When the Liver Echoes to the Heart: Assessing Subclinical Cardiac Dysfunction in NAFLD Using Speckle Tracking Echocardiography—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Risk of Bias Assessment in Individual Studies
2.4. Summary Measures and Synthesis of Results
3. Results
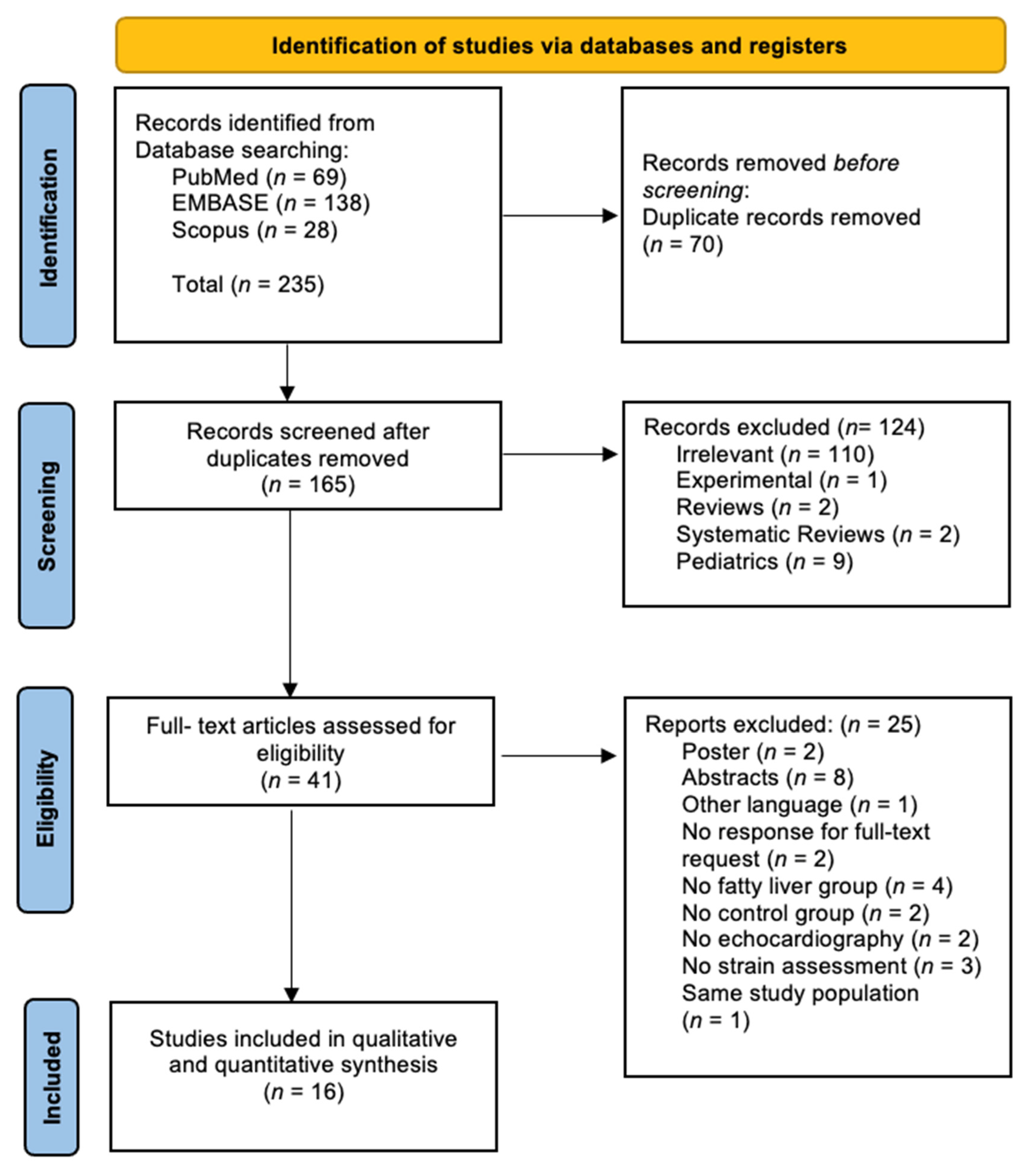
3.1. General Results
3.2. Study Characteristics
3.3. Definition of Hepatic Steatosis
3.4. Assessment of Strain Metrics and Additional Parameters
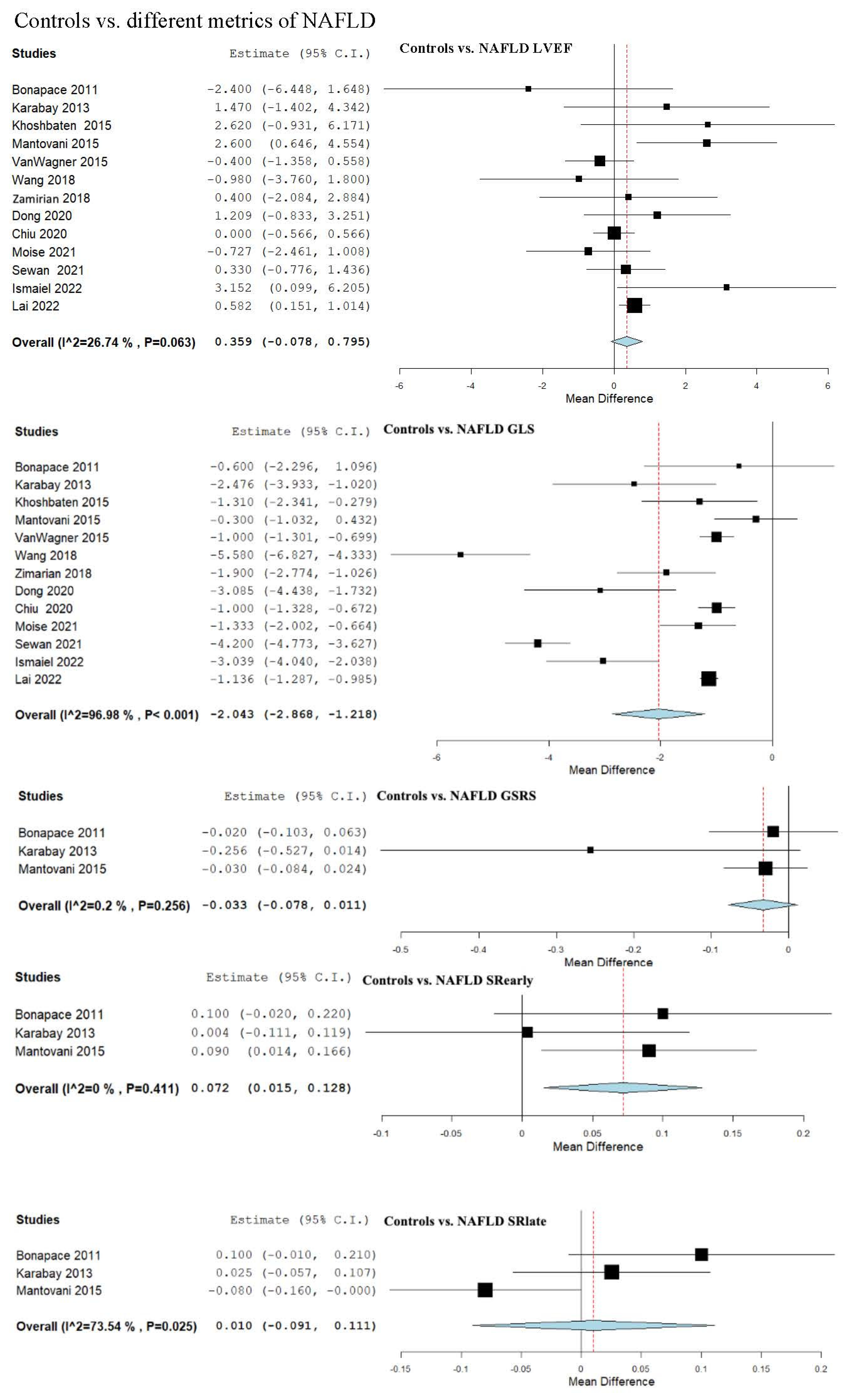
3.5. Strain and LVEF Assessment in Controls vs. NAFLD
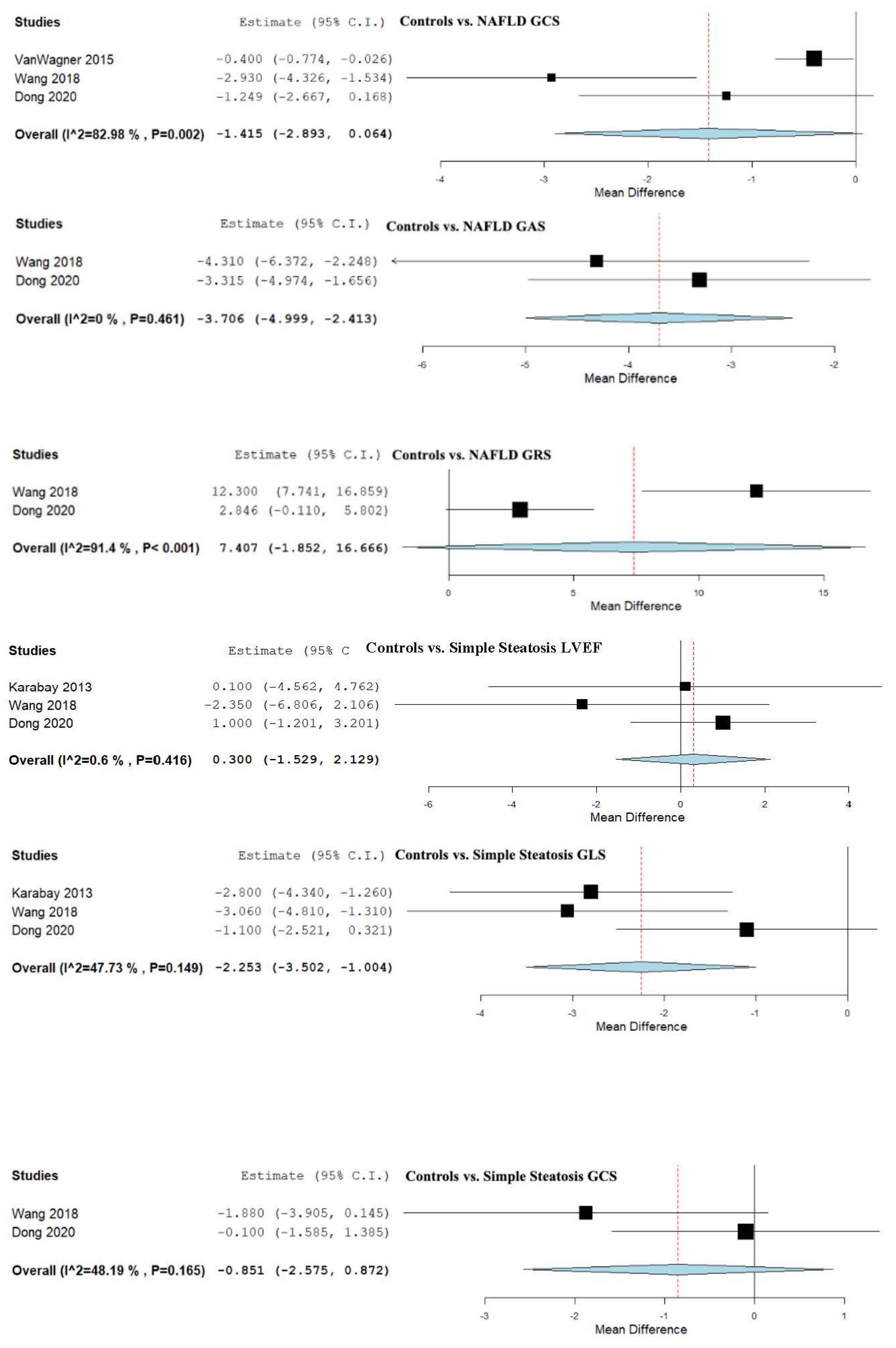
3.6. Strain and LVEF Assessment in Controls vs. Simple Steatosis
3.7. Strain and LVEF Assessment in Controls vs. Moderate/Severe NAFLD
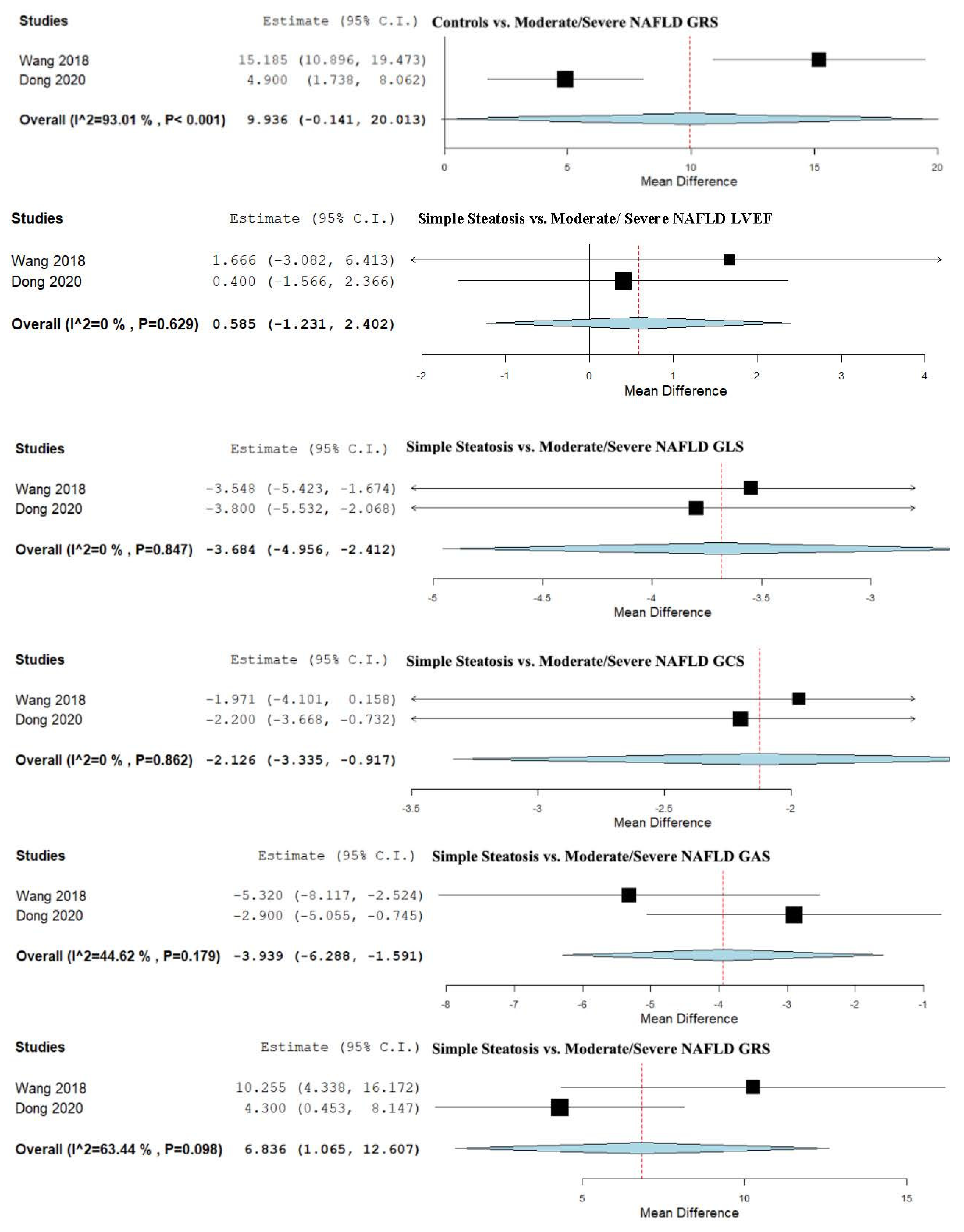
3.8. Strain and LVEF Assessment in Simple Steatosis vs. Moderate/Severe NAFLD
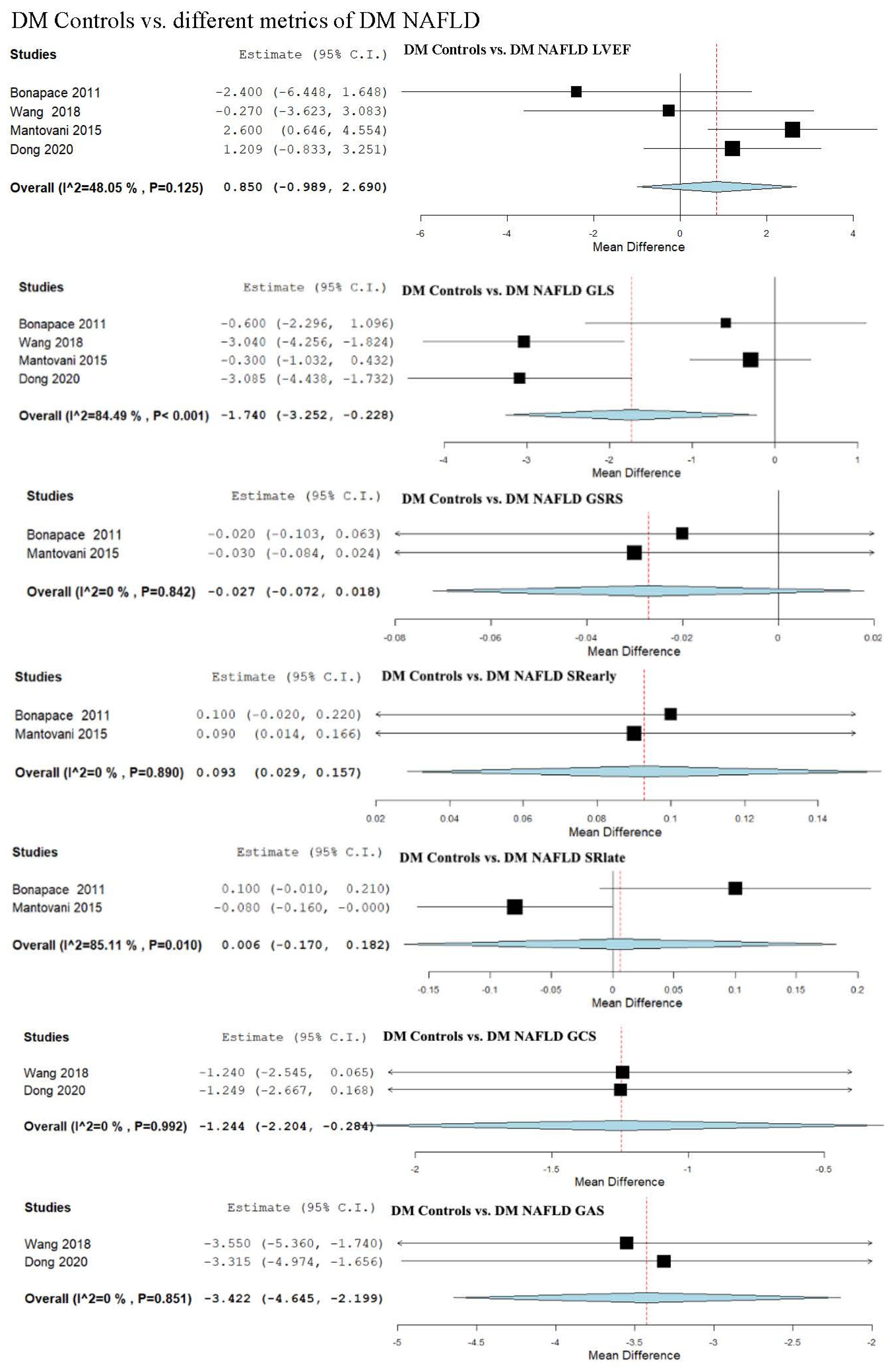
3.9. Strain Assessment in Diabetes Mellitus (DM) Patients vs. DM + NAFLD Patients
3.10. Strain and LVEF Assessment in Patients with DM Patients vs. DM + Mild NAFLD
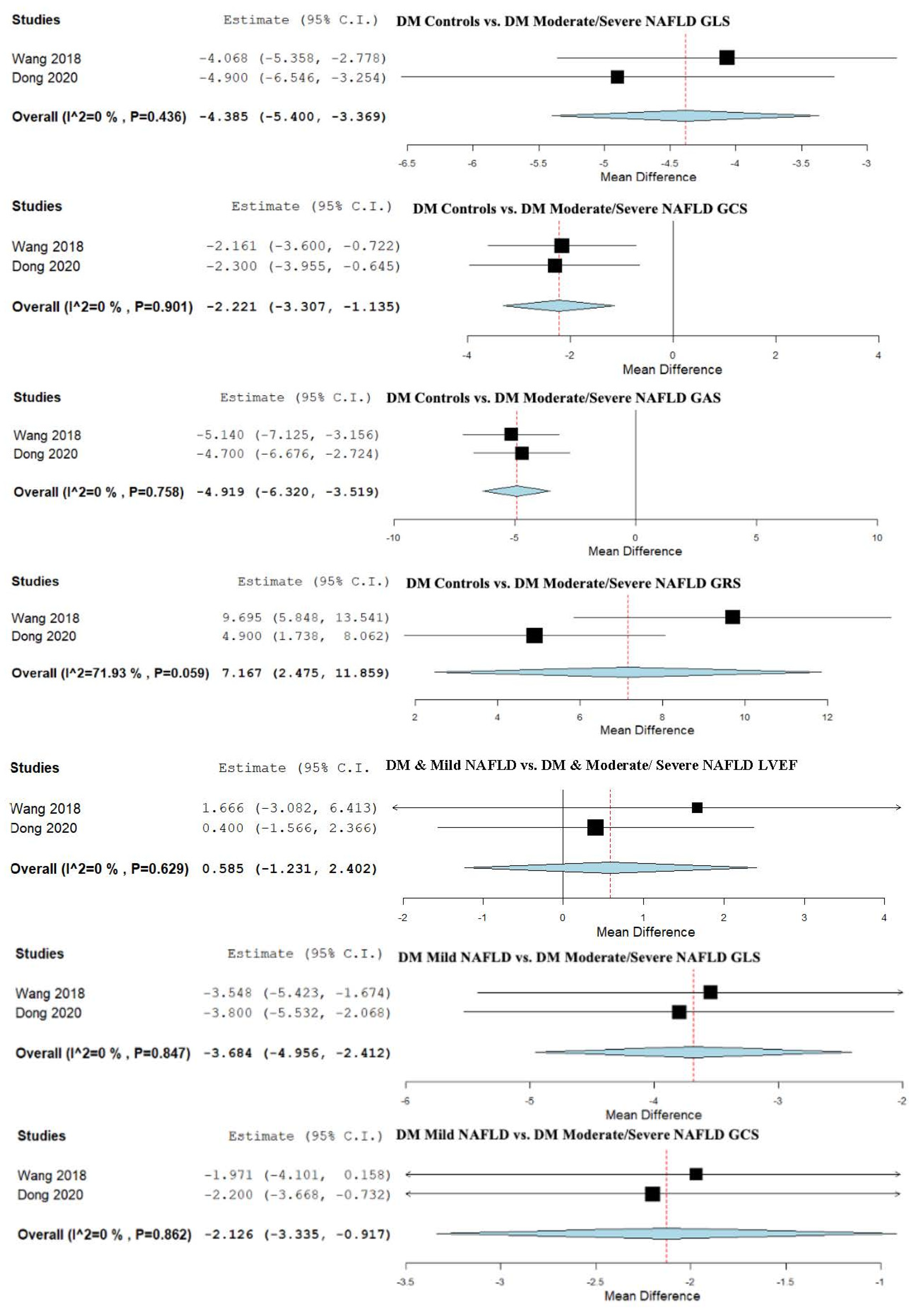
3.11. Strain and LVEF Assessment in DM Patients vs. DM + Moderate/Severe NAFLD
3.12. Strain and LVEF Assessment in Patients with DM and Mild NAFLD vs. Patients with DM and Moderate/Severe NAFLD
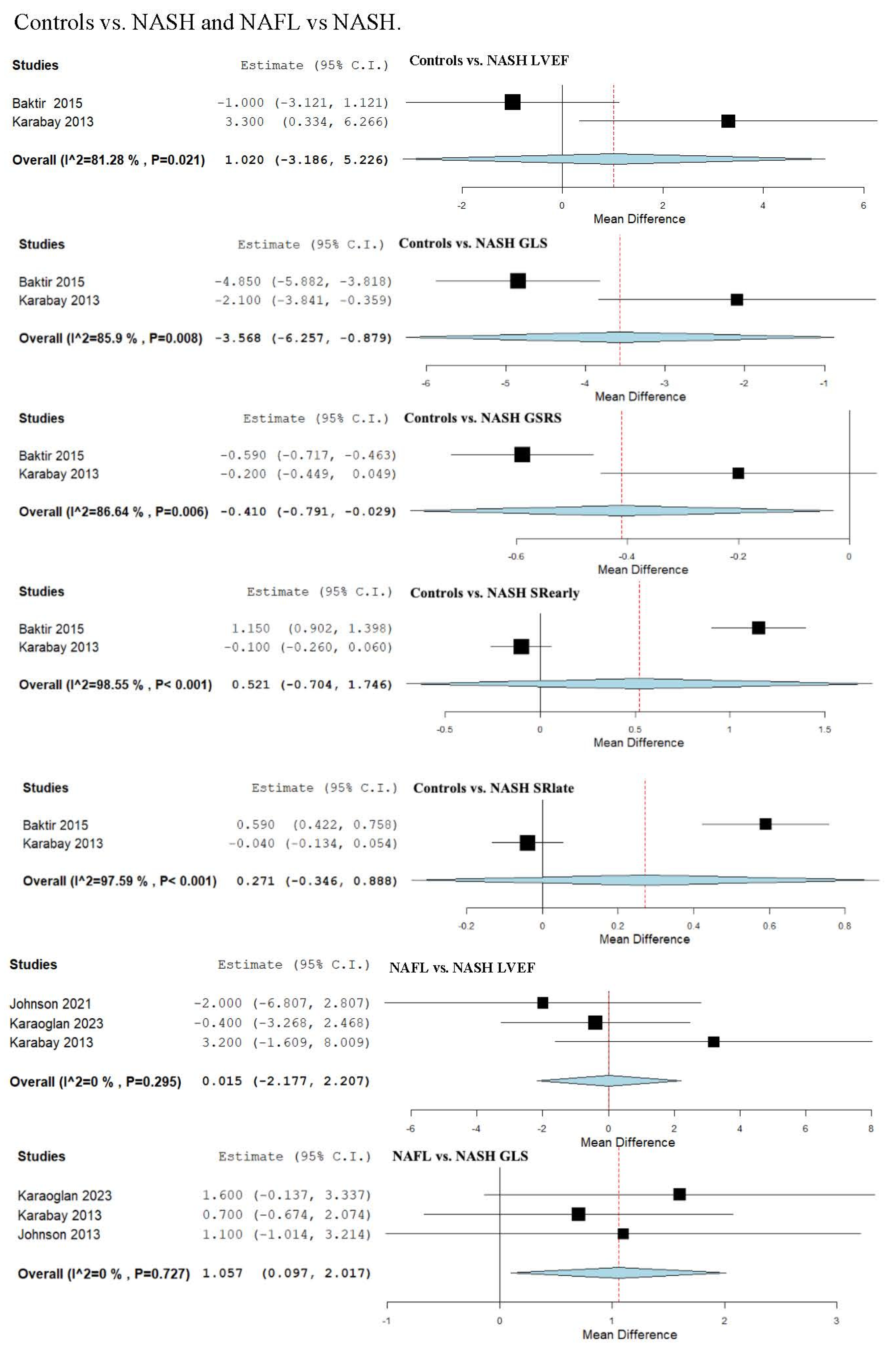
3.13. Strain and LVEF Assessment in Controls vs. NASH
3.14. Strain and LVEF Assessment in Non-Alcoholic Fatty Liver (NAFL) vs. NASH
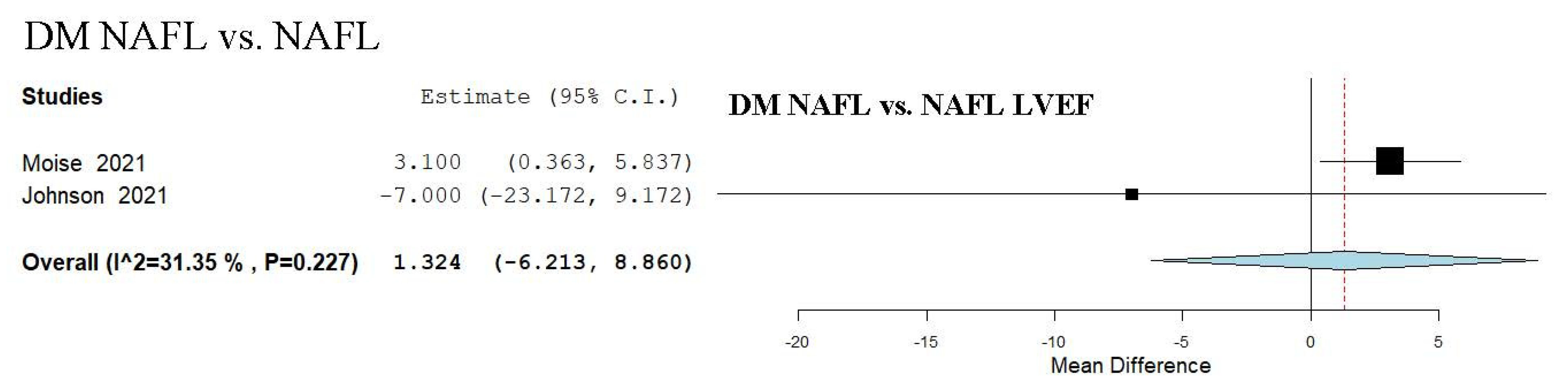
3.15. LVEF Assessment in DM NAFL vs. NAFL
3.16. Quality Assessment
4. Discussion
4.1. Main Findings
4.2. Controls vs. NAFLD and Severity Spectrum
4.3. Diabetes Subgroups and NAFLD Severity
4.4. NASH vs. NAFL and Controls
4.5. Diastolic Strain Parameter
4.6. Pathophysiological Mechanisms
4.7. Clinical Implications
4.8. Study Limitations and Strengths
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Ismaiel, A.; Al Srouji, N. Subclinical Left Ventricular Systolic Dysfunction Assessed Using Myocardial Strain Measured by Speckle Tracking in Non-Alcoholic Fatty Liver Disease—Systematic Review. Glob. J. Med. Ther. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Bisaccia, G.; Ricci, F.; Khanji, M.Y.; Sorella, A.; Melchiorre, E.; Iannetti, G.; Galanti, K.; Mantini, C.; Pizzi, A.D.; Tana, C.; et al. Cardiovascular morbidity and mortality related to non-alcoholic fatty liver disease: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2023, 48, 101643. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef]
- Hallsworth, K.; Hollingsworth, K.G.; Thoma, C.; Jakovljevic, D.; MacGowan, G.A.; Anstee, Q.M.; Taylor, R.; Day, C.P.; Trenell, M.I. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 757–762. [Google Scholar] [CrossRef]
- Ismaiel, A.; Dumitraşcu, D.L. Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis—Literature Review. Front. Med. 2019, 6, 202. [Google Scholar] [CrossRef]
- Ismaiel, A.; Colosi, H.A.; Rusu, F.; Dumitrașcu, D.L. Cardiac Arrhythmias and Electrocardiogram Modifications in Non-Alcoholic Fatty Liver Disease. A systematic Review. J. Gastrointest. Liver Dis. 2019, 28, 483–493. [Google Scholar] [CrossRef]
- Zamirian, M.; Samiee, E.; Moaref, A.; Abtahi, F.; Tahamtan, M. Assessment of Subclinical Myocardial Changes in Non-Alcoholic Fatty Liver Disease: A Case-Control Study Using Speckle Tracking Echocardiography. Iran. J. Med. Sci. 2018, 43, 466–472. [Google Scholar]
- Vancheri, F.; Longo, G.; Henein, M.Y. Left ventricular ejection fraction: Clinical, pathophysiological, and technical limitations. Front. Cardiovasc. Med. 2024, 11, 1340708. [Google Scholar] [CrossRef] [PubMed]
