Mitochondrial Permeability Transition Pore: The Cardiovascular Disease’s Molecular Achilles Heel
Abstract
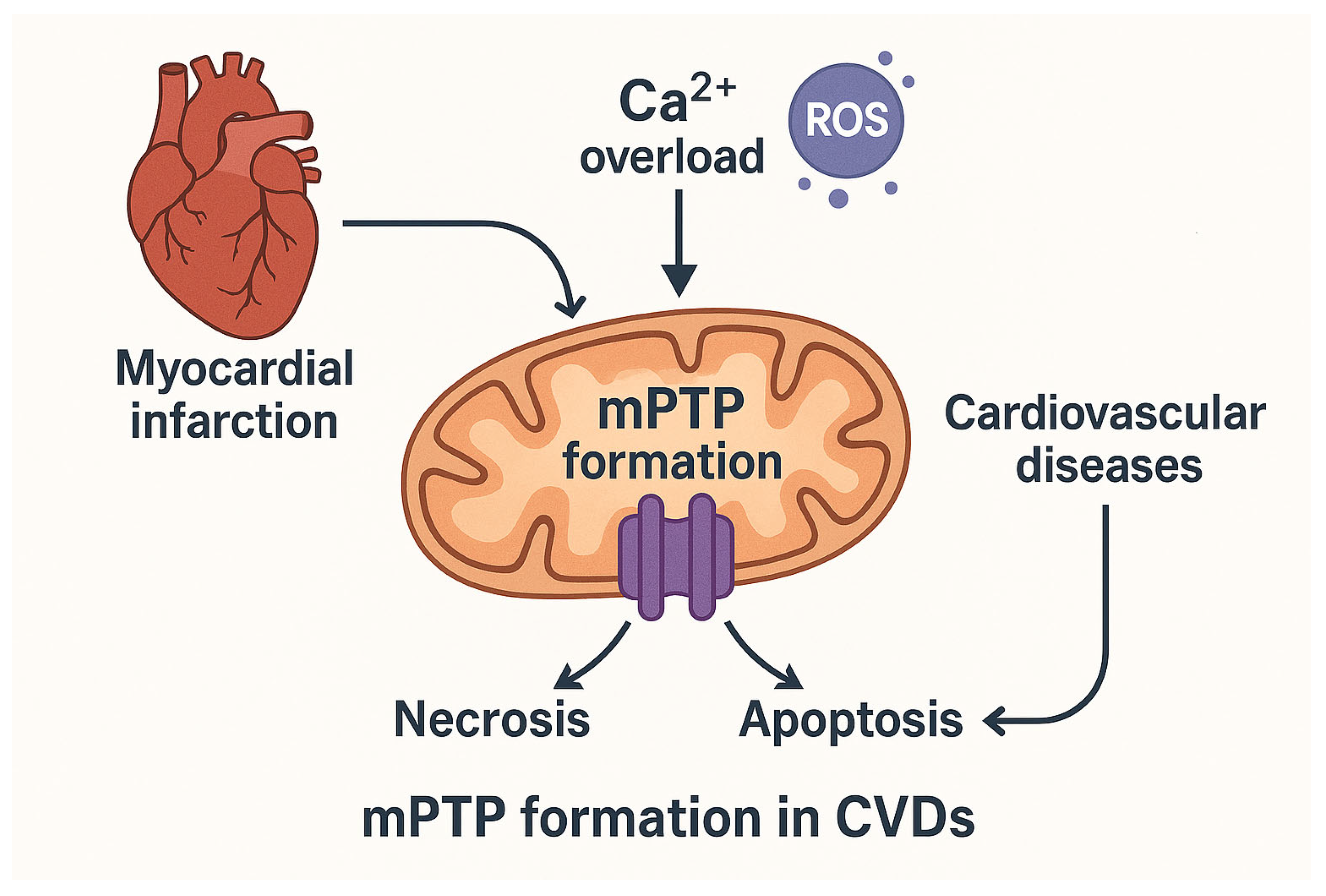
1. Introduction
2. Background of mPTP
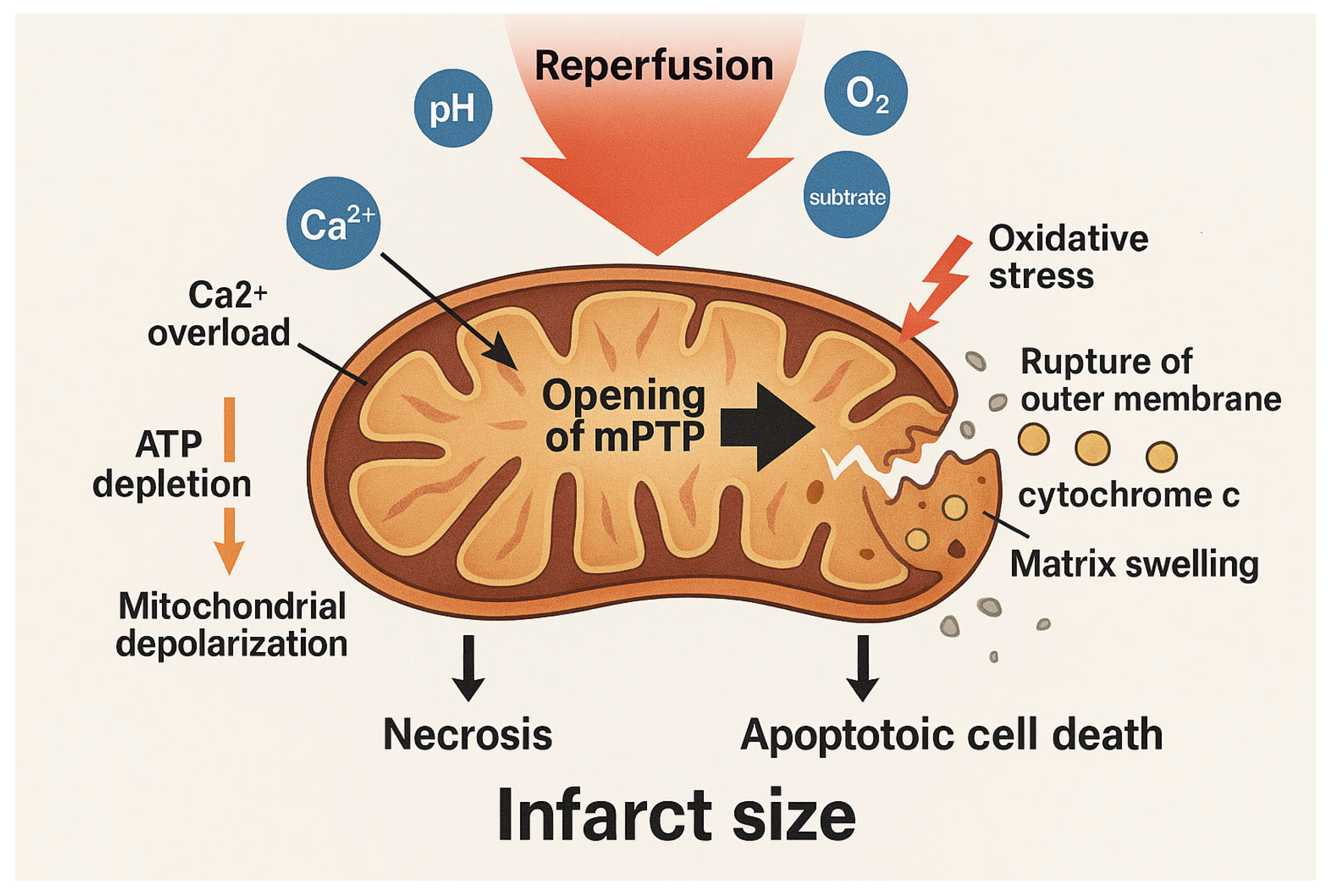
3. mPTP as a Critical Signalling Axis in Reperfusion Injury
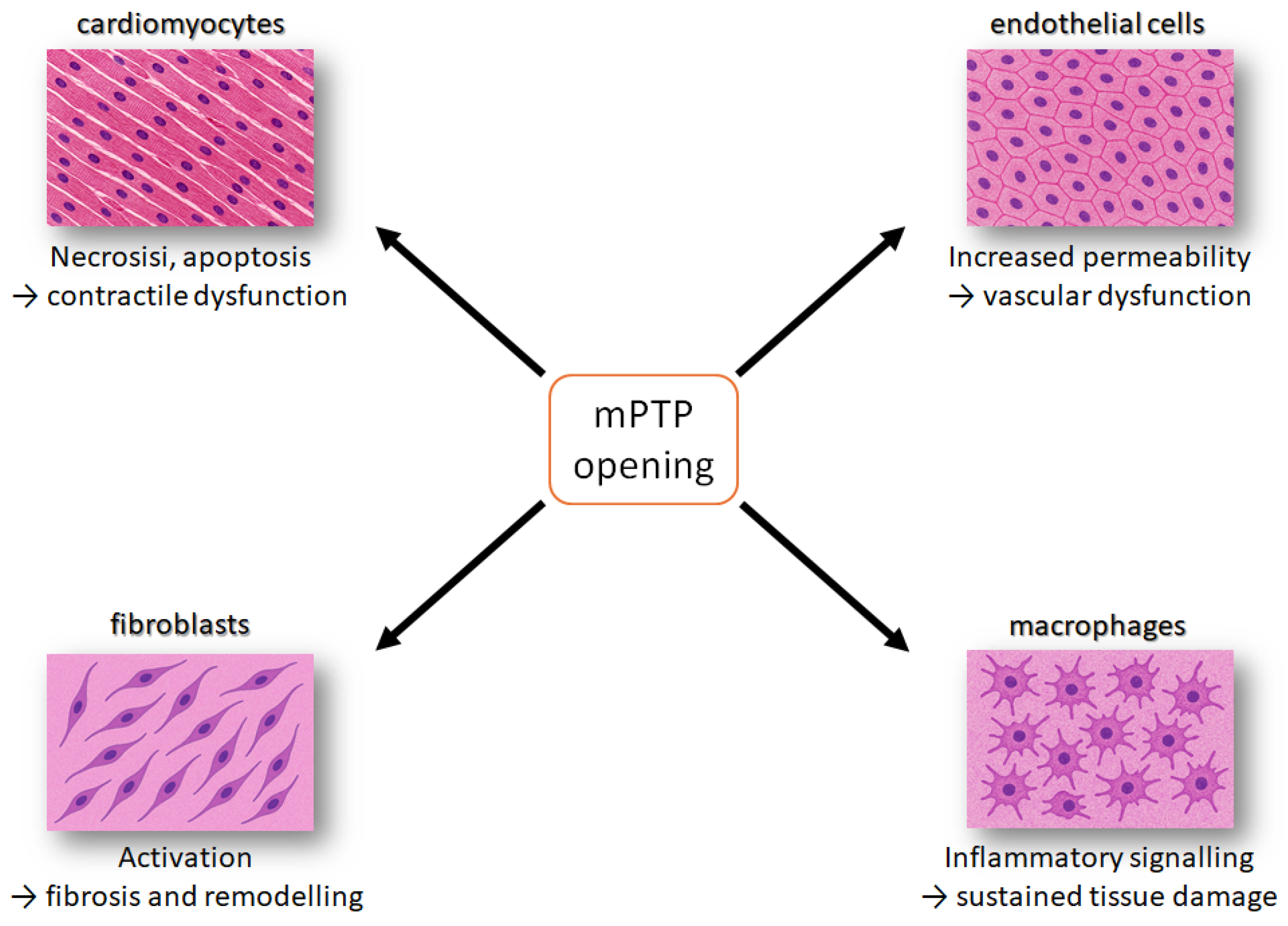
4. Pathomechanism of Mitochondrial Dysfunction Between Myocardial Cell Types
4.1. Cardiomyocytes
4.2. Endothelial Cells
4.3. Cardiac Fibroblasts
4.4. Immune Cells
4.5. Intercellular Crosstalk
5. Mechanism of Mitochondrial Homeostasis and Innovative Treatments to Improve Outcomes and Quality of Life
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANT | adenine nucleotide translocator |
| ATR | atractylate |
| BKA | bongkrekate |
| CaMKII | calcium/calmodulin-dependent protein kinase II;m |
| CATR | carboxyatractylate |
| CDVs | cardiovascular diseases |
| CFs | Cardiac fibroblasts |
| CMs | Cardiomyocytes |
| CyPD | cyclophilin D |
| DAMPs | damage-associated molecular patterns |
| ECs | endothelial cells |
| I/R | ischemia/reperfusion |
| IMM | inner mitochondrial membrane |
| MI | myocardial infarction |
| MLKL | mixed lineage kinase domain-like protein |
| mPTP | mitochondrial permeability transition pore |
| OXPHOS | oxidative phosphorylation |
| RCD | Regulated cell death |
| RIPK3 | regulatory proteins eceptor-interacting protein kinase 3 |
| ROS | reactive oxygen species |
References
- Murphy, E.; Eisner, D.A. How Does Mitochondrial Ca2+ Change during Ischemia and Reperfusion? Implications for Activation of the Permeability Transition Pore. J. Gen. Physiol. 2025, 157, e202313520. [Google Scholar] [CrossRef]
- Yang, H.-M. Mitochondrial Dysfunction in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 1917. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Karch, J. Regulation of Cell Death in the Cardiovascular System. Int. Rev. Cell Mol. Biol. 2020, 353, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, Y.; Xiong, J.-W. Rewiring Cell Identity and Metabolism to Drive Cardiomyocyte Proliferation. Cell Regen. 2025, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, I.Z.O.G.L.; Gayibov, U.G.; Omonturdiev, S.Z.; Azamjonovna, S.F.; Gayibova, S.N.; Aripov, T.F. Molecular Pathways in Cardiovascular Disease under Hypoxia: Mechanisms, Biomarkers, and Therapeutic Targets. J. Biomed. Res. 2025, 39, 254. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Nesci, S. Mitochondrial Permeability Transition, F1FO-ATPase and Calcium: An Enigmatic Triangle. EMBO Rep. 2017, 18, 1265–1267. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Nesci, S. The Mitochondrial Permeability Transition Pore in Cell Death: A Promising Drug Binding Bioarchitecture. Med. Res. Rev. 2020, 40, 811–817. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular Mechanisms and Consequences of Mitochondrial Permeability Transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, Structure, and Function of the Mitochondrial Permeability Transition Pore: Controversies, Consensus, Recent Advances, and Future Directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Tommasin, L.; Carrer, A.; Nata, F.B.; Frigo, E.; Fogolari, F.; Lippe, G.; Carraro, M.; Bernardi, P. Adenine Nucleotide Translocator and ATP Synthase Cooperate in Mediating the Mitochondrial Permeability Transition. J. Physiol. 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S. ATP Synthase as a Negative Regulator versus a Functional-Structural Component of the High Conductance State of Mitochondrial Permeability Transition Pore. J. Biochem. Mol. Toxicol. 2024, 38, e23821. [Google Scholar] [CrossRef]
- Karch, J.; Molkentin, J.D. Identity of the Elusive Mitochondrial Permeability Transition Pore: What It Might Be, What It Was, and What It Still Could Be. Curr. Opin. Physiol. 2018, 3, 57–62. [Google Scholar] [CrossRef]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-Dependent Mitochondrial Permeability Transition Regulates Some Necrotic but Not Apoptotic Cell Death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, D.; Lopez, S.J.; Mendoza, A.; Ramirez, D.; Lin, S.-C.J.; Molkentin, J.D.; Peixoto, P.M.; Karch, J. Atad3 Is Essential for Mitochondrial Permeability Transition Pore Opening and Cardiac Ischemia Reperfusion Injury. bioRxiv 2025. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Nesci, S. The Mitochondrial F1FO-ATPase Exploits the Dithiol Redox State to Modulate the Permeability Transition Pore. Arch. Biochem. Biophys. 2021, 712, 109027. [Google Scholar] [CrossRef]
- Nicolli, A.; Petronilli, V.; Bernardi, P. Modulation of the Mitochondrial Cyclosporin A-Sensitive Permeability Transition Pore by Matrix pH. Evidence That the Pore Open-Closed Probability Is Regulated by Reversible Histidine Protonation. Biochemistry 1993, 32, 4461–4465. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Nesci, S. Phenylglyoxal Inhibition of the Mitochondrial F1FO-ATPase Activated by Mg2+ or by Ca2+ Provides Clues on the Mitochondrial Permeability Transition Pore. Arch. Biochem. Biophys. 2020, 681, 108258. [Google Scholar] [CrossRef]
- Johans, M.; Milanesi, E.; Franck, M.; Johans, C.; Liobikas, J.; Panagiotaki, M.; Greci, L.; Principato, G.; Kinnunen, P.K.J.; Bernardi, P.; et al. Modification of Permeability Transition Pore Arginine(s) by Phenylglyoxal Derivatives in Isolated Mitochondria and Mammalian Cells. Structure-Function Relationship of Arginine Ligands. J. Biol. Chem. 2005, 280, 12130–12136. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Llaguno, M.C.; Yang, Y.; Yan, Y.; Weber, J.; Sigworth, F.J.; Jonas, E.A. A Mitochondrial Megachannel Resides in Monomeric F1FO ATP Synthase. Nat. Commun. 2019, 10, 5823. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Fabbri, M.; Nesci, S. The Inhibition of Gadolinium Ion (Gd3+) on the Mitochondrial F1FO-ATPase Is Linked to the Modulation of the Mitochondrial Permeability Transition Pore. Int. J. Biol. Macromol. 2021, 184, 250–258. [Google Scholar] [CrossRef]
