Flexible Loading Phase Treat-and-Extend Regimen with Faricimab for Neovascular Age-Related Macular Degeneration: A Real-World Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Treatment Protocol
2.3. Assessments
2.4. Main Outcome Measures
2.5. Statistical Analysis
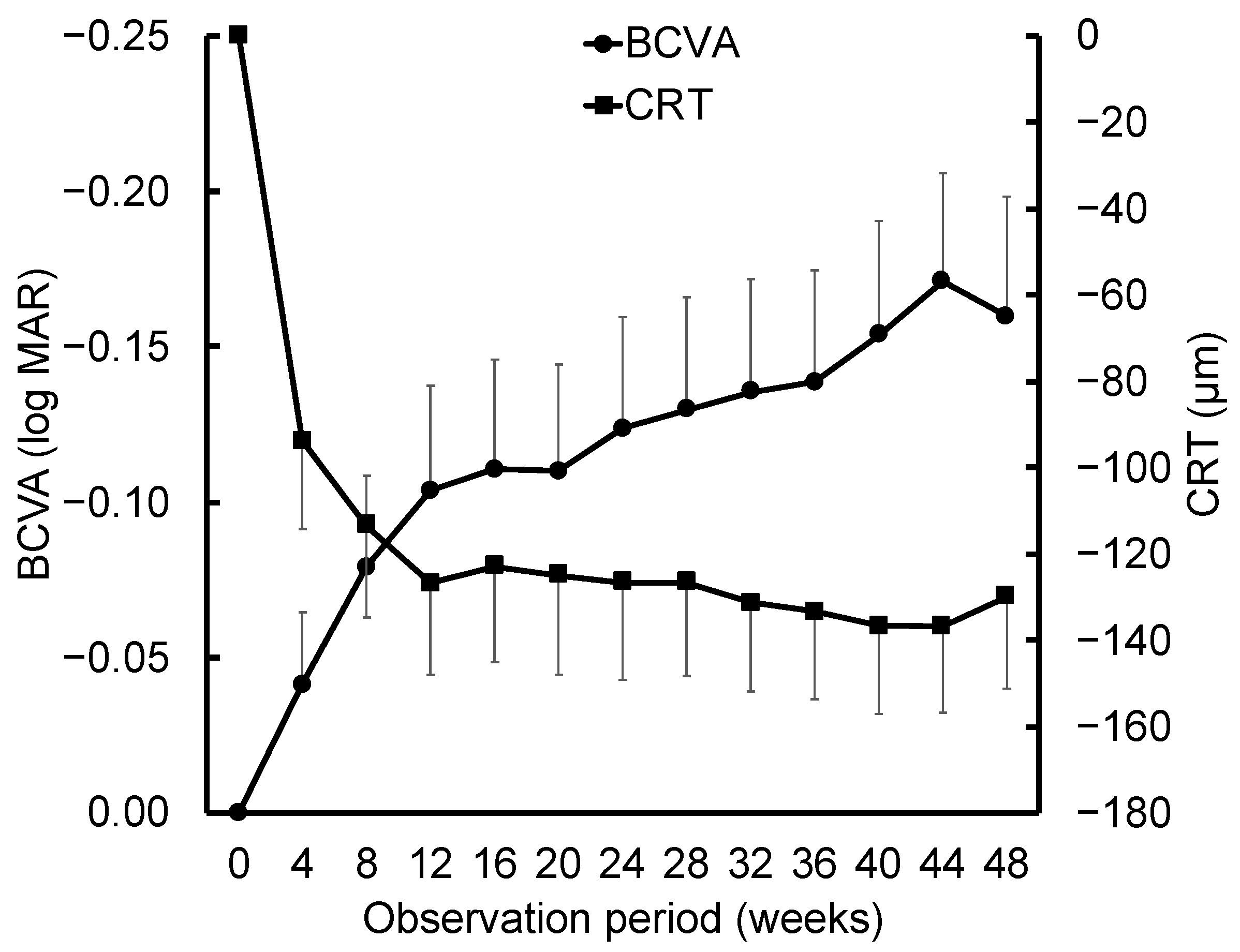
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Meeting Presentations
Abbreviations
| nAMD | Neovascular age-related macular degeneration |
| VEGF | Vascular endothelial growth factor |
| AMD | Age-related macular degeneration |
| MNV | Macular neovascularization |
| TAE | Treat-and-extend |
| PED | Pigment epithelial detachment |
| BCVA | Best-corrected visual acuity |
| PCV | Polypoidal choroidal vasculopathy |
| CRT | Central retinal thickness |
| OCT | Optical coherence |
| SRF | Subretinal fluid |
References
- de Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- CATT Research Group; Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- The IVAN Study Investigators; Chakravarthy, U.; Harding, S.P.; Rogers, C.A.; Downes, S.M.; Lotery, A.J.; Wordsworth, S.; Reeves, B.C. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology 2012, 119, 1399–1411. [Google Scholar] [CrossRef]
- Honda, S.; Yanagi, Y.; Koizumi, H.; Chen, Y.; Tanaka, S.; Arimoto, M.; Imai, K. Impact of neovascular age-related macular degeneration: Burden of patients receiving therapies in Japan. Sci. Rep. 2021, 11, 13152. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Lanzetta, P.; Korobelnik, J.F.; Heier, J.S.; Leal, S.; Holz, F.G.; Clark, W.L.; Eichenbaum, D.; Iida, T.; Xiaodong, S.; Berliner, A.J.; et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet 2024, 403, 1141–1152. [Google Scholar] [CrossRef]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Aziz, A.A.; Khan, H.; Gupta, A.; Mojumder, O.; Saulebayeva, A.; Abbey, A.M.; Almeida, D.R.P.; Avery, R.L.; Banda, H.K.; et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: The TRUCKEE study—6 month results. Eye 2023, 37, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, R.; Falfeli, T.; Bogdanova-Bennet, A.; Varma, D.; Habib, M.; Kotagiri, A.; Steel, D.H.; Grinton, M. Outcomes of treatment resistant neovascular-age related macular degeneration switched from Aflibercept to Faricimab. Ophthalmol. Retin. 2024, 8, 537–544, Erratum in Ophthalmol. Retin. 2025, 9, 1131. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Regillo, C.D.; Brown, D.M.; Abraham, P.; Yue, H.; Ianchulev, T.; Schneider, S.; Shams, N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am. J. Ophthalmol. 2008, 145, 239–248. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Eldem, B.; Guymer, R.; Korobelnik, J.F.; Schlingemann, R.O.; Axer-Siegel, R.; Wiedemann, P.; Simader, C.; Gekkieva, M.; Weichselberger, A.; et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: The EXCITE study. Ophthalmology 2011, 118, 831–839. [Google Scholar] [CrossRef]