- Klaeboe, L.G.; Edvardsen, T. Echocardiographic assessment of left ventricular systolic function. J. Echocardiogr. 2019, 17, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.J.; Altman, M.; Stanton, T.; Thomas, L. Echocardiographic Strain in Clinical Practice. Heart Lung Circ. 2019, 28, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Scatteia, A.; Silverio, A.; Padalino, R.; De Stefano, F.; America, R.; Cappelletti, A.M.; Vecchia, L.A.D.; Guarini, P.; Donatelli, F.; Caiazza, F.; et al. Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand? J. Pers. Med. 2021, 11, 1153. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, W.; Xia, J. Nonalcoholic Fatty Liver Is Associated With Further Left Ventricular Abnormalities in Patients With Type 2 Diabetes Mellitus: A 3-Dimensional Speckle-Tracking Study. J. Ultrasound Med. 2018, 37, 1899–1911. [Google Scholar] [CrossRef]
- Chiu, L.S.; Pedley, A.; Massaro, J.M.; Benjamin, E.J.; Mitchell, G.F.; McManus, D.D.; Aragam, J.; Vasan, R.S.; Cheng, S.; Long, M.T. The Association of Non-Alcoholic Fatty Liver Disease and Cardiac Structure and Function—Framingham Heart Study. Liver Int. 2020, 40, 2445–2454. [Google Scholar] [CrossRef]
- Orde, S.; Huang, S.J.; Mclean, A.S. Speckle tracking echocardiography in the critically ill: Enticing research with minimal clinical practicality or the answer to non-invasive cardiac assessment? Anaesth. Intensiv. Care 2016, 44, 542–551. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Wilcox, J.E.; Colangelo, L.A.; Lloyd-Jones, D.M.; Carr, J.J.; Lima, J.A.; Lewis, C.E.; Shah, S.J. Associations of Nonalcoholic Fatty Liver Disease with Subclinical Myocardial Dysfunction: The CARDIA Study. Circulation 2014, 129, A52. [Google Scholar] [CrossRef]
- Sewan, H.S.; Aljumaily, H.S. Frequency of Left Ventricle Dysfunction in non-Alcoholic Fatty Liver Disease (NAFLD) Patients Detected by Global Longitudinal Strain and Tissue Doppler Imaging in Babylon Province in Iraq. Med.-Leg. Update 2021, 21, 1380–1386. [Google Scholar]
- Khoshbaten, M.; Parkhideh, S.; Toufan, M. Comparison of Left Ventricular Function Between Patients with Non-Alcoholic Fatty Liver Disease and Healthy Individuals. Biomed. Pharmacol. J. 2015, 8, 749–754. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Henkens, M.T.H.M.; Wang, P.; Schummers, G.; Raafs, A.G.; Krapels, I.P.C.; van Empel, V.; Heymans, S.R.; Rocca, H.B.; Knackstedt, C. A global longitudinal strain cut-off value to predict adverse outcomes in individuals with a normal ejection fraction. ESC Heart Fail. 2021, 8, 4343–4345. [Google Scholar] [CrossRef]
- Song, Q.-R.; Liu, S.-L.; Bi, Y.-G.; Chen, S.-H.; Wu, S.-L.; Cai, J. Non-alcoholic fatty liver disease is associated with cardiovascular outcomes in subjects with prediabetes and diabetes: A prospective community-based cohort study. Front. Cardiovasc. Med. 2022, 9, 889597. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Chang, W.; Li, Y.; Li, G. Three-dimensional speckle tracking echocardiography in evaluation on left ventricular function in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease. Chin. J. Med. Imaging Technol. 2019, 35, 837–842. [Google Scholar]
- Armandi, A.; Andreis, A.; Bellettini, M.; Caviglia, G.F.; Castelnuovo, G.; Poggiolini, I.; Rosso, C.; Del Campo, N.P.D.; Abdulle, A.; Gjini, K.; et al. Echocardiography-based markers of subclinical cardiac dysfunction in individuals with Non-Alcoholic Fatty Liver Disease and preserved ejection fraction: Interim data from a prospective study. Dig. Liver Dis. 2023, 55, S36. [Google Scholar] [CrossRef]
- Catena, C.; Brosolo, G.; Da Porto, A.; Donnini, D.; Bulfone, L.; Vacca, A.; Soardo, G.; Sechi, L.A. Association of non-alcoholic liver disease with left ventricular changes in treatment-naive patients with uncomplicated hypertension. Front. Cardiovasc. Med. 2022, 9, 1030968. [Google Scholar] [CrossRef]