- Hunter, D.R.; Haworth, R.A. The Ca2+-Induced Membrane Transition in Mitochondria. I. The Protective Mechanisms. Arch. Biochem. Biophys. 1979, 195, 453–459. [Google Scholar] [CrossRef]
- Karch, J.; Bround, M.J.; Khalil, H.; Sargent, M.A.; Latchman, N.; Terada, N.; Peixoto, P.M.; Molkentin, J.D. Inhibition of Mitochondrial Permeability Transition by Deletion of the ANT Family and CypD. Sci. Adv. 2019, 5, eaaw4597. [Google Scholar] [CrossRef]
- Crompton, M.; Ellinger, H.; Costi, A. Inhibition by Cyclosporin A of a Ca2+-Dependent Pore in Heart Mitochondria Activated by Inorganic Phosphate and Oxidative Stress. Biochem. J. 1988, 255, 357–360. [Google Scholar]
- Algieri, V.; Algieri, C.; Maiuolo, L.; De Nino, A.; Pagliarani, A.; Tallarida, M.A.; Trombetti, F.; Nesci, S. 1,5-Disubstituted-1,2,3-Triazoles as Inhibitors of the Mitochondrial Ca2+-Activated F1FO-ATP(Hydrol)Ase and the Permeability Transition Pore. Ann. N. Y. Acad. Sci. 2021, 1485, 43–55. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Marchi, S.; Forte, M.; Tallarida, M.A.; Bianchi, F.; La Mantia, D.; Algieri, V.; Stanzione, R.; Cotugno, M.; et al. 1,5-Disubstituted-1,2,3-Triazoles Counteract Mitochondrial Dysfunction Acting on F1FO-ATPase in Models of Cardiovascular Diseases. Pharmacol. Res. 2023, 187, 106561. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Cugliari, A.; Granata, S.; Trombetti, F.; Glogowski, P.A.; Fabbri, M.; Morciano, G.; Pedriali, G.; Pinton, P.; et al. Melatonin Rescues Cell Respiration Impaired by Hypoxia/Reoxygenation in Aortic Endothelial Cells and Affects the Mitochondrial Bioenergetics Targeting the F1FO-ATPase. Redox Biol. 2025, 82, 103605. [Google Scholar] [CrossRef]
- Nesci, S.; Algieri, C.; Tallarida, M.A.; Stanzione, R.; Marchi, S.; Pietrangelo, D.; Trombetti, F.; D’Ambrosio, L.; Forte, M.; Cotugno, M.; et al. Molecular Mechanisms of Naringenin Modulation of Mitochondrial Permeability Transition Acting on F1FO-ATPase and Counteracting Saline Load-Induced Injury in SHRSP Cerebral Endothelial Cells. Eur. J. Cell Biol. 2024, 103, 151398. [Google Scholar] [CrossRef]
- Algieri, V.; Algieri, C.; Costanzo, P.; Fiorani, G.; Jiritano, A.; Olivito, F.; Tallarida, M.A.; Trombetti, F.; Maiuolo, L.; De Nino, A.; et al. Novel Regioselective Synthesis of 1,3,4,5-Tetrasubstituted Pyrazoles and Biochemical Valuation on F1FO-ATPase and Mitochondrial Permeability Transition Pore Formation. Pharmaceutics 2023, 15, 498. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Bernardini, C.; Fabbri, M.; Forni, M.; Nesci, S. Mitochondrial Ca2+-Activated F1FO-ATPase Hydrolyzes ATP and Promotes the Permeability Transition Pore. Ann. N. Y. Acad. Sci. 2019, 1457, 142–157. [Google Scholar] [CrossRef]
