Unlocking the Sugar Code: Implications and Consequences of Glycosylation in Alzheimer’s Disease and Other Tauopathies
Abstract
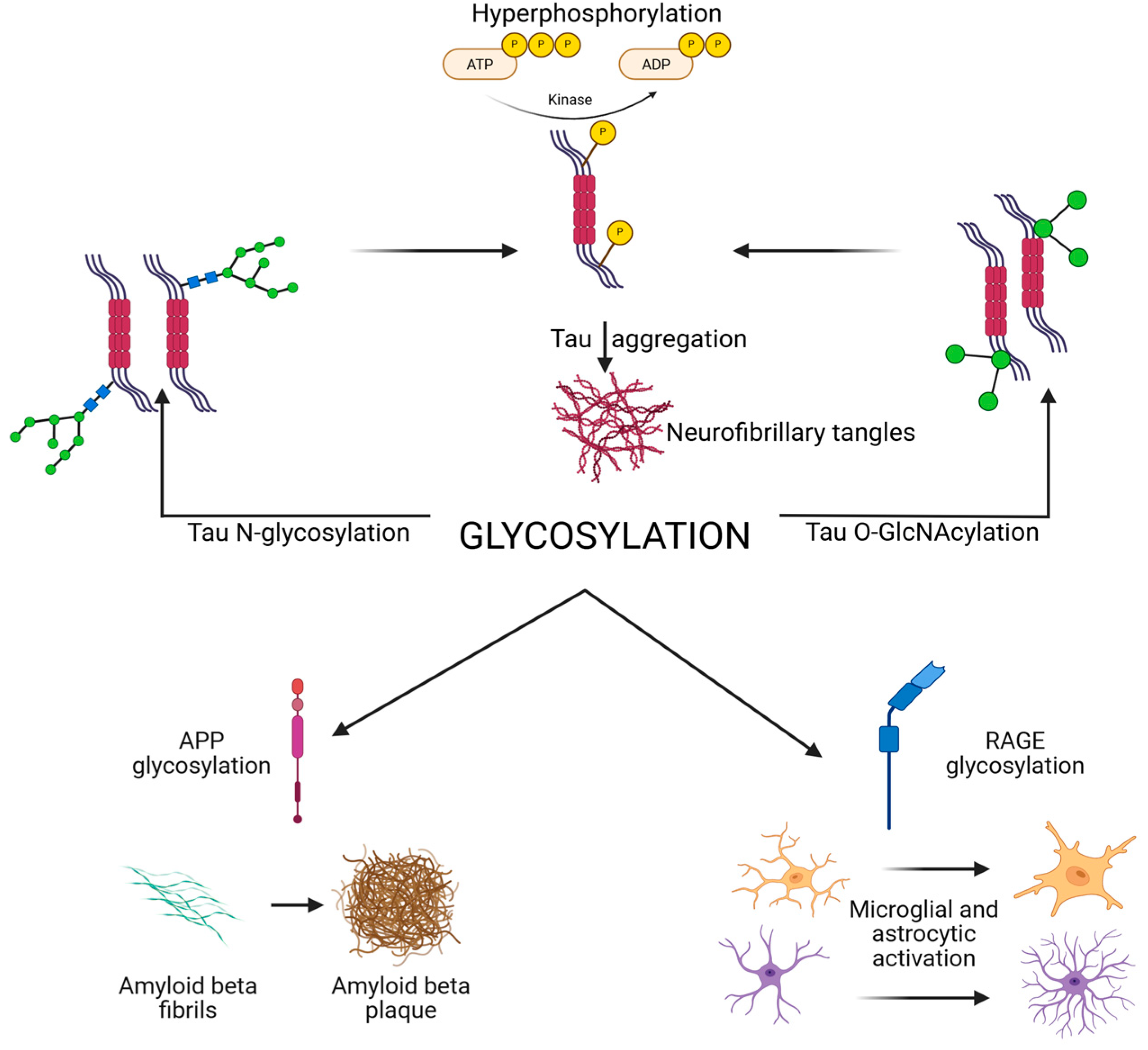
1. Introduction
2. Tau Glycosylation in Alzheimer’s Disease and Related Tauopathies
2.1. N-Glycosylation of Tau
2.2. O-GlcNAcylation of Tau
2.3. Glycosylation in Other Tauopathies
2.4. Interplay Between Glycosylation and Phosphorylation
2.5. Comparative Analysis of Tau Glycosylation and Phosphorylation Sites
3. Glycosylation and Amyloid-β Pathology
3.1. Amyloid Precursor Protein N-Glycosylation and Trafficking
3.2. O-Glycosylation and Secretase Regulation
3.3. Aberrant Glycosylation of Amyloid-β Peptides
3.4. Implications for Therapy
4. Glycosylation, Synaptic Function, and Neuroinflammation
4.1. Glycosylation of Synaptic Receptors and Adhesion Molecules
4.2. Immune Receptor Glycosylation and Microglial Activation
4.3. Cytokines, Chemokines, and Glycosylation
4.4. Glycosylation and the Complement System
4.5. Neuroinflammation Beyond Amyloid and Tau
4.6. Therapeutic Implications
5. Advances in Glycoproteomics and Biomarker Discovery
5.1. Glycoproteomic Alterations in Alzheimer’s Disease
5.2. Glycosylation as a Diagnostic and Prognostic Biomarker
5.3. Mass Spectrometry and Glycoproteomic Technologies
5.4. Immunoglobulin Glycosylation and Systemic Biomarkers
5.5. Integration with Multimodal Biomarkers
5.6. Challenges and Opportunities
6. Enzymatic Regulators of Glycosylation in Neurodegeneration
6.1. Glycosyltransferases in AD and Tauopathies
6.2. Glycosidases and Tau Pathology
6.3. Hexosamine Biosynthetic Pathway and Metabolic Regulation
6.4. Crosstalk with Phosphorylation Pathways
6.5. Enzymatic Dysregulation as Biomarkers
6.6. Clinical Correlations of Tau Glycosylation with Cognitive Decline and Disease Staging
6.7. Therapeutic Implications
7. Discussion
7.1. Glycosylation and the Hierarchy of Pathological Events
7.2. Protective Versus Pathogenic Roles
7.3. Crosstalk with Metabolism and Phosphorylation
7.4. Neuroinflammation as a Glycosylation-Driven Amplifier
7.5. Biomarker Potential and Translational Challenges
7.6. Therapeutic Perspectives
7.7. Remaining Controversies
7.8. Glycosylation-Focused Guidelines for Tauopathies: Toward Consensus on Tau Pathology
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, G.; Li, C.; Fan, W.; Zhang, M.; Li, X.; Chen, W.; Li, W.; Liang, R.; Li, Z.; Zhu, X. Brilliant Glycans and Glycosylation: Seq and Ye Shall Find. Int. J. Biol. Macromol. 2021, 189, 279–291. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Kim, Y.; Kim, S.-H.; Lee, J.H.; Yang, H.; Kim, S.J.; Li, C.M.; Lee, H.; Na, D.-H.; et al. Pathogenic Role of RAGE in Tau Transmission and Memory Deficits. Biol. Psychiatry 2023, 93, 829–841. [Google Scholar] [CrossRef]
