Association of Monoamine Oxidase A Gene Promoter Region (30 bp μVNTR) Polymorphism with Serum Levels in Multiple Psychiatric Disorders
Abstract
1. Introduction
2. Materials and Methodology
2.1. Ethical Approval
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Subjects Recruitment and Blood Sample Collection
2.5. Genotyping
2.6. MAOA Serum Level Assessment
2.7. Statistical Analysis
3. Results
3.1. Genetic Association of MAOA-30 bp µVNTR
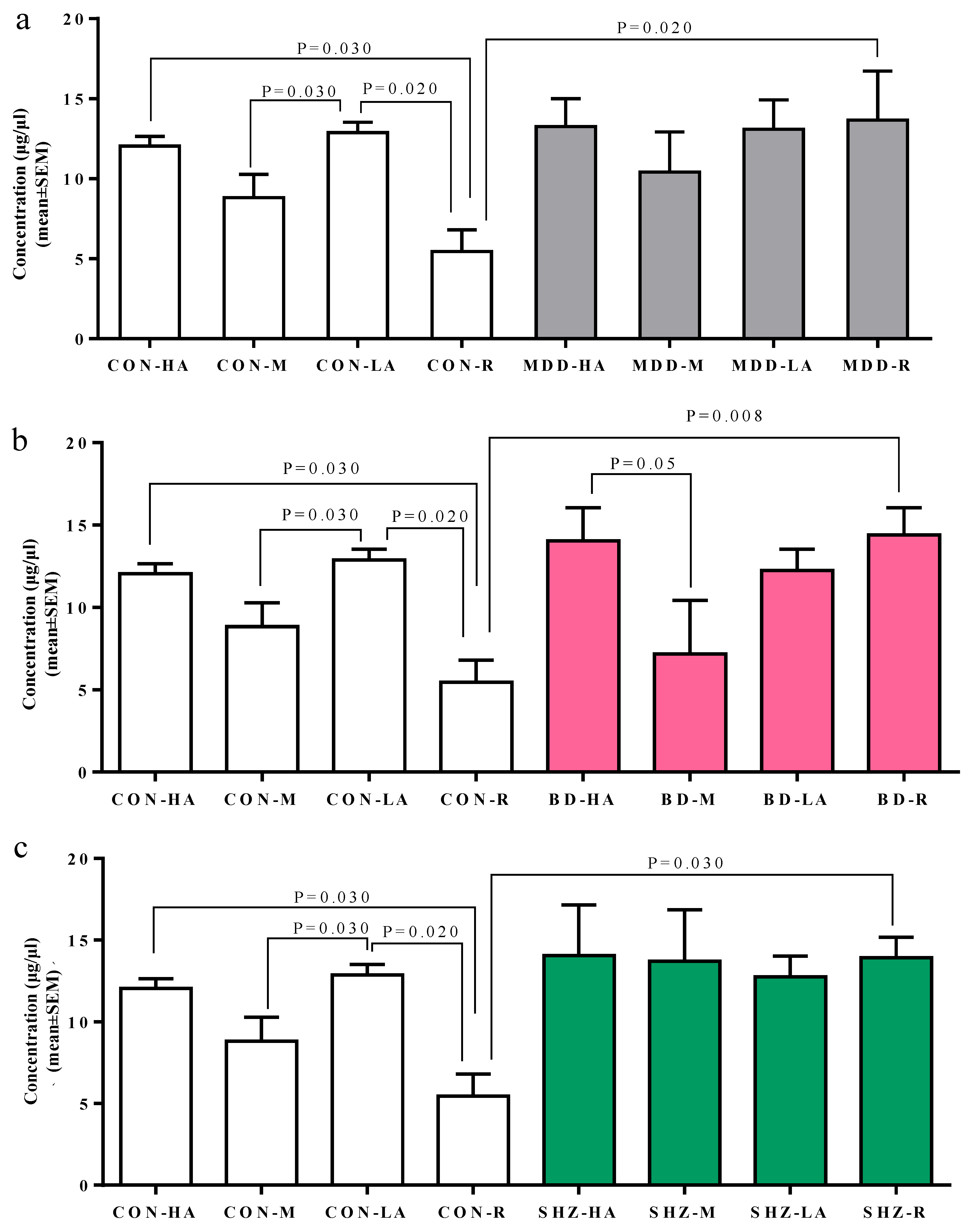
3.2. Serum-Based Analysis of MAOA Protein Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, L.; Faludi, G.; Palkovits, M.; Sotonyi, P.; Bakish, D.; Hrdina, P.D. High activity-related allele of MAO-A gene associated with depressed suicide in males. Neuroreport 2002, 13, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, L.; Luo, X.-J.; Wu, L.; Li, M. MAOA Variants and Genetic Susceptibility to Major Psychiatric Disorders. Mol. Neurobiol. 2016, 53, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ming, Q.; Zhong, X.; Dong, D.; Li, C.; Xiong, G.; Cheng, C.; Cao, W.; He, J.; Wang, X.; et al. The MAOA Gene Influences the Neural Response to Psychosocial Stress in the Human Brain. Front. Behav. Neurosci. 2020, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Sherif, F.; Marcusson, J.; Oreland, L. Brain gamma-aminobutyrate transaminase and monoamine oxidase activities in suicide victims. Eur. Arch. Psychiatry Clin. Neurosci. 1991, 241, 139–144. [Google Scholar] [CrossRef]
- Byrd, A.L.; Manuck, S.B. MAOA, childhood maltreatment, and antisocial behavior: Meta-analysis of a gene-environment interaction. Biol. Psychiatry 2014, 75, 9–17. [Google Scholar] [CrossRef]
- Preisig, M.; Bellivier, F.; Fenton, B.T.; Baud, P.; Berney, A.; Courtet, P.; Hardy, P.; Golaz, J.; Leboyer, M.; Mallet, J.; et al. Association between bipolar disorder and monoarnine oxidase a gene polymorphisms: Results of a multicenter study. Am. J. Psychiatry 2000, 157, 948–955. [Google Scholar] [CrossRef]
- Jacob, C.P.; Müller, J.; Schmidt, M.; Hohenberger, K.; Gutknecht, L.; Reif, A.; Schmidtke, A.; Mössner, R.; Lesch, K.P. Cluster B personality disorders are associated with allelic variation of monoamine oxidase A activity. Neuropsychopharmacology 2005, 30, 1711–1718. [Google Scholar] [CrossRef]
- Culej, J.; Gabaj, N.N.; Štefanović, M.; Karlović, D. Prediction of schizophrenia using MAOA-uVNTR polymorphism: A case-control study. Indian J. Psychiatry 2020, 62, 80–86. [Google Scholar] [CrossRef]
