Comparative Efficacy of Subcutaneous Versus Intravenous Interleukin 12/23 Inhibitors for the Remission of Moderate to Severe Crohn’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Sources
2.3. Search Terms
2.3.1. Search Terms for Interleukin 12/23 Inhibitors
2.3.2. Search Terms for Crohn’s Disease
2.3.3. Search Terms for Route of Administration
2.4. Procedure for the Selection of Studies
2.5. Eligibility
2.6. Outcomes
2.7. Data Extraction
2.8. Assessing the Quality of Included Studies
2.9. Data Synthesis
2.10. Ethics
3. Results
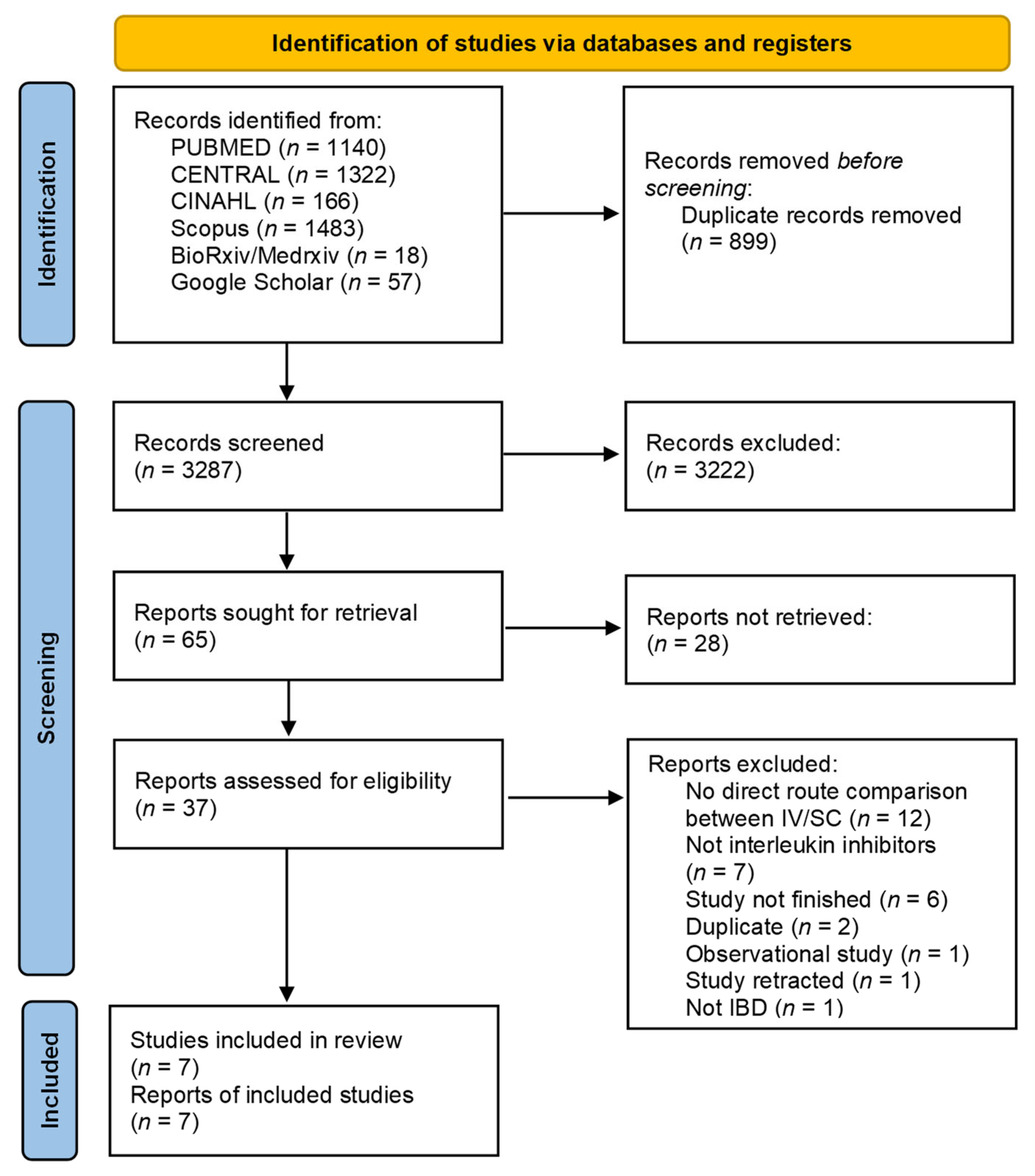
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Assessment of the Quality of Included Studies
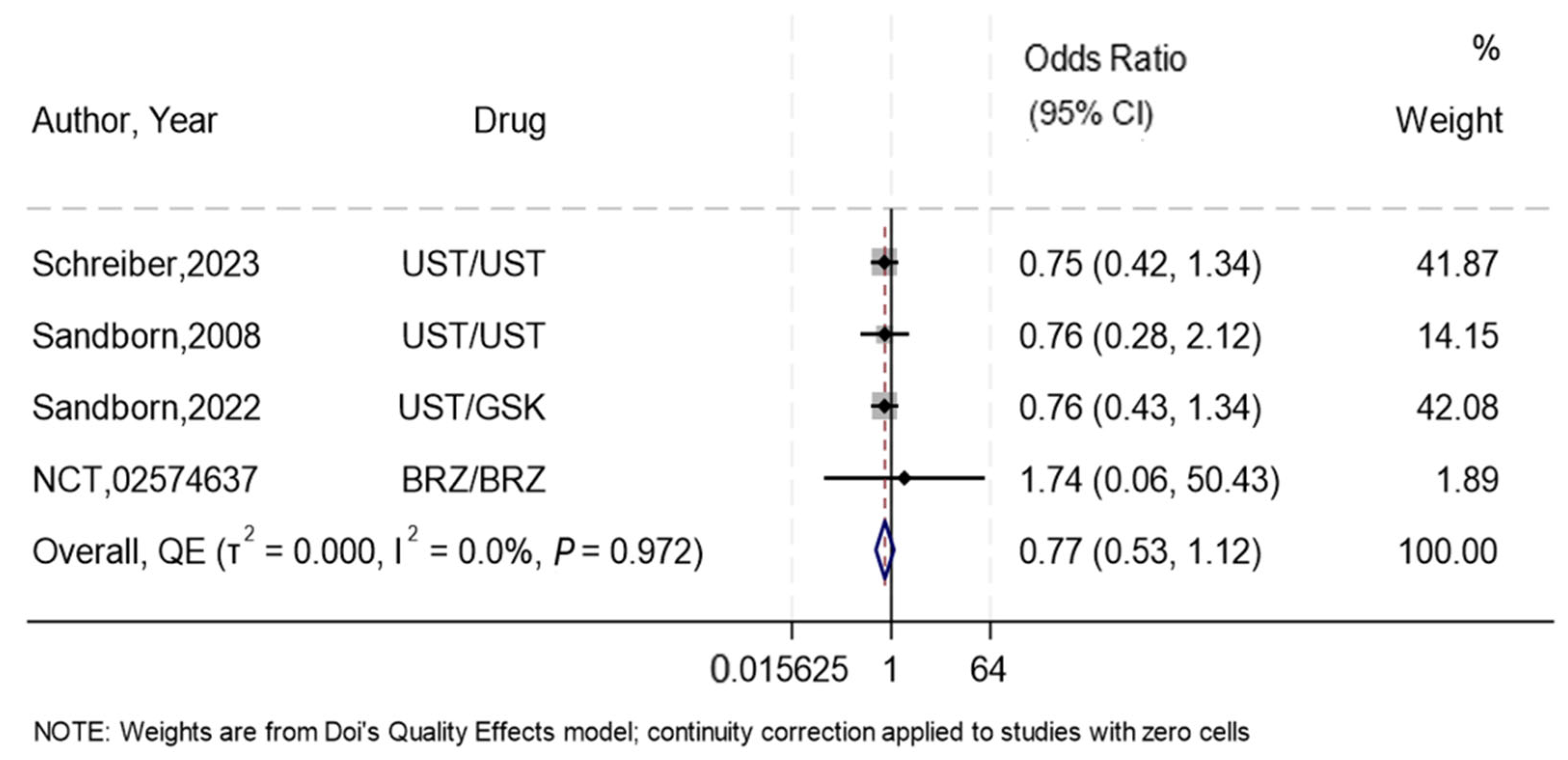
3.4. Induction of Remission
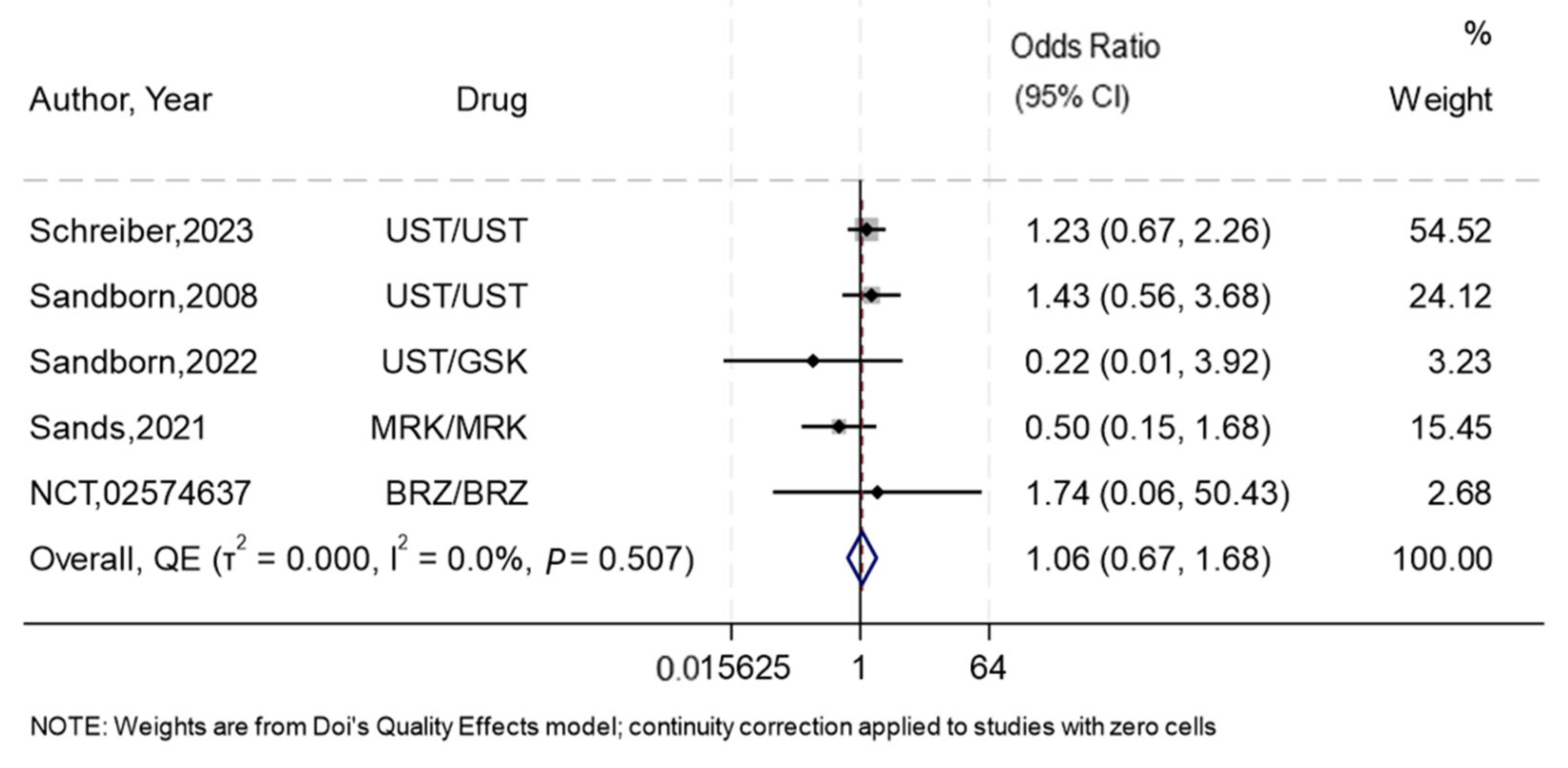
3.5. Maintenance of Remission
3.6. Endoscopic Response and Remission
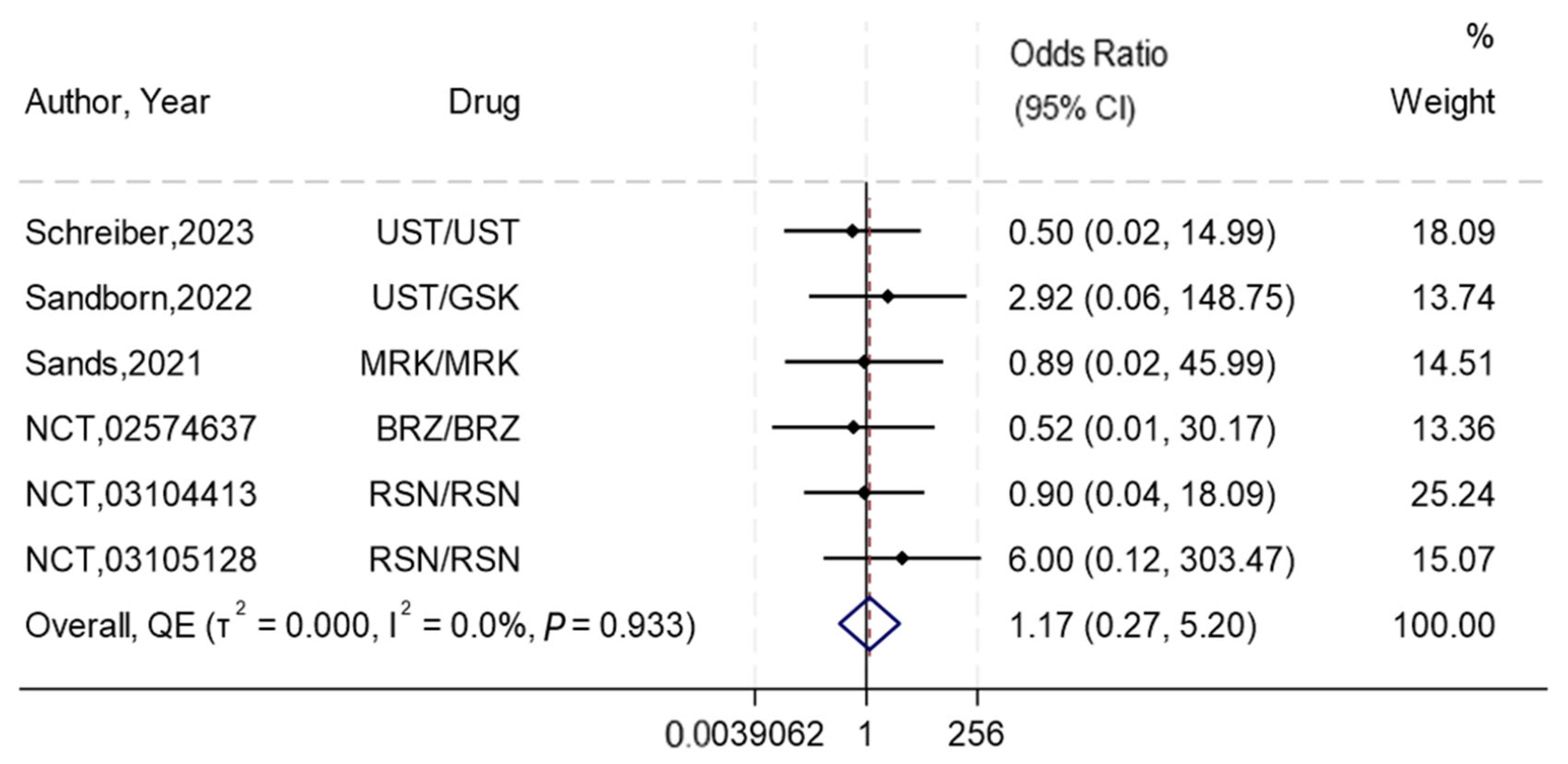
3.7. Any Adverse Events and Serious Adverse Events
3.7.1. Treatment Discontinuation
3.7.2. Hospitalization