- Lalwani, G.A.; Rosenfeld, P.J.; Fung, A.E.; Dubovy, S.R.; Michels, S.; Feuer, W.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PrONTO Study. Am. J. Ophthalmol. 2009, 148, 43–58.e1. [Google Scholar] [CrossRef]
- Gupta, B.; Adewoyin, T.; Patel, S.K.; Sivaprasad, S. Comparison of two intravitreal ranibizumab treatment schedules for neovascular age-related macular degeneration. Br. J. Ophthalmol. 2011, 95, 386–390. [Google Scholar] [CrossRef]
- Spaide, R. Ranibizumab according to need: A treatment for age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 679–680. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Ou, W.C.; Brown, D.M.; Croft, D.E.; Wang, R.; Payne, J.F.; Clark, W.L.; Abdelfattah, N.S.; Sadda, S.R.; TREX-AMD Study Group. Randomized Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: 2-Year Results of the TREX-AMD Study. Ophthalmol. Retin. 2017, 1, 314–321. [Google Scholar] [CrossRef]
- Rosenberg, D.; Deonarain, D.M.; Gould, J.; Sothivannan, A.; Phillips, M.R.; Sarohia, G.S.; Sivaprasad, S.; Wykoff, C.C.; Cheung, C.M.G.; Sarraf, D.; et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 2023, 37, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Horie-Inoue, K.; Gehlbach, P.L.; Takita, H.; Kabasawa, S.; Kawasaki, I.; Ohkubo, T.; Kurihara, S.; Iizuka, H.; Miyashita, Y.; et al. Phenotype and genotype characteristics of age-related macular degeneration in a Japanese population. Ophthalmology 2010, 117, 928–938. [Google Scholar] [CrossRef]
- Heier, J.S.; Singh, R.P.; Wykoff, C.C.; Csaky, K.G.; Lai, T.Y.Y.; Loewenstein, A.; Schlottmann, P.G.; Paris, L.P.; Westenskow, P.D.; Quezada-Ruiz, C.; et al. The Angiopoietin/Tie Pathway in Retinal Vascular Diseases: A Review. Retina 2021, 41, 1–19. [Google Scholar] [CrossRef]
- Joussen, A.M.; Ricci, F.; Paris, L.P.; Korn, C.; Quezada-Ruiz, C.; Zarbin, M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: A review of preclinical data. Eye 2021, 35, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Arnold-Vangsted, A.; Schou, M.G.; Balaratnasingam, C.; Cehofski, L.J.; Chhablani, J.; van Dijk, E.H.C.; Eriksen, N.S.; Grauslund, J.; Hajari, J.N.; Sabaner, M.C.; et al. Efficacy of intravitreal faricimab therapy for polypoidal choroidal vasculopathy: A systematic review and meta-analysis. Acta Ophthalmol. 