- Chiu, L.S.; Pedley, A.; Massaro, J.; Mitchell, G.F.; Vasan, R.; Benjamin, E.J.; Cheng, S.; Long, M.T. The Association of Non-Alcoholic Fatty Liver Disease and Altered Cardiac Structure and Function. Hepatology 2023, 78 (Suppl. S1). [Google Scholar] [CrossRef]
- Gupta, U.K.; Karoliya, V.; Gattani, R.K.; Das, D.; Dixit, V.K.; Shukla, S.K.; Yadav, D.P.; Kumar, V.; Tiwari, A. Assessment of Subclinical Cardiovascular Dis-ease in NAFLD Patients. J. Clin. Exp. Hepatol. 2023, 13, S126. [Google Scholar] [CrossRef]
- Hei, A.V.; Bush, K.; Fentanes, E.; Aden, J.; Paredes, A.; Harrison, S.; Thomas, D. Structural and Functional Changes by 3-Dimensional (3D) Transthoracic Echocardiography and Global Longitudinal Strain in Asymptomatic Patients with Biopsy-Proven Nafld: Results from the Prospective Prevlence Study. J. Am. Coll. Cardiol. 2019, 73 (Suppl. S1), 1596. [Google Scholar] [CrossRef]
- Medina, C.; VanWagner, L.; Daruwalla, V.; Kia, L.; Boyd, D.; Friedman, J.; Unger, E.; Shah, S.J.; Rinella, M. Determinants of abnormal cardiac mechan-ics in liver transplant candidates with hepatic steatosis. Circulation 2014, 130 (Suppl. S2), A20341. [Google Scholar]
- Vanni, E.; Mezzabotta, L.; Faletti, R.; Morello, M.; Marengo, A.; Battisti, G.; Frea, S.; Cannillo, M.; Mosso, E.; Rosso, C.; et al. P1056: Insulin resistance and liver damage are associated with early signs of left ventricular systolic dysfunction in patients with nonalcoholic fatty liver disease, independently of diabetes, hypertension and dyslipidemia. J. Hepatol. 2015, 62 (Suppl. S2), 744–745. [Google Scholar] [CrossRef]
- Baktır, A.O.; Sarli, B.; Altekin, E.R.; Arinc, H.; Saglam, H.; Dogan, Y. Non Alcoholic Steatohepatitis is Associated with Subclinical Impairment in Left Ventricular Function Measured by Speckle Tracking Echocardiography. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S2), C159. [Google Scholar] [CrossRef]
- Dimitroglou, Y.; Aggeli, C.; Alexopoulou, A.; Alexopoulos, T.; Nitsa, A.; Apostolou, I.; Patsourakos, D.; Vasilieva, L.; Dourakis, S.P.; Tousoulis, D. P1768 The value of global longitudinal strain in non-alcoholic steatohepatitis-associated cirrhosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21 (Suppl. S1), jez319.1126. [Google Scholar] [CrossRef]
- Demková, K.; Varga, T.; Tokarcík, J. Association of non-alcoholic fatty liver disease with cardiac structural impairment. J. Gastroenterol. Hepatol. 2022, 76, 341–346. [Google Scholar] [CrossRef]
- Tom, N.B.; Harshvardhan, L.; Singhal, M.; Agarwal, M.; Mathur, A.; Jain, T. A Study of Left Ventricular Dysfunction in Normotensive Non Diabetic Patients with Non Alcoholic Fatty Liver Disease. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Altekin, R.E.; Caglar, B.; Karakas, M.S.; Ozel, D.; Deger, N.; Demir, I. Evaluation of subclinical left ventricular systolic dysfunction using two-dimensional speckle-tracking echocardiography in patients with non-alcoholic cirrhosis. Hellenic J. Cardiol. 2014, 55, 402–410. [Google Scholar] [PubMed]
- Apostu, A.; Malita, D.; Arnautu, S.-F.; Tomescu, M.-C.; Gaiță, D.; Popescu, A.; Mare, R.; Gidea, R.; Arnautu, D.-A. Significant Association between Subclinical Left Cardiac Dysfunction and Liver Stiffness in Metabolic Syndrome Patients with Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease. Medicina 2023, 59, 328. [Google Scholar] [CrossRef] [PubMed]