3.7.3. Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungaro, R.C.; Aggarwal, S.; Topaloglu, O.; Lee, W.J.; Clark, R.; Colombel, J.F. Systematic review and meta-analysis: Efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 51, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.; Arif, M.; Al-Malki, M.; Hourani, R.; Al-Maadeed, T.; Khodr, N.; Al-Kuwari, G.; Al-Siddiqi, M.; Kane, T.; Chivese, T. The Association Between Inflammatory Bowel Disease and Exposure to Tobacco Smoking: A Case-Control Study in Qatar. Int. J. Gen. Med. 2023, 16, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chivese, T.; Esterhuizen, T.M.; Basson, A.R.; Watermeyer, G. Correction: The Influence of Second-Hand Cigarette Smoke Exposure during Childhood and Active Cigarette Smoking on Crohn’s Disease Phenotype Defined by the Montreal Classification Scheme in a Western Cape Population, South Africa. PLoS ONE 2018, 13, e0190822. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Radford, S.J.; McGing, J.; Czuber-Dochan, W.; Moran, G. Systematic review: The impact of inflammatory bowel disease-related fatigue on health-related quality of life. Frontline Gastroenterol. 2021, 12, 11–21. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Wan, Q.; Zhao, R.; Xia, L.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X.T. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: A meta-analysis of 26 observational studies. J. Cancer Res. Clin. Oncol. 2021, 147, 1077–1087. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 2020, 11, 2040622319899297. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Shi, H.Y.; Tang, W.; Law, C.C.; Sung, J.J.; Chan, F.K.; Ng, S.C. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 1224–1236. [Google Scholar] [CrossRef]
- Parigi, T.L.; D’Amico, F.; Abreu, M.T.; Dignass, A.; Dotan, I.; Magro, F.; Griffiths, A.M.; Jairath, V.; Iacucci, M.; Mantzaris, G.J.; et al. Difficult-to-treat inflammatory bowel disease: Results from an international consensus meeting. Lancet Gastroenterol. Hepatol. 2023, 8, 853–859. [Google Scholar] [CrossRef]
- Breedveld, F.C. Therapeutic monoclonal antibodies. Lancet 2000, 355, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients with Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J. Crohn’s Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef]