2025, 103, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Machida, A.; Oishi, A.; Ikeda, J.; Kurihara, J.; Yoneda, A.; Tsuiki, E.; Hirata, Y.; Murakami, R.; Kitaoka, T. Factors associated with success of switching to faricimab for neovascular age-related macular degeneration refractory to intravitreal aflibercept. Life 2024, 14, 476. [Google Scholar] [CrossRef]
- Hwang, J.U.; Yang, S.J.; Yoon, Y.H.; Lee, J.Y.; Kim, J.G. Recurrent submacular hemorrhage in patients with neovascular age-related macular degeneration. Retina 2012, 32, 652–657. [Google Scholar] [CrossRef]
- Kaufmann, G.T.; Boucher, N.; Sharma, C.; Aggarwal, N.; Starr, M.R. Submacular hemorrhage rates following anti-vascular endothelial growth factor injections for exudative age-related macular degeneration. Am. J. Ophthalmol. 2025, 270, 172–182. [Google Scholar] [CrossRef]
- Mori, R.; Honda, S.; Gomi, F.; Tsujikawa, A.; Koizumi, H.; Ochi, H.; Ohsawa, S.; Okada, A.A.; TENAYA and LUCERNE Investigators. Efficacy, durability, and safety of faricimab up to every 16 weeks in patients with neovascular age-related macular degeneration: 1-year results from the Japan subgroup of the phase 3 TENAYA trial. Jpn. J. Ophthalmol. 2023, 67, 301–310, Erratum in Jpn. J. Ophthalmol. 2023, 67, 311. [Google Scholar] [CrossRef] [PubMed]
- Menghini, M.; Kloeckener-Gruissem, B.; Fleischhauer, J.; Kurz-Levin, M.M.; Sutter, F.K.; Berger, W.; Barthelmes, D. Impact of loading phase, initial response and CFH genotype on the long-term outcome of treatment for neovascular age-related macular degeneration. PLoS ONE 2012, 7, e42014. [Google Scholar] [CrossRef]
- Khanani, A.M.; Kotecha, A.; Chang, A.; Chen, S.J.; Chen, Y.; Guymer, R.; Heier, J.S.; Holz, F.G.; Iida, T.; Ives, J.A.; et al. TENAYA and LUCERNE: Two-year results from the Phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmology 2024, 131, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Gomi, F.; Tsujikawa, A.; Honda, S.; Mori, R.; Ochi, H.; Iwasaki, K.; Okada, A.A.; TENAYA and LUCERNE Investigators. Efficacy, durability, and safety of faricimab up to every 16 weeks in patients with neovascular age-related macular degeneration: 2-year results from the Japan subgroup of the phase III TENAYA trial. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Machida, A.; Kurihara, J.; Murakami, R.; Oka, A.; Yoneda, A.; Tsuiki, E.; Hirata, Y.; Oishi, A. One-year outcomes of a faricimab Treat-and-Extend regimen without loading phase in neovascular age-related macular degeneration. In Proceedings of the 63rd Annual Meeting of the Japanese Retina and Vitreous Society, Osaka, Japan, 6–8 December 2024. [Google Scholar]