- Parvanescu, T.; Vitel, A.; Sporea, I.; Mare, R.; Buz, B.; Bordejevic, D.A.; Tomescu, M.C.; Arnautu, S.F.; Morariu, V.I.; Citu, I.M. Significant Association Between Left Ventricular Diastolic Dysfunction, Left Atrial Performance and Liver Stiffness in Patients with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Vitel, A.; Sporea, I.; Mare, R.; Banciu, C.; Bordejevic, D.-A.; Parvanescu, T.; Citu, I.M.; Tomescu, M.C. Association Between Subclinical Left Ventricular Myocardial Systolic Dysfunction Detected by Strain and Strain-Rate Imaging and Liver Steatosis and Fibrosis Detected by Elastography and Controlled Attenuation Parameter in Patients with Metabolic Syndrome. Diabetes Metab. Syndr. Obes. 2020, 13, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Cerini, F.; Cerrone, A.; Argiento, L.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; Rumi, M.G.; Viganò, M. Liver stiffness measurement identi-fies subclinical myocardial dysfunction in non-advanced non-alcoholic fatty liver disease patients without overt heart disease. Intern. Emerg. Med. 2022, 17, 1425–1438. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cerini, F.; Nicolosi, G.L.; Lombardo, M.; Rumi, M.G.; Viganò, M. Left ventricular strain predicts subclinical atherosclerosis in nonadvanced nonalcoholic fatty liver disease patients. Eur. J. Gastroenterol. Hepatol. 2022, 34, 707–716. [Google Scholar] [CrossRef]
- Huang, S.; Shi, K.; Li, Y.; Wang, J.; Jiang, L.; Gao, Y.; Yan, W.; Shen, L.; Yang, Z. Effect of Metabolic Dysfunction-Associated Fatty Liver Disease on Left Ventricular Deformation and Atrioventricular Coupling in Patients With Metabolic Syndrome Assessed by MRI. J. Magn. Reson. Imaging 2023, 58, 1098–1107. [Google Scholar] [CrossRef]
- de Freitas Diniz, T.B.; de Jesus, R.N.; Jimenez, L.S.; Pareja, J.C.; Chaim, E.A.; Cazzo, E. Non-Alcoholic Fatty Liver Disease Is Associated with Impairment of Ejection Fraction Among Individuals with Obesity Undergoing Bariatric Surgery: Results of a Cross-Sectional Study. Obes. Surg. 2020, 30, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Fotbolcu, H.; Yakar, T.; Duman, D.; Karaahmet, T.; Tigen, K.; Cevik, C.; Kurtoglu, U.; Dindar, I. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol. J. 2010, 17, 457–463. [Google Scholar]
- Goland, S.; Shimoni, S.; Zornitzki, T.; Knobler, H.; Azoulai, O.; Lutaty, G.; Melzer, E.; Orr, A.M.; Caspi, A.; Malnick, S.M. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: Echocardiographic and tissue Doppler imaging assessment. J. Clin. Gastroenterol. 2006, 40, 949–955. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Wilcox, J.E.; Ning, H.; Lewis, C.E.; Carr, J.J.; Rinella, M.E.; Shah, S.J.; Lima, J.A.C.; Lloyd-Jones, D.M. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J. Am. Heart Assoc. 2020, 9, e014279. [Google Scholar] [CrossRef]
- Moise, C.G.; Donoiu, I.; Târtea, G.C.; Mirea, O.; Rogoveanu, I. Contribution of Modern Echocardiographic Techniques in the Detection of Subclinical Heart Dysfunction in Young Adults with Non-Alcoholic Fatty Liver Disease. Curr. Health Sci. J. 2021, 47, 275–283. [Google Scholar] [PubMed]
- Lai, Y.-H.; Su, C.-H.; Hung, T.-C.; Yun, C.-H.; Tsai, C.-T.; Yeh, H.-I.; Hung, C.-L. Association of Non-Alcoholic Fatty Liver Disease and Hepatic Fibrosis with Epicardial Adipose Tissue Volume and Atrial Deformation Mechanics in a Large Asian Population Free from Clinical Heart Failure. Diagnostics 2022, 12, 916. [Google Scholar] [CrossRef]
- Bonapace, S.; Perseghin, G.; Molon, G.; Canali, G.; Bertolini, L.; Zoppini, G.; Barbieri, E.; Targher, G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care 2012, 35, 389–395. [Google Scholar] [CrossRef]
- Karabay, C.Y.; Kocabay, G.; Kalayci, A.; Colak, Y.; Oduncu, V.; Akgun, T.; Kalkan, S.; Guler, A.; Kirma, C. Impaired left ventricular mechanics in non-alcoholic fatty liver disease: A speckle-tracking echocardiography study. Eur. J. Gastroenterol. Hepatol. 2014, 26, 325–331. [Google Scholar] [CrossRef]