- Schulze, T.G.; Müller, D.J.; Krauss, H.; Scherk, H.; Ohlraun, S.; Syagailo, Y.V.; Windemuth, C.; Neidt, H.; Grässle, M.; Papassotiropoulos, A.; et al. Association between a functional polymorphism in the monoamine oxidase A gene promoter and major depressive disorder. Am. J. Med. Genet. Neuropsychiatr. Genet. 2000, 96, 801–803. [Google Scholar] [CrossRef]
- Manuck, S.B.; Flory, J.D.; Ferrell, R.E.; Mann, J.J.; Muldoon, M.F. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000, 95, 9–23. [Google Scholar] [CrossRef]
- Kim-Cohen, J.; Caspi, A.; Taylor, A.; Williams, B.; Newcombe, R.; Craig, I.W.; Moffitt, T.E. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol. Psychiatry 2006, 11, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Ou, X.M.; Roettger, M.; Shih, J.C. The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: Associations and MAOA promoter activity. Eur. J. Hum. Genet. 2008, 16, 624–634. [Google Scholar] [CrossRef]
- Voltas, N.; Aparicio, E.; Arija, V.; Canals, J. Association study of monoamine oxidase-A gene promoter polymorphism (MAOA-uVNTR) with self-reported anxiety and other psychopathological symptoms in a community sample of early adolescents. J. Anxiety Disord. 2015, 31, 65–72. [Google Scholar] [CrossRef]
- Brunner, H.G.; Nelen, M.; Breakefield, X.O.; Ropers, H.H.; Van Oost, B.A. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 1993, 262, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Chester, D.S.; DeWall, C.N.; Derefinko, K.J.; Estus, S.; Peters, J.R.; Lynam, D.R.; Jiang, Y. Monoamine oxidase A (MAOA) genotype predicts greater aggression through impulsive reactivity to negative affect. Behav. Brain Res. 2015, 283, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.W.; Sjöberg, R.L.; Damberg, M.; Leppert, J.; Öhrvik, J.; Alm, P.O.; Lindström, L.; Oreland, L. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol. Psychiatry 2006, 59, 121–127. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases, Tenth Revision (ICD-10); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular cloning: A laboratory manual (3-volume set). In Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2001. [Google Scholar]
- R Core Team. The R Project for Statistical Computing; R Core TeamVienna, Austria. 2020. Available online: https://www.r-project.org/ (accessed on 17 December 2024).