| Total | Type 1 | Type 2 | Type 3 | ||
|---|---|---|---|---|---|
| Without polyps | With polyps | ||||
| Number of eyes | n = 43 | n = 13 | n = 26 | n = 3 | n = 1 |
| Clinicodemographic characteristics | |||||
| Age (years), mean (SD) | 76.6 (8.9) | 78.1 (12.4) | 75.6 (7.2) | 78.1 (8.4) | 80.3 (NA) |
| Sex: Female, n (%) | 12 (27.9) | 3 (23.1) | 6 (23.1) | 3 (100.0) | 0 (0.0) |
| Hypertension, n (%) | 29 (67.4) | 8 (61.5) | 17 (65.4) | 3 (100.0) | 1 (100.0) |
| Diabetes mellitus, n (%) | 8 (18.6) | 3 (23.1) | 4 (15.4) | 1 (33.3) | 0 (100.0) |
| Malignancy, n (%) | 9 (20.9) | 2 (15.4) | 6 (23.1) | 0 (0.0) | 1 (100.0) |
| History of cardiovascular events, n (%) | 6 (14.0) | 1 (7.7) | 3 (11.5) | 1 (33.3) | 1 (100.0) |
| Anticoagulant use, n (%) | 9 (20.9) | 3 (23.1) | 3 (11.5) | 2 (66.7) | 1 (100.0) |
| Smoking history, n (%) | 23 (53.5) | 7 (53.8) | 15 (57.7) | 0 (0.0) | 1 (100.0) |
| BCVA, logMAR, mean (SD) | 0.35 (0.32) | 0.32 (0.28) | 0.32 (0.33) | 0.58 (0.39) | 0.70 (NA) |
| Anatomical parameters at baseline | |||||
| CRT (μm), mean (SD) | 312.4 (140.5) | 251.4 (76.0) | 332.4 (164.0) | 353.7 (27.4) | 460.0 (NA) |
| CCT (μm), mean (SD) | 258.6 (99.1) | 240.1 (113.5) | 274.3 (93.9) | 196.7 (86.6) | 277.0 (NA) |
| PED height at fovea (μm), mean (SD) | 67.1 (125.0) | 40.2 (39.1) | 82.8 (154.9) | 10.7 (18.5) | 177.0 (NA) |
| Disrupted EZ at fovea, n (%) | 28 (65.1) | 8 (61.5) | 16 (61.5) | 3 (100.0) | 1 (100.0) |
| SRH, within 6 mm, n (%) | 18 (41.9) | 4 (30.8) | 11 (42.3) | 2 (66.7) | 1 (100.0) |
| SHRM within 6 mm, n (%) | 29 (67.4) | 7 (53.8) | 19 (73.1) | 3 (100.0) | 0 (0.0) |
| HRF within 6 mm, n (%) | 24 (55.8) | 5 (38.5) | 17 (65.4) | 2 (66.7) | 0 (0.0) |
| Intraretinal fluid in fovea, n (%) | 14 (32.6) | 4 (30.8) | 6 (23.1) | 3 (100.0) | 1 (100.0) |
| Subretinal fluid in fovea, n (%) | 36 (83.7) | 11 (84.6) | 22 (84.6) | 2 (66.7) | 1 (100.0) |
| Presence of PED in fovea, n (%) | 31 (72.1) | 10 (76.9) | 19 (73.1) | 1 (33.3) | 1 (100.0) |
| Number of Loading Phase Injections | ||||
|---|---|---|---|---|
| 2 Injections | 3 Injections | 4 Injections | p values | |
| Number of eyes, n (%) | 17 (39.5) | 10 (23.3) | 16 (37.2) | |
| Clinicodemographic characteristics | ||||
| Age (years), mean (SD) | 77.6 (8.9) | 74.4 (8.8) | 77.0 (9.4) | 0.656 |
| Sex: Female, n (%) | 4 (23.5) | 4 (40.0) | 4 (25.0) | 0.685 |
| Hypertension, n (%) | 12 (70.6) | 5 (50.0) | 12 (75.0) | 0.415 |