- Vanwagner, L.B.; Wilcox, J.E.; Colangelo, L.A.; Lloyd-Jones, D.M.; Carr, J.J.; Lima, J.A.; Lewis, C.E.; Rinella, M.E.; Shah, S.J. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology 2015, 62, 773–783. [Google Scholar] [CrossRef]
- Mantovani, A.; Pernigo, M.; Bergamini, C.; Bonapace, S.; Lipari, P.; Pichiri, I.; Bertolini, L.; Valbusa, F.; Barbieri, E.; Zoppini, G.; et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135329. [Google Scholar] [CrossRef]
- Baktır, A.O.; Şarlı, B.; Emre Altekin, R.; Karaman, A.; Arınç, H.; Sağlam, H.; Dogan, Y.; Erden, A.; Karaman, H. Non alcoholic steatohepatitis is associated with subclinical impairment in left ventricular function measured by speckle tracking echocardiography. Anatol. J. Cardiol. 2015, 15, 137–142. [Google Scholar] [CrossRef]
- Johnson, P.C.; Cochet, A.A.; Gore, R.S.; Harrison, S.A.; Magulick, J.P.; Aden, J.K.; Paredes, A.H. Early Cardiac Dysfunction in Biopsy-proven Nonalcoholic Fatty Liver Disease. Korean J. Gastroenterol. 2021, 78, 161–167. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, D.; Sun, L.; Wang, Y.; Li, Y.; Chang, W.; Li, G.; Cui, H. Assessment of left ventricular function in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease using three-dimensional speckle-tracking echocardiography. Anatol. J. Cardiol. 2019, 23, 41–48. [Google Scholar]
- Ismaiel, A.; Spinu, M.; Socaciu, C.; Budisan, L.; Leucuta, D.-C.; Popa, S.-L.; Chis, B.A.; Berindan-Neagoe, I.; Olinic, D.M.; Dumitrascu, D.L. Metabolic biomarkers related to cardiac dysfunction in metabolic-dysfunction-associated fatty liver disease: A cross-sectional analysis. Nutr. Diabetes 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlan, B.B.; Tulunay, C.; Uzun, Ç.; Peker, E.; Özyüncü, N.; Ellik, Z.; Kuru, D.; Turhan, S.; Savas, B.; Erden, A.; et al. Determining Subclinical Cardiovascular and Cardiac Diseases in Patients with Non-Alcoholic Fatty Liver Disease. Turk. J. Gastroenterol. 2023, 34, 242–253. [Google Scholar] [CrossRef]
- Dandel, M.; Lehmkuhl, H.; Knosalla, C.; Suramelashvili, N.; Hetzer, R. Strain and Strain rate imaging by echocardiog-raphy-basic concepts and clinical applicability. Curr. Cardiol. Rev. 2009, 5, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Pandey, A.; Soloveva, A.; Abdelmalek, M.F.; Diehl, A.M.; Moylan, C.A.; Wegermann, K.; Rao, V.N.; Hernandez, A.F.; Tedford, R.J.; et al. Relationship of Nonalcoholic Fatty Liver Disease and Heart Failure With Preserved Ejection Fraction. JACC Basic Transl. Sci. 2021, 6, 918–932. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2020, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rashid, M.A.A.; El Sebaie, M.H.; Nasr Fouda, M.R.; Salem, A.M. Evaluation of subclinical LV systolic dysfunction by speckle tracking echocardiography in patients with Non-alcoholic fatty liver disease. Int. J. Health Sci. 2025, 31, 945–953. [Google Scholar] [CrossRef]
- Houghton, D.; Zalewski, P.; Hallsworth, K.; Cassidy, S.; Thoma, C.; Avery, L.; Slomko, J.; Hardy, T.; Burt, A.D.; Tiniakos, D.; et al. The degree of hepatic steatosis associates with impaired cardiac and autonomic function. J. Hepatol. 2019, 70, 1203–1213. [Google Scholar] [CrossRef]
- Chang, T.W.; Hsu, H.C.; Tsai, W.C. Association of left ventricular global area strain derived from resting 3D speckle-tracking echocardiography and exercise capacity in individuals undergoing treadmill exercise test. Int. J. Med. Sci. 2022, 19, 1576–1585. [Google Scholar] [CrossRef]