- Nesci, S.; Algieri, C.; Trombetti, F.; Ventrella, V.; Fabbri, M.; Pagliarani, A. Sulfide Affects the Mitochondrial Respiration, the Ca2+-Activated F1FO-ATPase Activity and the Permeability Transition Pore but Does Not Change the Mg2+-Activated F1FO-ATPase Activity in Swine Heart Mitochondria. Pharmacol. Res. 2021, 166, 105495. [Google Scholar] [CrossRef]
- Algieri, C.; Oppedisano, F.; Trombetti, F.; Fabbri, M.; Palma, E.; Nesci, S. Selenite Ameliorates the ATP Hydrolysis of Mitochondrial F1FO-ATPase by Changing the Redox State of Thiol Groups and Impairs the ADP Phosphorylation. Free Radic. Biol. Med. 2024, 210, 333–343. [Google Scholar] [CrossRef]
- Morciano, G.; Preti, D.; Pedriali, G.; Aquila, G.; Missiroli, S.; Fantinati, A.; Caroccia, N.; Pacifico, S.; Bonora, M.; Talarico, A.; et al. Discovery of Novel 1,3,8-Triazaspiro[4.5]Decane Derivatives That Target the c Subunit of F1/FO-Adenosine Triphosphate (ATP) Synthase for the Treatment of Reperfusion Damage in Myocardial Infarction. J. Med. Chem. 2018, 61, 7131–7143. [Google Scholar] [CrossRef]
- Albanese, V.; Pedriali, G.; Fabbri, M.; Ciancetta, A.; Ravagli, S.; Roccatello, C.; Guerrini, R.; Morciano, G.; Preti, D.; Pinton, P.; et al. Design and Synthesis of 1,4,8-Triazaspiro[4.5]Decan-2-One Derivatives as Novel Mitochondrial Permeability Transition Pore Inhibitors. J. Enzyme Inhib. Med. Chem. 2025, 40, 2505907. [Google Scholar] [CrossRef] [PubMed]
- Fantinati, A.; Morciano, G.; Turrin, G.; Pedriali, G.; Pacifico, S.; Preti, D.; Albanese, V.; Illuminati, D.; Cristofori, V.; Giorgi, C.; et al. Identification of Small-Molecule Urea Derivatives as PTPC Modulators Targeting the c Subunit of F1/FO-ATP Synthase. Bioorg. Med. Chem. Lett. 2022, 72, 128822. [Google Scholar] [CrossRef]
- Clarke, S.J.; McStay, G.P.; Halestrap, A.P. Sanglifehrin A Acts as a Potent Inhibitor of the Mitochondrial Permeability Transition and Reperfusion Injury of the Heart by Binding to Cyclophilin-D at a Different Site from Cyclosporin A. J. Biol. Chem. 2002, 277, 34793–34799. [Google Scholar] [CrossRef] [PubMed]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol. Sci. 2019, 40, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Gutiérrez-Aguilar, M. The Still Uncertain Identity of the Channel-Forming Unit(s) of the Mitochondrial Permeability Transition Pore. Cell Calcium 2018, 73, 121–130. [Google Scholar] [CrossRef]
- Argaud, L.; Gateau-Roesch, O.; Muntean, D.; Chalabreysse, L.; Loufouat, J.; Robert, D.; Ovize, M. Specific Inhibition of the Mitochondrial Permeability Transition Prevents Lethal Reperfusion Injury. J. Mol. Cell. Cardiol. 2005, 38, 367–374. [Google Scholar] [CrossRef]
- Chang, Y.; Zou, Q. Mitochondrial Calcium Homeostasis and Atrial Fibrillation: Mechanisms and Therapeutic Strategies Review. Curr. Probl. Cardiol. 2025, 50, 102988. [Google Scholar] [CrossRef]