- Yi, L.; Fu, M.; Shao, Y.; Tang, K.; Yan, Y.; Ding, C.-F. Bifunctional Super-Hydrophilic Mesoporous Nanocomposite: A Novel Nanoprobe for Investigation of Glycosylation and Phosphorylation in Alzheimer’s Disease. J. Chromatogr. A 2022, 1676, 463236. [Google Scholar] [CrossRef]
- Maria, C.; Rauter, A.P. Nucleoside Analogues: N-Glycosylation Methodologies, Synthesis of Antiviral and Antitumor Drugs and Potential against Drug-Resistant Bacteria and Alzheimer’s Disease. Carbohydr. Res. 2023, 532, 108889. [Google Scholar] [CrossRef] [PubMed]
- Tachida, Y.; Iijima, J.; Takahashi, K.; Suzuki, H.; Kizuka, Y.; Yamaguchi, Y.; Tanaka, K.; Nakano, M.; Takakura, D.; Kawasaki, N.; et al. O-GalNAc Glycosylation Determines Intracellular Trafficking of APP and Aβ Production. J. Biol. Chem. 2023, 299, 104905. [Google Scholar] [CrossRef]
- Li, W.; Li, H.-L.; Wang, J.-Z.; Liu, R.; Wang, X. Abnormal Protein Post-Translational Modifications Induces Aggregation and Abnormal Deposition of Protein, Mediating Neurodegenerative Diseases. Cell Biosci. 2024, 14, 22. [Google Scholar] [CrossRef]
- Tsay, H.-J.; Huang, Y.-C.; Chen, Y.-J.; Lee, Y.-H.; Hsu, S.-M.; Tsai, K.-C.; Yang, C.-N.; Huang, F.-L.; Shie, F.-S.; Lee, L.-C.; et al. Identifying N-Linked Glycan Moiety and Motifs in the Cysteine-Rich Domain Critical for N-Glycosylation and Intracellular Trafficking of SR-AI and MARCO. J. Biomed. Sci. 2016, 23, 27. [Google Scholar] [CrossRef]
- Akasaka-Manya, K.; Manya, H. The Role of APP O-Glycosylation in Alzheimer’s Disease. Biomolecules 2020, 10, 1569. [Google Scholar] [CrossRef]
- Wang, A.C.; Jensen, E.H.; Rexach, J.E.; Vinters, H.V.; Hsieh-Wilson, L.C. Loss of O-GlcNAc Glycosylation in Forebrain Excitatory Neurons Induces Neurodegeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 15120–15125. [Google Scholar] [CrossRef]
- Mashal, Y.; Abdelhady, H.; Iyer, A.K. Comparison of Tau and Amyloid-β Targeted Immunotherapy Nanoparticles for Alzheimer’s Disease. Biomolecules 2022, 12, 1001. [Google Scholar] [CrossRef]
- Fastenau, C.; Bunce, M.; Keating, M.; Wickline, J.; Hopp, S.C.; Bieniek, K.F. Distinct Patterns of Plaque and Microglia Glycosylation in Alzheimer’s Disease. Brain Pathol. 2024, 34, e13267. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, Y.; Liu, X.; Ren, X.; Zhang, W.; Dong, M.; Ge, Y.; Yu, Y.; Ye, M. Development of Complementary Enrichment Strategies for Analysis of N-Linked Intact Glycopeptides and Potential Site-Specific Glycoforms in Alzheimer’s Disease. Talanta 2026, 297, 128595. [Google Scholar] [CrossRef]