- Støylen, A.; Mølmen, H.E.; Dalen, H. Left ventricular global strains by linear measurements in three dimensions: Interrelations and relations to age, gender and body size in the HUNT Study. Open Heart 2019, 6, e001050. [Google Scholar] [CrossRef] [PubMed]
- Mihos, C.G.; Liu, J.E.; Anderson, K.M.; Pernetz, M.A.; O’Driscoll, J.M.; Aurigemma, G.P.; Ujueta, F.; Wessly, P.; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular and Stroke Nursing; et al. Speckle-tracking strain echocardiography for the assessment of left ventricular structure and function: A scientific statement from the American Heart Association. Circulation 2025, 152, e96–e109. [Google Scholar] [CrossRef] [PubMed]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, L.; Hu, X.; Gao, L.; Ji, M.; He, Q.; Xie, M.; Li, Y. Clinical usefulness of speckle-tracking echocardiography in patients with heart failure with preserved ejection fraction. Diagnostics 2023, 13, 2923. [Google Scholar] [CrossRef]
- Tang, X.; Shi, R.; Jiang, L.; Yan, W.F.; Han, P.L.; Qian, W.L.; Yang, Z.G.; Li, Y. Additive effect of metabolic dysfunction-associated fatty liver disease on left ventricular function and global strain in type 2 diabetes mellitus patients: A 3.0 T cardiac magnetic resonance feature tracking study. Cardiovasc. Diabetol. 2024, 23, 317. [Google Scholar] [CrossRef]
- Akasheva, D.U.; Utina, T.G.; Dzhioeva, O.N.; Drapkina, O.M. Subclinical left ventricular dysfunction over seven-year follow-up in type 2 diabetes patients without cardiovascular diseases. Biomedicines 2024, 12, 2031. [Google Scholar] [CrossRef]
- Ghoreyshi-Hefzabad, S.M.; Jeyaprakash, P.; Gupta, A.; Vo, H.Q.; Pathan, F.; Negishi, K. Three-Dimensional Global Left Ventricular Myocardial Strain Reduced in All Directions in Subclinical Diabetic Cardiomyopathy: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e020811. [Google Scholar] [CrossRef]
- Cassidy, S.; Hallsworth, K.; Thoma, C.; MacGowan, G.A.; Hollingsworth, K.G.; Day, C.P.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. Cardiac structure and function are altered in type 2 diabetes and non-alcoholic fatty liver disease and associate with glycemic control. Cardiovasc. Diabetol. 2015, 14, 23. [Google Scholar] [CrossRef]
- Brar, P.C.; Chun, A.; Fan, X.; Jani, V.; Craft, M.; Bhatla, P.; Kutty, S. Impaired myocardial deformation and ventricular vascular coupling in obese adolescents with dysglycemia. Cardiovasc. Diabetol. 2019, 18, 172. [Google Scholar] [CrossRef]
- Heinemann, F.; Gross, P.; Zeveleva, S.; Qian, H.S.; Hill, J.; Höfer, A.; Jonigk, D.; Diehl, A.M.; Abdelmalek, M.; Lenter, M.C.; et al. Deep learning-based quantification of NAFLD/NASH progression in human liver biopsies. Sci. Rep. 2022, 12, 19236. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Yu, Z.; Wang, M.; Shi, J.; Sun, L.; Zhang, Y.; Zhao, W.; Chen, C.; Tang, J.; Wang, C.; et al. Association of metabolic dysfunction-associated fatty liver disease with left ventricular diastolic function and cardiac morphology. Front. Endocrinol. 2022, 13, 935390. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, G.; Kalinowski, P.; Michałowski, L.; Paluszkiewicz, R.; Ziarkiewicz-Wróblewska, B.; Zieniewicz, K.; Tataj, E.; Rabczenko, D.; Szmigielski, C.A.; Sinski, M. Cardiac morphology, function, and hemodynamics in patients with morbid obesity and nonalcoholic steatohepatitis. J. Am. Heart Assoc. 2021, 10, e017371. [Google Scholar] [CrossRef]
- Varghese, J.; Devadas, K.; Vinayakumar, N.; Nahaz, N.; Hareendran, A.; Oommen, T.T.; George, B. Cardiac dysfunction in a cohort of biopsy proven nonalcoholic steatohepatitis in comparison to nonalcoholic fatty liver. Egypt. Liver J. 2023, 13, 3. [Google Scholar] [CrossRef]