- Andrienko, T.; Pasdois, P.; Rossbach, A.; Halestrap, A.P. Real-Time Fluorescence Measurements of ROS and [Ca2+] in Ischemic/Reperfused Rat Hearts: Detectable Increases Occur Only after Mitochondrial Pore Opening and Are Attenuated by Ischemic Preconditioning. PLoS ONE 2016, 11, e0167300. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J. Signalling Pathways in Ischaemic Postconditioning. Thromb. Haemost. 2009, 101, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Li, B.; Mewton, N.; Sanchez, I.; Piot, C.; Elbaz, M.; Ovize, M. Inhibition of Mitochondrial Permeability Transition Pore Opening: Translation to Patients. Cardiovasc. Res. 2009, 83, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Gateau-Roesch, O.; Argaud, L.; Ovize, M. Mitochondrial Permeability Transition Pore and Postconditioning. Cardiovasc. Res. 2006, 70, 264–273. [Google Scholar] [CrossRef]
- Piper, H.M.; Abdallah, Y.; Schäfer, C. The First Minutes of Reperfusion: A Window of Opportunity for Cardioprotection. Cardiovasc. Res. 2004, 61, 365–371. [Google Scholar] [CrossRef]
- Bers, D.M.; Barry, W.H.; Despa, S. Intracellular Na+ Regulation in Cardiac Myocytes. Cardiovasc. Res. 2003, 57, 897–912. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Choya-Foces, C.; Carregal-Romero, S.; Ramos, E.; Oliva, T.; Villa-Piña, T.; Moreno, L.; Izquierdo-Álvarez, A.; Cabrera-García, J.D.; Cortés, A.; et al. Na+ Controls Hypoxic Signalling by the Mitochondrial Respiratory Chain. Nature 2020, 586, 287–291. [Google Scholar] [CrossRef]
- Lenaz, G.; Nesci, S.; Genova, M.L. Understanding Differential Aspects of Microdiffusion (Channeling) in the Coenzyme Q and Cytochrome c Regions of the Mitochondrial Respiratory System. Mitochondrion 2024, 74, 101822. [Google Scholar] [CrossRef]
- Halestrap, A. Biochemistry: A Pore Way to Die. Nature 2005, 434, 578–579. [Google Scholar] [CrossRef]
- Argaud, L.; Gateau-Roesch, O.; Raisky, O.; Loufouat, J.; Robert, D.; Ovize, M. Postconditioning Inhibits Mitochondrial Permeability Transition. Circulation 2005, 111, 194–197. [Google Scholar] [CrossRef]
- Ruan, Z.-H.; Xu, Z.-X.; Zhou, X.-Y.; Zhang, X.; Shang, L. Implications of Necroptosis for Cardiovascular Diseases. Curr. Med. Sci. 2019, 39, 513–522. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Kalmykov, V.A.; Maksaeva, A.O.; Rozhkova, U.V.; Lapshina, K.O.; Orekhov, A.N. Necroptosis in Myocardial Ischaemia-Reperfusion Injury: Current Update on Mechanisms, Therapeutic Targets, and Translational Potential. Apoptosis 2025, 30, 1216–1234. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, W.; Wang, Y.; Zhang, J.; Qiu, S.; Guan, Z.; Shi, C.; Ma, J.; Xu, Y. MLKL–Mediated Necroptosis Predominantly Contributes to Immune-Associated Myocardial Damage. Inflammation 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, Q.; Liu, W.; Wang, L.; Li, X.; Zhao, C.; Wu, N.; Ma, C. Mitochondrial Apoptosis in Response to Cardiac Ischemia-Reperfusion Injury. J. Transl. Med. 2025, 23, 125. [Google Scholar] [CrossRef]
- Kinnally, K.W.; Antonsson, B. A Tale of Two Mitochondrial Channels, MAC and PTP, in Apoptosis. Apoptosis 2007, 12, 857–868. [Google Scholar] [CrossRef]
- Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. [Google Scholar] [CrossRef]
- Myint, M.; Oppedisano, F.; De Giorgi, V.; Kim, B.-M.; Marincola, F.M.; Alter, H.J.; Nesci, S. Inflammatory Signaling in NASH Driven by Hepatocyte Mitochondrial Dysfunctions. J. Transl. Med. 2023, 21, 757. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Liu, M.; Chen, Y.; Wu, Y.; Li, Q.; Ma, T.; Gao, J.; Xia, Y.; Fan, M.; et al. Protective Effect of HINT2 on Mitochondrial Function via Repressing MCU Complex Activation Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury. Basic Res. Cardiol. 2021, 116, 65. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and Endothelial Function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial Dysfunction in Vascular Endothelial Cells and Its Role in Atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Burke, R.M.; Burgos Villar, K.N.; Small, E.M. Fibroblast Contributions to Ischemic Cardiac Remodeling. Cell. Signal. 2021, 77, 109824. [Google Scholar] [CrossRef] [PubMed]
- Bretherton, R.; Bugg, D.; Olszewski, E.; Davis, J. Regulators of Cardiac Fibroblast Cell State. Matrix Biol. 2020, 91–92, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Vivar, R.; Soto, C.; Copaja, M.; Mateluna, F.; Aranguiz, P.; Muñoz, J.P.; Chiong, M.; Garcia, L.; Letelier, A.; Thomas, W.G.; et al. Phospholipase C/Protein Kinase C Pathway Mediates Angiotensin II-Dependent Apoptosis in Neonatal Rat Cardiac Fibroblasts Expressing AT1 Receptor. J. Cardiovasc. Pharmacol. 2008, 52, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Fan, J.; Zhang, J.; Ren, M.; Feng, L. Immune in Myocardial Ischemia/Reperfusion Injury: Potential Mechanisms and Therapeutic Strategies. Front. Immunol. 2025, 16, 1558484. [Google Scholar] [CrossRef]
- Cai, W.; Lian, L.; Li, A.; Zhang, Q.; Li, M.; Zhang, J.; Xie, Y. Cardiac Resident Macrophages: The Core of Cardiac Immune Homeostasis. Cell. Signal. 2024, 119, 111169. [Google Scholar] [CrossRef]
- Banerjee, D.; Tian, R.; Cai, S. The Role of Innate Immune Cells in Cardiac Injury and Repair: A Metabolic Perspective. Curr. Cardiol. Rep. 2023, 25, 631–640. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA Fragments Exit Mitochondria via mPTP- and VDAC-Dependent Channels to Activate NLRP3 Inflammasome and Interferon Signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef]
- Arslan, F.; de Kleijn, D.P.; Pasterkamp, G. Innate Immune Signaling in Cardiac Ischemia. Nat. Rev. Cardiol. 2011, 8, 292–300. [Google Scholar] [CrossRef]
- Weiss, J.N.; Korge, P.; Honda, H.M.; Ping, P. Role of the Mitochondrial Permeability Transition in Myocardial Disease. Circ. Res. 2003, 93, 292–301. [Google Scholar] [CrossRef]
- Bonora, M.; Wieckowski, M.R.; Sinclair, D.A.; Kroemer, G.; Pinton, P.; Galluzzi, L. Targeting Mitochondria for Cardiovascular Disorders: Therapeutic Potential and Obstacles. Nat. Rev. Cardiol. 2019, 16, 33–55. [Google Scholar] [CrossRef]