- Kuepper, Y.; Grant, P.; Wielpuetz, C.; Hennig, J. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behav. Brain Res. 2013, 247, 73–78. [Google Scholar] [CrossRef]
- Al-Tayie, S.R.; Ali, A.A. Allelic diversity of VNTR polymorphism in monoamine oxidase a (MAOA) gene in iraqi population. J. Pharm. Sci. Res. 2018, 10, 3099–3102. [Google Scholar]
- Müller, D.J.; Serretti, A.; Sicard, T.; Tharmalingain, S.; King, N.; Artioli, P.; Mandelli, L.; Lorenzi, C.; Kennedy, J.L. Further evidence of MAO-A gene variants associated with bipolar disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144, 37–40. [Google Scholar] [CrossRef]
- Norton, N.; Kirov, G.; Zammit, S.; Jones, G.; Jones, S.; Owen, R.; Krawczak, M.; Williams, N.M.; O’Donovan, M.C.; Owen, M.J. Schizophrenia and functional polymorphisms in the MAOA and COMT genes: No evidence for association or epistasis. Am. J. Med. Genet. Neuropsychiatr. Genet. 2002, 114, 491–496. [Google Scholar] [CrossRef]
- Lawson, D.C.; Turic, D.; Langley, K.; Pay, H.M.; Govan, C.F.; Norton, N.; Hamshere, M.L.; Owen, M.J.; O’Donovan, M.C.; Thapar, A. Association analysis of monoamine oxidase A and attention deficit hyperactivity disorder. Am. J. Med. Genet. Neuropsychiatr. Genet. 2003, 116B, 84–89. [Google Scholar] [CrossRef]
- Manor, I.; Tyano, S.; Mel, E.; Eisenberg, J.; Bachner-Melman, R.; Kotler, M.; Ebstein, R.P. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): Preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA). Mol. Psychiatry 2002, 7, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Domschke, K.; Sheehan, K.; Lowe, N.; Kirley, A.; Mullins, C.; O’sullivan, R.; Freitag, C.; Becker, T.; Conroy, J.; Fitzgerald, M.; et al. Association analysis of the monoamine oxidase A and B genes with attention deficit hyperactivity disorder (ADHD) in an Irish sample: Preferential transmission of the MAO-A 941G allele to affected children. Am. J. Med. Genet.—Neuropsychiatr. Genet. 2005, 134B, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Brookes, K.; Chen, C.K.; Huang, Y.S.; Wu, Y.Y.; Asherson, P. Association study between the monoamine oxidase A gene and attention deficit hyperactivity disorder in Taiwanese samples. BMC Psychiatry 2007, 7, 10. [Google Scholar] [CrossRef]
- Sabol, S.Z.; Hu, S.; Hamer, D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum. Genet. 1998, 103, 273–279. [Google Scholar] [CrossRef]
- Denney, R.M.; Koch, H.; Craig, I.W. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum. Genet. 1999, 105, 542–551. [Google Scholar] [CrossRef]