- Goliopoulou, A.; Theofilis, P.; Oikonomou, E.; Anastasiou, A.; Pantelidis, P.; Gounaridi, M.I.; Zakynthinos, G.E.; Katsarou, O.; Kassi, E.; Lambadiari, V.; et al. Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 14292. [Google Scholar] [CrossRef] [PubMed]
- Gohil, N.V.; Tanveer, N.; Makkena, V.K.; Jaramillo, A.P.; Awosusi, B.L.; Ayyub, J.; Dabhi, K.N.; Nath, T.S. Non-alcoholic fatty liver disease and its association with left ventricular diastolic dysfunction: A systematic review. Cureus 2023, 15, e43013. [Google Scholar] [CrossRef]
- Ratti, C.; Malaguti, M.; Emanuele, D.A.; Bellasi, A.; Sanna, G. Understanding MASLD—From molecular pathogenesis to cardiovascular risk: A concise review for the clinical cardiologist. Atherosclerosis 2025, 409, 120495. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hinds, T.D.; Stec, D.E. Mechanisms linking metabolic-associated fatty liver disease (MAFLD) to cardiovascular disease. Curr. Hypertens. Rep. 2023, 25, 151–162. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical utility of three-dimensional speckle-tracking echocardiog-raphy in heart failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cerini, F.; Fagiani, V.; Nicolosi, G.L.; Rumi, M.G.; Lombardo, M.; Muti, P. Effect of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) on Left Ventricular Mechanics in Patients Without Overt Cardiac Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging-a clinical overview of Ultra-sound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Alamolhoda, M.; Ayatollahi, S.M.T.; Bagheri, Z. A comparative study of the impacts of unbalanced sample sizes on the four synthesized methods of meta-analytic structural equation modeling. BMC Res. Notes 2017, 10, 446. [Google Scholar] [CrossRef]
- Sitia, S.; Tomasoni, L.; Turiel, M. Speckle tracking echocardiography: A new approach to myocardial function. World J. Cardiol. 2010, 2, 1–5. [Google Scholar] [CrossRef]
- Zou, H.; Ma, X.; Pan, W.; Xie, Y. Comparing similarities and differences between NAFLD, MAFLD, and MASLD in the general U.S. population. Front. Nutr. 2024, 11, 1411802. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in cardiovascular disease mortality rates and excess deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Tavernese, A.; Rizza, V.; Cammalleri, V.; Mollace, R.; Carresi, C.; Antonelli, G.; Cocco, N.; D’antonio, L.; Gelfusa, M.; Piccirillo, F.; et al. Early Echocardiographic Markers in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Dev. Dis. 2025, 12, 229. [Google Scholar] [CrossRef] [PubMed]



| Study (Author, Year) | Country | Study Design | Total Subjects (N) | NAFLD Diagnosis Method | Key Comparison Groups | Key GLS Finding | NOS Score (/10) |
|---|---|---|---|---|---|---|---|
| Bonapace S et al., 2011 [49] | Italy | Cross-sectional | 50 | Ultrasound (US) | Controls vs. NAFLD (all DM) | Reduced in NAFLD | 7 |
| Karabay C et al., 2013 [50] | Turkey | Cross-sectional | 76 | Biopsy | Controls vs. Simple Steatosis vs. NASH | Reduced in all NAFLD groups | 7 |
| Khoshtbaten M et al., 2015 [18] | Iran | Cross-sectional | 60 | US | Controls vs. NAFLD | Reduced in NAFLD | 7 |
| Baktir A et al., 2015 [53] | Turkey | Cross-sectional | 56 | Biopsy | Controls vs. NASH | Reduced in NASH | 7 |
| Mantovani A et al., 2015 [52] | Italy | Cross-sectional | 222 | US | DM Controls vs. DM NAFLD | Reduced in NAFLD | 7 |
| VanWagner L et al., 2015 [51] | USA | Cross-sectional | 2713 | CT | Controls vs. NAFLD | Reduced in NAFLD | 7 |
| Wang Q et al., 2018 [13] | China | Cross-sectional | 120 | US | Controls vs. DM vs. DM + NAFLD | Stepwise worsening with NAFLD severity | 7 |
| Zamirian M et al., 2018 [8] | Iran | Cross-sectional | 60 | Biopsy | Controls vs. NAFLD | Reduced in NAFLD | 8 |