- Fanizza, J.; D’Amico, F.; Lusetti, F.; Fasulo, E.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Radice, S.; Peyrin-Biroulet, L.; et al. The Role of IL-23 Inhibitors in Crohn’s Disease. J. Clin. Med. 2023, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.F.; Zhu, L.L.; Chen, M.; Xu, H.M.; Wang, H.F.; Feng, X.Q.; Zhu, X.P.; Zhou, Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942. [Google Scholar] [CrossRef]
- Xu, Z.; Leu, J.H.; Xu, Y.; Nnane, I.; Liva, S.G.; Wang-Lin, S.X.; Kudgus-Lokken, R.; Vermeulen, A.; Ouellet, D. Development of Therapeutic Proteins for a New Subcutaneous Route of Administration After the Establishment of Intravenous Dosages: A Systematic Review. Clin. Pharmacol. Ther. 2023, 113, 1011–1029. [Google Scholar] [CrossRef]
- Ordás, I.; Mould, D.R.; Feagan, B.G.; Sandborn, W.J. Anti-TNF monoclonal antibodies in inflammatory bowel disease: Pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 2012, 91, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Stoner, K.L.; Harder, H.; Fallowfield, L.J.; Jenkins, V.A. Intravenous versus Subcutaneous Drug Administration. Which Do Patients Prefer? A Systematic Review. Patient 2014, 8, 145–153. [Google Scholar] [CrossRef] [PubMed]
- De Cock, E.; Pivot, X.; Hauser, N.; Verma, S.; Kritikou, P.; Millar, D.; Knoop, A. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016, 5, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Ben-Horin, S.; Leszczyszyn, J.; Dudkowiak, R.; Lahat, A.; Gawdis-Wojnarska, B.; Pukitis, A.; Horynski, M.; Farkas, K.; Kierkus, J.; et al. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2340–2353. [Google Scholar] [CrossRef] [PubMed]
- NCT02574637. Evaluation of Efficacy and Safety of Brazikumab (MEDI2070) in Participants with Active, Moderate to Severe Crohn’s Disease. 2021. Available online: https://clinicaltrials.gov/study/NCT02574637 (accessed on 3 February 2024).
- NCT03104413. A Study to Assess the Efficacy and Safety of Risankizumab in Participants with Moderately to Severely Active Crohn’s Disease Who Failed Prior Biologic Treatment. 2022. Available online: https://clinicaltrials.gov/study/NCT03104413 (accessed on 28 February 2024).
- NCT03105128. A Study of the Efficacy and Safety of Risankizumab in Participants with Moderately to Severely Active Crohn’s Disease. 2022. Available online: https://clinicaltrials.gov/study/NCT03105128 (accessed on 28 February 2024).