- Deckert, J.; Catalano, M.; Syagailo, Y.V.; Bosi, M.; Okladnova, O.; Di Bella, D.; Nöthen, M.M.; Maffei, P.; Franke, P.; Fritze, J.; et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum. Mol. Genet. 1999, 8, 621–624. [Google Scholar] [CrossRef]
- Manca, M.; Pessoa, V.; Lopez, A.I.; Harrison, P.T.; Miyajima, F.; Sharp, H.; Pickles, A.; Hill, J.; Murgatroyd, C.; Bubb, V.J.; et al. The Regulation of Monoamine Oxidase A Gene Expression by Distinct Variable Number Tandem Repeats. J. Mol. Neurosci. 2018, 64, 459–470. [Google Scholar] [CrossRef]
- Lung, F.W.; Tzeng, D.S.; Huang, M.F.; Lee, M.B. Association of the maoa promoter uvntr polymorphism with suicide attempts in patients with major depressive disorder. BMC Med. Genet. 2011, 12, 74. [Google Scholar] [CrossRef]
- Edwards, A.C.; Dodge, K.A.; Latendresse, S.J.; Lansford, J.E.; Bates, J.E.; Pettit, G.S.; Budde, J.P.; Goate, A.M.; Dick, D.M. MAOA-uVNTR and early physical discipline interact to influence delinquent behavior. J. Child Psychol. Psychiatry Allied Discip. 2010, 51, 679–687. [Google Scholar] [CrossRef]
- Reif, A.; Richter, J.; Straube, B.; Höfler, M.; Lueken, U.; Gloster, A.T.; Weber, H.; Domschke, K.; Fehm, L.; Ströhle, A.; et al. MAOA and mechanisms of panic disorder revisited: From bench to molecular psychotherapy. Mol. Psychiatry 2014, 19, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Philibert, R.A.; Gunter, T.D.; Beach SR, H.; Brody, G.H.; Madan, A. Rapid publication: MAOA methylation is associated with nicotine and alcohol dependence in women. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Wei, Y.; Wong CC, Y.; Sjöholm, L.K.; Åberg, E.; Mill, J.; Schalling, M.; Forsell, Y.; Lavebratt, C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int. J. Neuropsychopharmacol. 2013, 16, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Frazzetto, G.; Di Lorenzo, G.; Carola, V.; Proietti, L.; Sokolowska, E.; Siracusano, A.; Gross, C.; Troisi, A. Early trauma and increased risk for physical aggression during adulthood: The moderating role of MAOA genotype. PLoS ONE 2007, 2, e486. [Google Scholar] [CrossRef]
- Fan, J.B.; Sklar, P. Meta-analysis reveals association between serotonin transporter gene STin2 VNTR polymorphism and schizophrenia. Mol. Psychiatry 2005, 10, 928–938. [Google Scholar] [CrossRef]
- Ho, L.W.; Furlong, R.A.; Rubinsztein, J.S.; Walsh, C.; Paykel, E.S.; Rubinsztein, D.C. Genetic associations with clinical characteristics in bipolar affective disorder and recurrent unipolar depressive disorder. Am. J. Med. Genet. Neuropsychiatr. Genet. 2000, 96, 36–42. [Google Scholar] [CrossRef]

