, P.; Asadourian, M.; Moon, B.H.; Ayson, A.; Borneman, J.; et al. A Disease-Associated Microbial and Metabolomics State in Relatives of Pediatric Inflammatory Bowel Disease Patients. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 750–766. [Google Scholar] [CrossRef]
- Forrest, C.M.; Gould, S.R.; Darlington, L.G.; Stone, T.W. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv. Exp. Med. Biol. 2003, 527, 395–400. [Google Scholar]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, E.; Wiest, R.; Scharl, M.; Rogler, G.; Casbas, A.G.; Yilmaz, B.; Wawrzyniak, M.; Francés, R. Dysbiotic microbiota interactions in Crohn’s disease. Gut Microbes 2021, 13, 1949096. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Rau, S.; Gregg, A.; Yaceczko, S.; Berkeley, L.i.m.k.e.t.k.a.i. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Qin, Y.-Q.; Wang, L.-Y.; Yang, X.-Y.; Xu, Y.-J.; Fan, G.; Fan, Y.-G.; Ren, J.-N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef]
- Paudel, D.; Nair, D.V.T.; Joseph, G.; Castro, R.; Tiwari, A.K.; Singh, V. Gastrointestinal microbiota-directed nutritional and therapeutic interventions for inflammatory bowel disease: Opportunities and challenges. Gastroenterol. Rep. 2023, 12, goae033. [Google Scholar] [CrossRef]
- Bretin, A.; Zou, J.; Yeoh, B.S.; Ngo, V.L.; Winer, S.; Winer, D.A.; Reddivari, L.; Pellizzon, M.; Walters, W.A.; Patterson, A.D.; et al. Psyllium Fiber Protects Against Colitis Via Activation of Bile Acid Sensor Farnesoid X Receptor. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Hamideh, M.; Shah, R.; Sauk, J.S.; Jaffe, N. Dietary Patterns and Their Association with Symptoms Activity in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2022, 28, 1627–1636. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Godoy-Brewer, G.; Parian, A.M.; Noorian, S.; Krishna, M.; Shah, N.D.; White, J.; Mullin, G.E. Dietary Interventions for the Treatment of Inflammatory Bowel Diseases: An Updated Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 21, 2508–2525. [Google Scholar] [CrossRef]
- Kaur, L.; Gordon, M.; Baines, P.A.; Iheozor-Ejiofor, Z.; Sinopoulou, V.; Akobeng, A.K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 3, CD005573. [Google Scholar] [PubMed]
- Nguyen, N.; Zhang, B.; Holubar, S.D.; Pardi, D.S.; Singh, S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst. Rev. 2019, 5, CD001176. [Google Scholar] [PubMed]
- Arora, U.; Kedia, S.; Ahuja, V. The practice of fecal microbiota transplantation in inflammatory bowel disease. Intest. Res. 2023, 22, 44–64. [Google Scholar] [CrossRef]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. Systematic review of donor and recipient predictive biomarkers of response to faecal microbiota transplantation in patients with ulcerative colitis. eBioMedicine 2022, 81, 104088. [Google Scholar]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2016, 67, 108–119. [Google Scholar]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Quera, R.; Espinoza, R.; Estay, C.; Rivera, D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J. Crohn’s Colitis 2014, 8, 252–253. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule– vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection. JAMA 2017, 318, 1985. [Google Scholar] [CrossRef]