- Schreiber, S.W.; Lee, S.; van der Woude, C.J.; Marín-Jiménez, I.; Wolf, D.; Schnoy, E.; Salzberg, B.; Busse, C.; Nazar, M.; Langholff, W.; et al. P436 Efficacy and safety of intravenous ustekinumab re-induction therapy in Crohn’s disease patients with secondary loss of response to ustekinumab maintenance therapy: Week 16 results from the POWER trial. J. Crohn’s Colitis 2023, 17 (Suppl. S1), i564–i566. [Google Scholar] [CrossRef]
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results From the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e8. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Fedorak, R.N.; Scherl, E.; Fleisher, M.R.; Katz, S.; Johanns, J.; Blank, M.; Rutgeerts, P. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008, 135, 1130–1141. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Kierkus, J.; Higgins, P.D.R.; Fischer, M.; Jairath, V.; Hirai, F.; D’Haens, G.; Belin, R.M.; Miller, D.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients with Crohn’s Disease. Gastroenterology 2021, 162, 495–508. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Vuitton, L.; Marteau, P.; Sandborn, W.J.; Levesque, B.G.; Feagan, B.; Vermeire, S.; Danese, S.; D’Haens, G.; Lowenberg, M.; Khanna, R.; et al. IOIBD technical review on endoscopic indices for Crohn’s disease clinical trials. Gut 2016, 65, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.C.; Glass, K.; Clark, J.; Ritskes-Hoitinga, M.; Munn, Z.; Tugwell, P.; Doi, S.A.R. The MethodologicAl STandards for Epidemiological Research (MASTER) scale demonstrated a unified framework for bias assessment. J. Clin. Epidemiol. 2021, 134, 52–64. [Google Scholar] [CrossRef]
- Doi, S.A.; Thalib, L. A quality-effects model for meta-analysis. Epidemiology 2008, 19, 94–100. [Google Scholar] [CrossRef]
- Doi, S.A.; Furuya-Kanamori, L.; Xu, C.; Lin, L.; Chivese, T.; Thalib, L. Controversy and Debate: Questionable utility of the relative risk in clinical research: Paper 1: A call for change to practice. J. Clin. Epidemiol. 2022, 142, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.C.; Glass, K.; Munn, Z.; Tugwell, P.; Doi, S.A.R. Comparison of bias adjustment methods in meta-analysis suggests that quality effects modeling may have less limitations than other approaches. J. Clin. Epidemiol. 2020, 117, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); John Wiley & Sons: Chichester, UK, 2019; Available online: www.training.cochrane.org/handbook (accessed on 18 June 2023).
- Thompson, S.G. Systematic Review: Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994, 309, 1351. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Maida, M.; Ventimiglia, M.; Cottone, M.; Orlando, A. Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: A meta-analysis of observational studies. Expert. Opin. Biol. Ther. 2020, 20, 193–203. [Google Scholar] [CrossRef]
- Yang, H.H.; Huang, Y.; Zhou, X.C.; Wang, R.N. Efficacy and safety of adalimumab in comparison to infliximab for Crohn’s disease: A systematic review and meta-analysis. World J. Clin. Cases 2022, 10, 6091–6104. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Bossuyt, P.; Bettenworth, D.; Loftus, E.V., Jr.; Anjie, S.I.; D’Haens, G.; Saruta, M.; Arkkila, P.; Park, H.; Choi, D.; et al. Comparative Efficacy of Subcutaneous and Intravenous Infliximab and Vedolizumab for Maintenance Treatment of TNF-naive Adult Patients with Inflammatory Bowel Disease: A Systematic Literature Review and Network Meta-analysis. Dig. Dis. Sci. 2024, 69, 1808–1825. [Google Scholar] [CrossRef]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as induction therapy for Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 2022, 399, 2015–2030. [Google Scholar] [CrossRef] [PubMed]