| Demographic Factors | CON | MDD | BD | SHZ | MDD vs. CON p | BD vs. CON p | SHZ vs. CON p | |
|---|---|---|---|---|---|---|---|---|
| Total (n) | 355 | 428 | 206 | 131 | NA | NA | NA | |
| 1 | Age (years) | 24.06 ± 6.11 | 32.93 ± 11.61 | 30.20 ± 11.22 | 29.68 ± 10.01 | <2.2 × 10−16 | 4.15 × 10 12 | 1.03 × 10−8 |
| 2 | Age at onset (years) | NA | 29.10 ± 11.12 | 23.57 ± 9.72 | 23.32 ± 8.87 | NA | NA | NA |
| 3 | Sex (male) (female) | 204 (57.0%) 151 (43.0%) | 165 (39.0%) 263 (61.0%) | 122 (59.0%) 84 (41.0%) | 93 (71.0%) 38 (29.0%) | 1.11 × 10−7 * | 0.68 * | 0.005 * |
| 4 | Ethnicity (Punjabi) | 245 (69%) | 288 (67.30%) | 155 (75.00%) | 103 (79.00%) | 0.61 * | 0.11 * | 0.03 * |
| 5 | Tobacco users | 22 (6.20%) | 70 (16.35%) | 56 (27.18%) | 45 (34.35%) | 4.64 × 10−6 * | 1.60 × 10−9 * | 1.27 × 10−9 * |
| 6 | Cannabis abusers | 0 | 15 (3.50%) | 18 (8.74%) | 11 (8.40%) | NA | NA | NA |
| Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAOA-30 bp VNTR (rs1346551029) | Univariate Analysis | Multivariate Analysis * | ||||||||
| Phenotype | MDD vs. CON | BD vs. CON | SHZ vs. CON | MDD vs. CON | BD vs. CON | SHZ vs. CON | ||||
| Genotype | Control n = 151 | MDD n = 263 | BD n = 84 | SHZ n = 40 | OR (95% CI) p | OR (95% CI) p | OR (95% CI) p | OR (95% CI) p * | OR (95% CI) p * | OR (95% CI) p * |
| HA | 55 (36.42%) | 76 (28.90%) | 26 (30.95%) | 9 (22.5%) | 1.05 (0.98–1.12) 0.16 | 1.05 (0.96–1.1) 0.30 | 0.30 (0.98–1.15) 0.14 | 1.02 (0.96–1.08) 0.60 | 1.02 (0.94–1.11) 0.56 | 1.04 (0.96–1.13) 0.32 |
| HA/LA | 69 (45.70%) | 133 (50.57%) | 39 (46.43%) | 22 (55.0%) | ||||||
| LA | 27 (17.88%) | 54 (20.53%) | 19 (22.62%) | 9 (22.5%) | ||||||
| Males | ||||||||||
| MAOA-30 bp VNTR (rs1346551029) | Univariate Analysis | Multivariate Analysis * | ||||||||
| Phenotype | MDD vs. CON | BD vs. CON | SHZ vs. CON | MDD vs. CON | BD vs. CON | SHZ vs. CON | ||||
| Genotype | Control n = 198 | MDD n = 153 | BD n = 116 | SHZ n = 77 | OR (95% CI) p | OR (95% CI) p | OR (95% CI) p | OR (95% CI) p * | OR (95% CI) p * | OR (95% CI) p * |
| HA | 114 (57.58%) | 83 (54.25%) | 68 (58.62%) | 45 (58.44%) | 1.03 (0.93–1.15) 0.53 | 0.99 (0.89–1.10) 0.86 | 0.99 (0.89–1.10) 0.90 | 1.04 (0.95–1.15) 0.37 | 0.98 (0.88–1.08) 0.68 | 1.01 (0.91–1.12) 0.84 |
| LA | 84 (42.42%) | 70 (45.75%) | 48 (41.38%) | 32 (41.56%) | ||||||
| Subjects | CON | MDD | BD | SHZ | MDD vs. CON | BD vs. CON | SHZ vs. CON |
|---|---|---|---|---|---|---|---|
| n | 22 | 22 | 22 | 22 | NA | NA | NA |
| Sex (male) (female) | 8 | 8 | 11 | 13 | 1.00 | 0.54 | 0.23 |
| Age (years) ** (Mean ± SD) | 22.10 ± 2.40 | 30.04 ± 7.58 | 31.59 ± 12.4 | 34.54 ± 10.26 | 8.196 × 10−5 | 0.002 | 1.166 × 10−5 |