- Keller, J.J.; Ooijevaar, R.E.; Hvas, C.L.; Terveer, E.M.; Lieberknecht, S.C.; Högenauer, C.; Arkkila, P.; Sokol, H.; Gridnyev, O.; Mégraud, F.; et al. A standardised model for stool banking for faecal microbiota transplantation: A consensus report from a multidisciplinary UEG working group. United Eur. Gastroenterol. J. 2021, 9, 229–247. [Google Scholar] [CrossRef]
- Peng, Z.; Xiang, J.; He, Z.; Zhang, T.; Xu, L.; Cui, B.; Li, P.; Huang, G.; Ji, G.; Nie, Y.; et al. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc. Int. Open 2016, 4, E610–E613. [Google Scholar] [CrossRef]
- Reinisch, W. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Dig. Dis. 2017, 35, 123. [Google Scholar] [CrossRef] [PubMed]
- Cully, M. Microbiome therapeutics go small molecule. Nat. Rev. Drug Discov. 2019, 18, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Sadman Sakib Zou, S. Attenuation of Chronic Inflammation in Intestinal Organoids with Graphene Oxide-Mediated Tumor Necrosis Factor-α_Small Interfering RNA Delivery. Langmuir 2024, 40, 3402–3413. [Google Scholar]
- Veiga, N.; Goldsmith, M.; Granot, Y.; Rosenblum, D.; Dammes, N.; Kedmi, R.; Ramishetti, S.; Peer, D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 2018, 9, 4493. [Google Scholar] [CrossRef]
- Karp, J.M.; Peer, D. Focus on RNA interference: From nanoformulations to in vivo delivery. Nanotechnology 2017, 29, 010201. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.R.; Ramishetti, S.; Peshes-Yaloz, N.; Goldsmith, M.; Wohl, A.; Zibly, Z.; Peer, D. Localized RNAi Therapeutics of Chemoresistant Grade IV Glioma Using Hyaluronan-Grafted Lipid-Based Nanoparticles. ACS Nano 2015, 9, 1581–1591. [Google Scholar] [CrossRef]
- Mizrahy, S.; Hazan-Halevy, I.; Dammes, N.; Landesman-Milo, D.; Peer, D. Current Progress in Non-viral RNAi-Based Delivery Strategies to Lymphocytes. Mol. Ther. 2017, 25, 1491–1500. [Google Scholar] [CrossRef]
- Romano, A.; Mortellaro, A. The New Frontiers of Gene Therapy and Gene Editing in Inflammatory Diseases. Hum. Gene Ther. 2024, 35, 219–231. [Google Scholar] [CrossRef]
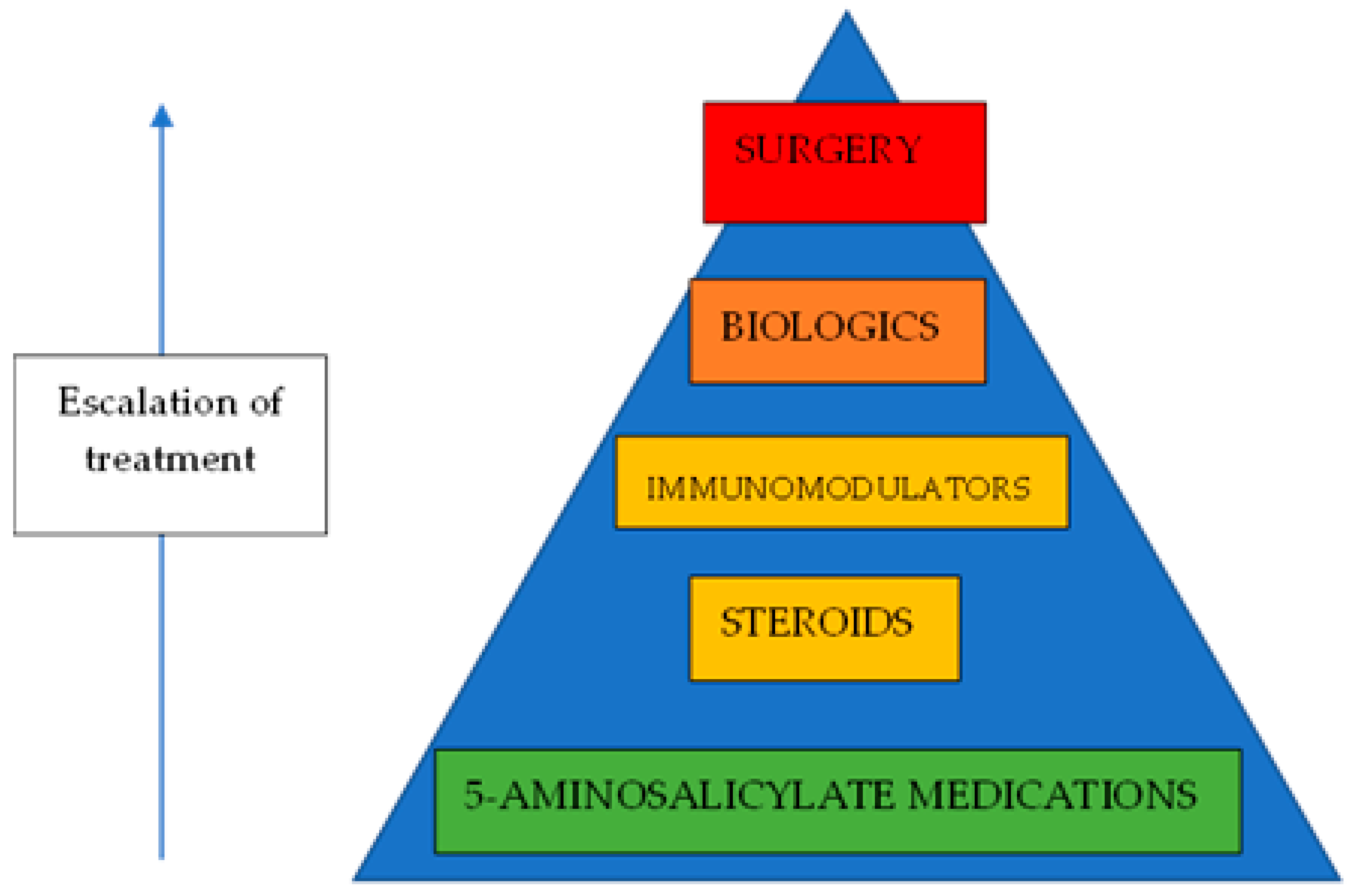
| Probiotics Pros | Prebiotics Pros | Probiotic Limitations | Prebiotic Limitations |
|---|---|---|---|
| Symptom relief | Anti-inflammatory | Strain and condition specific | Worsening of some symptoms |
| Enhancement of immunity | Reverses dysbiosis | Not universally effective | Limited evidence base |
| Variable quality | |||
| Risk in immunocompromised |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumar, N.; Krishnamoorthy, A.; Ryali, H.; Arasaradnam, R.P. Gut Microbial Targets in Inflammatory Bowel Disease: Current Position and Future Developments. Biomedicines 2025, 13, 716. https://doi.org/10.3390/biomedicines13030716
Sivakumar N, Krishnamoorthy A, Ryali H, Arasaradnam RP. Gut Microbial Targets in Inflammatory Bowel Disease: Current Position and Future Developments. Biomedicines. 2025; 13(3):716. https://doi.org/10.3390/biomedicines13030716
Chicago/Turabian StyleSivakumar, Naveen, Ashwin Krishnamoorthy, Harshita Ryali, and Ramesh P. Arasaradnam. 2025. "Gut Microbial Targets in Inflammatory Bowel Disease: Current Position and Future Developments" Biomedicines 13, no. 3: 716. https://doi.org/10.3390/biomedicines13030716
APA StyleSivakumar, N., Krishnamoorthy, A., Ryali, H., & Arasaradnam, R. P. (2025). Gut Microbial Targets in Inflammatory Bowel Disease: Current Position and Future Developments. Biomedicines, 13(3), 716. https://doi.org/10.3390/biomedicines13030716