- Overton, P.M.; Shalet, N.; Somers, F.; Allen, J.A. Patient Preferences for Subcutaneous versus Intravenous Administration of Treatment for Chronic Immune System Disorders: A Systematic Review. Patient Prefer. Adherence 2021, 15, 811–834. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Type of IBD | Specific Biologic | SC/IV Sample Size | Phase of Remission | Follow-Up Period (Months) | Mechanism of Action |
|---|---|---|---|---|---|---|
| Schreiber, 2023 [28] (NCT,03782376) | Crohn’s Disease | SC: Ustekinumab: 90 mg IV: Ustekinumab: 6 mg/kg | SC:107 IV:108 | Induction | 8 | IL-12/IL-23 Inhibitor |
| Sandborn, 2008 [30] (NCT,00265122) | Crohn’s Disease | SC: Ustekinumab: 90 mg IV: Ustekinumab: 4.5 mg/kg | SC:65 IV:66 | Induction and Maintenance | 5.22 | IL-12/IL-23 Inhibitor |
| Sandborn, 2022 [29] (NCT,03466411) | Crohn’s Disease | SC: Ustekinumab: 90 mg IV: Guselkumab: 200 mg, 600 mg, 1200 mg | SC:63 IV:185 | Induction | 2.78 | IL-12/IL-23 Inhibitor |
| Sands, 2021 [31] (NCT,02891226) | Crohn’s Disease | SC: Mirikizumab: 300 mg IV: Mirikizumab: 1000 mg | SC:46 IV:41 | Maintenance | 12 | IL-23 Inhibitor |
| NCT,02574637 [25] | Crohn’s Disease | SC: Brazikumab: 35, 70, 105, 210 mg IV: Brazikumab: 700 mg | SC:10 IV:5 | Induction | 6.4 | IL-23 Inhibitor |
| NCT,03104413 [26] | Crohn’s Disease | SC: Risankizumab: 180, 360 mg IV: Risankizumab: 1200 mg | SC: 83 IV: 453 | Adverse events | 10.1 | IL-23 Inhibitor |
| NCT,03105128 [27] | Crohn’s Disease | SC: Risankizumab: 180, 360 mg IV: Risankizumab: 1200 mg | SC:135 IV:812 | Adverse events | 10.1 | IL-23 Inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwisi, N.; Ismail, R.; Al-Kuwari, H.; Al-Ansari, K.H.; Al-Matwi, M.A.; Aweer, N.A.; Al-Marri, W.N.; Al-Kubaisi, Y.; Al-Mohannadi, M.; Hamran, S.; et al. Comparative Efficacy of Subcutaneous Versus Intravenous Interleukin 12/23 Inhibitors for the Remission of Moderate to Severe Crohn’s Disease: A Systematic Review and Meta-Analysis. Biomedicines 2025, 13, 702. https://doi.org/10.3390/biomedicines13030702
Alwisi N, Ismail R, Al-Kuwari H, Al-Ansari KH, Al-Matwi MA, Aweer NA, Al-Marri WN, Al-Kubaisi Y, Al-Mohannadi M, Hamran S, et al. Comparative Efficacy of Subcutaneous Versus Intravenous Interleukin 12/23 Inhibitors for the Remission of Moderate to Severe Crohn’s Disease: A Systematic Review and Meta-Analysis. Biomedicines. 2025; 13(3):702. https://doi.org/10.3390/biomedicines13030702
Chicago/Turabian StyleAlwisi, Nouran, Rana Ismail, Hissa Al-Kuwari, Khalifa H. Al-Ansari, Mohammed A. Al-Matwi, Noor A. Aweer, Wejdan N. Al-Marri, Yousif Al-Kubaisi, Muneera Al-Mohannadi, Shahd Hamran, and et al. 2025. "Comparative Efficacy of Subcutaneous Versus Intravenous Interleukin 12/23 Inhibitors for the Remission of Moderate to Severe Crohn’s Disease: A Systematic Review and Meta-Analysis" Biomedicines 13, no. 3: 702. https://doi.org/10.3390/biomedicines13030702
APA StyleAlwisi, N., Ismail, R., Al-Kuwari, H., Al-Ansari, K. H., Al-Matwi, M. A., Aweer, N. A., Al-Marri, W. N., Al-Kubaisi, Y., Al-Mohannadi, M., Hamran, S., Doi, S. A. R., Farooqui, H. H., & Chivese, T. (2025). Comparative Efficacy of Subcutaneous Versus Intravenous Interleukin 12/23 Inhibitors for the Remission of Moderate to Severe Crohn’s Disease: A Systematic Review and Meta-Analysis. Biomedicines, 13(3), 702. https://doi.org/10.3390/biomedicines13030702