| Diabetes mellitus, n (%) | 4 (23.5) | 1 (10.0) | 3 (18.8) | 0.882 |
| Malignancy, n (%) | 4 (23.5) | 2 (20.0) | 3 (18.8) | 1.000 |
| History of cardiovascular events, n (%) | 4 (23.5) | 1 (10.0) | 1 (6.2) | 0.470 |
| Anticoagulant use, n (%) | 4 (23.5) | 2 (20.0) | 3 (18.8) | 1.000 |
| Smoking history, n (%) | 8 (47.1) | 6 (60.0) | 9 (56.2) | 0.793 |
| BCVA, logMAR, mean (SD) | 0.31 (0.28) | 0.39 (0.39) | 0.37 (0.32) | 0.818 |
| Anatomical parameters at baseline | ||||
| CRT (μm), mean (SD) | 257.8 (86.4) | 393.1 (182.6) | 319.9 (139.7) | 0.048 |
| CCT (μm), mean (SD) | 251.2 (103.0) | 251.1 (103.4) | 271.3 (97.5) | 0.820 |
| PED height at fovea (μm), mean (SD) | 99.0 (185.4) | 33.2 (38.0) | 54.3 (64.5) | 0.375 |
| Disrupted EZ at fovea, n (%) | 11 (64.7) | 8 (80.0) | 9 (56.2) | 0.514 |
| SRH, within 6 mm, n (%) | 6 (35.3) | 6 (60.0) | 6 (37.5) | 0.455 |
| SHRM within 6 mm, n (%) | 10 (58.8) | 10 (100) | 9 (56.2) | 0.030 |
| HRF within 6 mm, n (%) | 6 (35.3) | 7 (70.0) | 11 (68.8) | 0.098 |
| Intraretinal fluid in fovea, n (%) | 7 (41.2) | 4 (40.0) | 3 (18.8) | 0.346 |
| Subretinal fluid in fovea, n (%) | 11 (64.7) | 10 (100.0) | 15 (93.8) | 0.036 |
| Presence of PED in fovea, n (%) | 12 (70.6) | 6 (60.0) | 13 (81.2) | 0.516 |
| Subtype | 0.091 | |||
| Type 1 | 8 (47.1) | 2 (20.0) | 3 (18.8) | |
| Type 1 with polyps (PCV) | 6 (35.3) | 7 (70.0) | 13 (81.2) | 0.022 |
| Type 2 | 2 (11.8) | 1 (10.0) | 0 (0.0) | |
| Type 3 (RAP) | 1 (5.9) | 0 (0.0) | 0 (0.0) | |
| Clinical course at 1 year | ||||
| BCVA (logMAR), mean (SD) | 0.17 (0.35) | 0.20 (0.48) | 0.20 (0.25) | 0.974 |
| CRT (μm), mean (SD) | 167.6 (37.7) | 210.0 (51.1) | 191.4 (98.0) | 0.295 |
| Intraretinal fluid in fovea, n (%) | 0 (0.0) | 1 (10.0) | 1 (6.2) | 0.511 |
| Subretinal fluid in fovea, n (%) | 1 (5.9) | 2 (20.0) | 3 (18.8) | 0.540 |
| Treatment interval ≥12 weeks, n (%) | 14 (82.4) | 9 (90.0) | 9 (56.2) | 0.140 |
| Treatment interval ≥16 weeks, n (%) | 13 (76.5) | 6 (60.0) | 7 (43.8) | 0.163 |
| Total number of injections, mean (SD) | 5.7 (1.6) | 6.3 (0.7) | 7.8 (1.1) | 0.001 |
| Switched to another drug, n (%) | 1 (5.9) | 0 (0.0) | 3 (18.8) | 0.339 |
| Treatment Interval Extended to ≥16 Weeks | |||
|---|---|---|---|
| Success | Failed | p values | |
| Number of eyes, n (%) | 26 (60.5) | 17 (39.5) | |
| Clinicodemographic characteristics | |||
| Age (years), mean (SD) | 77.5 (9.4) | 75.2 (8.3) | 0.403 |
| Sex: Female, n (%) | 9 (34.6) | 3 (17.6) | 0.306 |
| Hypertension, n (%) | 19 (73.1) | 10 (58.8) | 0.507 |
| Diabetes mellitus, n (%) | 6 (23.1) | 2 (11.8) | 0.446 |