- Islam, M.N.; Mishra, V.K.; Munalisa, R.; Parveen, F.; Ali, S.F.; Akter, K.; Ahmed, T.; Ho, T.-J.; Huang, C.-Y. Mechanistic Insight of Mitochondrial Dysfunctions in Cardiovascular Diseases with Potential Biomarkers. Mol. Cell. Toxicol. 2024, 20, 441–463. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Functions and Dysfunctions of Mitochondrial Dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, M.; Naón, D. Mitochondria for Cardiovascular Therapy: A Deep Dive into Drug Targets and Therapeutic Approaches. Curr. Pharmacol. Rep. 2025, 11, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Jin, Y.; Chen, K.; Zhang, L.; Gao, X.; Li, M.; Yuan, Z.; Jia, J.; Sun, A.; et al. Mitochondrial Transplantation Augments the Reparative Capacity of Macrophages Following Myocardial Injury. Adv. Sci. 2025, 12, e06337. [Google Scholar] [CrossRef]
- Algieri, C.; Nesci, S.; Oppedisano, F. Mitochondrial Dysfunction Acts as a Modulator of the Immunometabolic Route for Activating the Cytosolic DNA Sensor Pathway in Triggering Innate Immunosurveillance. J. Transl. Med. 2025, 23, 1321. [Google Scholar] [CrossRef]
- Ferko, M.; Andelová, N.; Szeiffová Bačová, B.; Jašová, M. Myocardial Adaptation in Pseudohypoxia: Signaling and Regulation of mPTP via Mitochondrial Connexin 43 and Cardiolipin. Cells 2019, 8, 1449. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Ludwig, K.R.; Darvish, S.; Coppock, M.E.; Seals, D.R.; Rossman, M.J. Chronic Mitochondria Antioxidant Treatment in Older Adults Alters the Circulating Milieu to Improve Endothelial Cell Function and Mitochondrial Oxidative Stress. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H187–H194. [Google Scholar] [CrossRef]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl. Sci. 2019, 4, 147–157. [Google Scholar] [CrossRef]
- Antonucci, S.; Di Sante, M.; Sileikyte, J.; Deveraux, J.; Bauer, T.; Bround, M.J.; Menabò, R.; Paillard, M.; Alanova, P.; Carraro, M.; et al. A Novel Class of Cardioprotective Small-Molecule PTP Inhibitors. Pharmacol. Res. 2019, 151, 104548. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous Mitochondrial Transplantation for Dysfunction after Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Di Donfrancesco, A.; Massaro, G.; Di Meo, I.; Tiranti, V.; Bottani, E.; Brunetti, D. Gene Therapy for Mitochondrial Diseases: Current Status and Future Perspective. Pharmaceutics 2022, 14, 1287. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Troisio, I.; et al. The Impairment of Cell Metabolism by Cardiovascular Toxicity of Doxorubicin Is Reversed by Bergamot Polyphenolic Fraction Treatment in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 8977. [Google Scholar] [CrossRef]
- Saludas, L.; Oliveira, C.C.; Roncal, C.; Ruiz-Villalba, A.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M.J. Extracellular Vesicle-Based Therapeutics for Heart Repair. Nanomaterials 2021, 11, 570. [Google Scholar] [CrossRef]