| Age of onset (years) (Mean ± SD) | - | 26.84 ± 8.29 | 24.41 ± 11.10 | 25.18 ± 9.47 | - | - | - |
| Tobacco users | 2 (9.09%) | 4 (18.18%) | 6 (27.27%) | 8 (36.36%) | 0.66 | 0.241 | 0.07 |
| Suicidal | - | 19 (86.36%) | 18 (81.81%) | 15 (68.18%) | - | - | - |
| Aggression | - | 15 (68.18%) | 15 (68.18%) | 8 (36.36%) | - | - | - |
| Insomnia | - | 13 (59.09%) | 10 (45.45%) | 8 (36.36%) | - | - | - |
| Ethnicity (Punjabis) * | 11 (50.0%) | 11 (50.0%) | 15 (68.18%) | 18 (81.81%) | 1.00 | 0.36 | 0.06 |
| Total serum MAOA # levels ng/µL (Mean ± SD) | 8.78 ± 4.12 | 11.2 ± 6.12 | 11.8 ± 4.92 | 13.5 ± 4.89 | 0.159 | 0.061 | 0.004 |
| Comparative Analysis | p-Value |
|---|---|
| CON-HA vs. CON-LA | 0.48 |
| CON-HA vs. CON-M | 0.09 |
| CON-HA vs. CON-R | 0.03 |
| CON-LA vs. CON-M | 0.03 |
| CON-LA vs. CON-R | 0.02 |
| CON-M vs. CON-R | 0.28 |
| MDD-HA vs. MDD-LA | 0.85 |
| MDD-HA vs. MDD-M | 0.55 |
| MDD-HA vs. MDD-R | 0.83 |
| MDD-LA vs. MDD-M | 0.57 |
| MDD-LA vs. MDD-R | 0.76 |
| MDD-M vs. MDD-R | 0.51 |
| BD-HA vs. BD-LA | 0.43 |
| BD-HA vs. BD-M | 0.05 |
| BD-HA vs. BD-R | 0.94 |
| BD-LA vs. BD-M | 0.22 |
| BD-LA vs. BD-R | 0.52 |
| BD-M vs. BD-R | 0.19 |
| SHZ-HA vs. SHZ-LA | 0.43 |
| SHZ-HA vs. SHZ-M | 0.50 |
| SHZ-HA vs. SHZ-R | 0.68 |
| SHZ-LA vs. SHZ-M | 0.77 |
| SHZ-LA vs. SHZ-R | 0.49 |
| SHZ-M vs. SHZ-R | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashmi, A.N.; Taj, R.; Agha, Z.; Qamar, R.; Williams, J.B.; Azam, M. Association of Monoamine Oxidase A Gene Promoter Region (30 bp μVNTR) Polymorphism with Serum Levels in Multiple Psychiatric Disorders. Biomedicines 2025, 13, 698. https://doi.org/10.3390/biomedicines13030698
Hashmi AN, Taj R, Agha Z, Qamar R, Williams JB, Azam M. Association of Monoamine Oxidase A Gene Promoter Region (30 bp μVNTR) Polymorphism with Serum Levels in Multiple Psychiatric Disorders. Biomedicines. 2025; 13(3):698. https://doi.org/10.3390/biomedicines13030698
Chicago/Turabian StyleHashmi, Aisha Nasir, Rizwan Taj, Zehra Agha, Raheel Qamar, Jamal B. Williams, and Maleeha Azam. 2025. "Association of Monoamine Oxidase A Gene Promoter Region (30 bp μVNTR) Polymorphism with Serum Levels in Multiple Psychiatric Disorders" Biomedicines 13, no. 3: 698. https://doi.org/10.3390/biomedicines13030698
APA StyleHashmi, A. N., Taj, R., Agha, Z., Qamar, R., Williams, J. B., & Azam, M. (2025). Association of Monoamine Oxidase A Gene Promoter Region (30 bp μVNTR) Polymorphism with Serum Levels in Multiple Psychiatric Disorders. Biomedicines, 13(3), 698. https://doi.org/10.3390/biomedicines13030698