| Malignancy, n (%) | 4 (15.4) | 5 (29.4) | 0.445 |
| History of cardiovascular events, n (%) | 5 (19.2) | 1 (5.9) | 0.376 |
| Anticoagulant use, n (%) | 7 (26.9) | 2 (11.8) | 0.281 |
| Smoking history, n (%) | 13 (50.0) | 10 (58.8) | 0.756 |
| BCVA (logMAR), mean (SD) | 0.32 (0.25) | 0.40 (0.40) | 0.433 |
| Anatomical parameters at baseline | |||
| CRT (μm), mean (SD) | 294.9 (109.3) | 339.2 (178.6) | 0.317 |
| CCT (μm), mean (SD) | 251.3 (105.2) | 269.8 (90.9) | 0.555 |
| PED height at fovea (μm), mean (SD) | 35.3 (41.9) | 115.7 (184.8) | 0.038 |
| Disrupted EZ at fovea, n (%) | 13 (50.0) | 15 (88.2) | 0.020 |
| SRH, within 6 mm, n (%) | 8 (30.8) | 10 (58.8) | 0.114 |
| SHRM within 6 mm, n (%) | 17 (65.4) | 12 (70.6) | 1.000 |
| HRF within 6 mm, n (%) | 14 (53.8) | 10 (58.8) | 1.000 |
| Intraretinal fluid in fovea, n (%) | 9 (34.6) | 5 (29.4) | 1.000 |
| Subretinal fluid in fovea, n (%) | 22 (84.6) | 14 (82.4) | 1.000 |
| Presence of PED in fovea, n (%) | 18 (69.2) | 13 (76.5) | 0.735 |
| Subtype | 0.103 | ||
| Type 1 | 10 (38.5) | 3 (17.6) | |
| Type 1 with polyps (PCV) | 12 (46.2) | 14 (82.4) | 0.026 |
| Type 2 | 3 (11.5) | 0 (0.0) | |
| Type 3 (RAP) | 1 (3.8) | 0 (0.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machida, A.; Kurihara, J.; Hirata, Y.; Machida, E.; Murakami, R.; Oka, A.; Yoneda, A.; Tsuiki, E.; Oishi, A. Flexible Loading Phase Treat-and-Extend Regimen with Faricimab for Neovascular Age-Related Macular Degeneration: A Real-World Study. Biomedicines 2025, 13, 2909. https://doi.org/10.3390/biomedicines13122909
Machida A, Kurihara J, Hirata Y, Machida E, Murakami R, Oka A, Yoneda A, Tsuiki E, Oishi A. Flexible Loading Phase Treat-and-Extend Regimen with Faricimab for Neovascular Age-Related Macular Degeneration: A Real-World Study. Biomedicines. 2025; 13(12):2909. https://doi.org/10.3390/biomedicines13122909
Chicago/Turabian StyleMachida, Akira, Junko Kurihara, Yuki Hirata, Eriko Machida, Ryuya Murakami, Akari Oka, Ai Yoneda, Eiko Tsuiki, and Akio Oishi. 2025. "Flexible Loading Phase Treat-and-Extend Regimen with Faricimab for Neovascular Age-Related Macular Degeneration: A Real-World Study" Biomedicines 13, no. 12: 2909. https://doi.org/10.3390/biomedicines13122909
APA StyleMachida, A., Kurihara, J., Hirata, Y., Machida, E., Murakami, R., Oka, A., Yoneda, A., Tsuiki, E., & Oishi, A. (2025). Flexible Loading Phase Treat-and-Extend Regimen with Faricimab for Neovascular Age-Related Macular Degeneration: A Real-World Study. Biomedicines, 13(12), 2909. https://doi.org/10.3390/biomedicines13122909