- Khan, K.; Caron, C.; Mahmoud, I.; Derish, I.; Schwertani, A.; Cecere, R. Extracellular Vesicles as a Cell-Free Therapy for Cardiac Repair: A Systematic Review and Meta-Analysis of Randomized Controlled Preclinical Trials in Animal Myocardial Infarction Models. Stem Cell Rev. Rep. 2022, 18, 1143–1167. [Google Scholar] [CrossRef]
| Therapeutic Approach | Primary Mitochondrial Target/Mechanism | Evidence/Status in CVD | Ref. |
|---|---|---|---|
| Mitochondria-targeted antioxidants | Scavenges mitochondrial ROS to protect the respiratory chain and cardiolipin | Robust preclinical cardioprotection in I/R models; early clinical studies in vascular and cardiac dysfunction | [79] |
| Cardiolipin stabilizers/bioenergetic peptides | Stabilize the inner mitochondrial membrane and cardiolipin, improve electron transport and ATP synthesis | Phase II/III trials in I/R injury and primary mitochondrial disease; signals of improved energetics and functional capacity, with mixed outcome data | [80] |
| mPTP modulators | Inhibit or desensitize the mitochondrial permeability transition pore to prevent necrosis/apoptosis | Strong mechanistic and preclinical rationale | [27,81] |
| Mitophagy and quality-control enhancers | Promote selective removal of damaged mitochondria and improve mitochondrial turnover | Preclinical cardioprotective effects in I/R and pressure-overload models; early human data mainly from ageing/skeletal-muscle studies | [82] |
| Mitochondrial transfer/transplantation | Deliver viable, functional mitochondria or mitochondrial components to injured myocardium | Proof-of-concept animal studies and early clinical feasibility reports after cardiac surgery suggest improved contractile recovery and reduced remodelling | [83] |
| Gene- and RNA-based mitochondrial therapies | Modulate nuclear or mitochondrial genes controlling mitochondrial function, dynamics, or quality control | Currently at preclinical stage; major challenges include targeted delivery, long-term safety, and off-target effects, but offer potential for precision correction of mitochondrial defects | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesci, S.; Rubattu, S. Mitochondrial Permeability Transition Pore: The Cardiovascular Disease’s Molecular Achilles Heel. Biomedicines 2025, 13, 3014. https://doi.org/10.3390/biomedicines13123014
Nesci S, Rubattu S. Mitochondrial Permeability Transition Pore: The Cardiovascular Disease’s Molecular Achilles Heel. Biomedicines. 2025; 13(12):3014. https://doi.org/10.3390/biomedicines13123014
Chicago/Turabian StyleNesci, Salvatore, and Speranza Rubattu. 2025. "Mitochondrial Permeability Transition Pore: The Cardiovascular Disease’s Molecular Achilles Heel" Biomedicines 13, no. 12: 3014. https://doi.org/10.3390/biomedicines13123014
APA StyleNesci, S., & Rubattu, S. (2025). Mitochondrial Permeability Transition Pore: The Cardiovascular Disease’s Molecular Achilles Heel. Biomedicines, 13(12), 3014. https://doi.org/10.3390/biomedicines13123014