| Dong Y. et al., 2020 [55] | China | Cross-Fsectional | 97 | US | DM Controls vs. DM Mild vs. DM Mod/Sev NAFLD | Progressive impairment with NAFLD severity | 7 |
| Chiu L et al., 2020 [14] | USA | Cross-sectional | 2356 | CT | Controls vs. NAFLD | Reduced in NAFLD; exacerbated by DM | 6 |
| Johnson P et al., 2021 [54] | USA | Cross-sectional | 33 | Biopsy | NAFL vs. NASH (with/without DM) | Reduced in NAFLD patients | 7 |
| Moise C et al., 2021 [47] | Romania | Cross-sectional | 159 | US | Controls vs. NAFLD vs. NAFLD + DM | Reduced in NAFLD | 5 |
| Sewan H et al., 2021 [17] | Iraq | Cross-sectional | 60 | US | Controls vs. NAFLD | Reduced in NAFLD | 7 |
| Ismaiel A et al., 2022 [56] | Romania | Cross-sectional | 75 | US | Controls vs. MAFLD | Worsened with NAFLD severity/fibrosis | 6 |
| Lai Y et al., 2022 [48] | Taiwan | Cross-sectional | 2161 | US | Controls vs. NAFLD (low/high fibrosis) | Reduced in NAFLD | 6 |
| Karaoğlan B et al., 2023 [57] | Turkey | Cross-sectional | 61 | Biopsy | NAFL vs. NASH | Reduced in NAFLD patients | 8 |
| Comparison Group | LVEF (%) MD (95% CI) | GLS (%) MD (95% CI) | GCS (%) MD (95% CI) | GAS (%) MD (95% CI) | GRS (%) MD (95% CI) |
|---|---|---|---|---|---|
| Controls vs. NAFLD (Overall) | 0.359 (−0.078, 0.795) | −2.043 (−2.868, −1.218) | −1.415 (−2.893, 0.064) | −3.706 (−4.999, −2.413) | 7.407 (−1.852, 16.666) |
| Controls vs. Simple Steatosis | 0.300 (−1.529, 2.129) | −2.253 (−3.502, −1.004) | −0.851 (−2.575, 0.872) | −1.383 (−2.944, 0.178) | 2.128 (−1.927, 6.184) |
| Controls vs. Moderate/Severe NAFLD | 0.619 (−1.359, 2.596) | −5.828 (−7.496, −4.160) | −3.111 (−4.630, −1.593) | −5.231 (−6.707, −3.756) | 9.936 (−0.141, 20.013) |
| Simple Steatosis vs. Moderate/Severe NAFLD | 0.585 (−1.231, 2.402) | −3.684 (−4.956, −2.412) | −2.126 (−3.335, −0.917) | −3.939 (−6.288, −1.591) | 6.836 (1.065, 12.607) |
| DM Controls vs. DM NAFLD | 0.850 (−0.989, 2.690) | −1.740 (−3.252, −0.228) | −1.244 (−2.204, −0.284) | −3.422 (−4.645, −2.199) | 4.551 (0.704, 8.397) |
| Controls vs. NASH | 1.020 (−3.186, 5.226) | −3.568 (−6.257, −0.879) | NA | NA | NA |
| NAFL vs. NASH | 0.015 (−2.177, 2.207) | 1.057 (0.097, 2.017) | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, M.; Almasri, M.; Abdulredha, R.; Tecar, I.; Leucuta, D.-C.; Popa, S.-L.; Dumitrascu, D.L.; Ismaiel, A. When the Liver Echoes to the Heart: Assessing Subclinical Cardiac Dysfunction in NAFLD Using Speckle Tracking Echocardiography—A Systematic Review and Meta-Analysis. Biomedicines 2025, 13, 2908. https://doi.org/10.3390/biomedicines13122908
Gruber M, Almasri M, Abdulredha R, Tecar I, Leucuta D-C, Popa S-L, Dumitrascu DL, Ismaiel A. When the Liver Echoes to the Heart: Assessing Subclinical Cardiac Dysfunction in NAFLD Using Speckle Tracking Echocardiography—A Systematic Review and Meta-Analysis. Biomedicines. 2025; 13(12):2908. https://doi.org/10.3390/biomedicines13122908
Chicago/Turabian StyleGruber, Micha, Malaz Almasri, Rania Abdulredha, Iulia Tecar, Daniel-Corneliu Leucuta, Stefan-Lucian Popa, Dan L. Dumitrascu, and Abdulrahman Ismaiel. 2025. "When the Liver Echoes to the Heart: Assessing Subclinical Cardiac Dysfunction in NAFLD Using Speckle Tracking Echocardiography—A Systematic Review and Meta-Analysis" Biomedicines 13, no. 12: 2908. https://doi.org/10.3390/biomedicines13122908
APA StyleGruber, M., Almasri, M., Abdulredha, R., Tecar, I., Leucuta, D.-C., Popa, S.-L., Dumitrascu, D. L., & Ismaiel, A. (2025). When the Liver Echoes to the Heart: Assessing Subclinical Cardiac Dysfunction in NAFLD Using Speckle Tracking Echocardiography—A Systematic Review and Meta-Analysis. Biomedicines, 13(12), 2908. https://doi.org/10.3390/biomedicines13122908