- Gupta, R.; Sahu, M.; Srivastava, D.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Post-Translational Modifications: Regulators of Neurodegenerative Proteinopathies. Ageing Res. Rev. 2021, 68, 101336. [Google Scholar] [CrossRef]
- Krawczuk, D.; Kulczyńska-Przybik, A.; Mroczko, B. The Potential Regulators of Amyloidogenic Pathway of APP Processing in Alzheimer’s Disease. Biomedicines 2025, 13, 1513. [Google Scholar] [CrossRef]
- Kizuka, Y.; Kitazume, S.; Taniguchi, N. N-Glycan and Alzheimer’s Disease. Biochim. Biophys. Acta BBA—Gen. Subj. 2017, 1861, 2447–2454. [Google Scholar] [CrossRef]
- Suzuki, S.; Itoh, M. Synergistic Effects of Mutation and Glycosylation on Disease Progression. Front. Mol. Biosci. 2025, 12, 1550815. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, Z.; Yang, S.; Zhu, Y.; Chen, Z.; Can, D.; Lei, A.; Li, H.; Leng, L.; Zhang, J. Mannose Promotes β-Amyloid Pathology by Regulating BACE1 Glycosylation in Alzheimer’s Disease. Adv. Sci. 2025, 12, 2409105. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ishihara, S.; Nobuhara, M.; Higashide, H.; Funamoto, S. Glycosylation Status of Nicastrin Influences Catalytic Activity and Substrate Preference of γ-Secretase. Biochem. Biophys. Res. Commun. 2018, 502, 98–103. [Google Scholar] [CrossRef]
- Cathrine, R.; Lukose, B.; Rani, P. G82S RAGE Polymorphism Influences Amyloid-RAGE Interactions Relevant in Alzheimer’s Disease Pathology. PLoS ONE 2020, 15, e0225487. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, M.; Sun, J.; Guo, A.; Fernando, R.L.; Chen, Y.; Peng, P.; Zhao, G.; Deng, Y. DPP-4 Inhibitor Improves Learning and Memory Deficits and AD-like Neurodegeneration by Modulating the GLP-1 Signaling. Neuropharmacology 2019, 157, 107668. [Google Scholar] [CrossRef]
- Aljadaan, A.M.; AlSaadi, A.M.; Shaikh, I.A.; Whitby, A.; Ray, A.; Kim, D.-H.; Carter, W.G. Characterization of the Anticholinesterase and Antioxidant Properties of Phytochemicals from Moringa Oleifera as a Potential Treatment for Alzheimer’s Disease. Biomedicines 2025, 13, 2148. [Google Scholar] [CrossRef]
- Ma, L.; Allen, M.; Sakae, N.; Ertekin-Taner, N.; Graff-Radford, N.R.; Dickson, D.W.; Younkin, S.G.; Sevlever, D. Expression and Processing Analyses of Wild Type and p.R47H TREM2 Variant in Alzheimer’s Disease Brains. Mol. Neurodegener. 2016, 11, 72. [Google Scholar] [CrossRef]
- Li, J.Q.; Wang, L.H.; Zhan, Q.W.; Liu, Y.L.; Zhang, Q.; Li, J.F.; Fan, F.F. Mapping Quantitative Trait Loci for Five Forage Quality Traits in a Sorghum-Sudangrass Hybrid. Genet. Mol. Res. 2015, 14, 13266–13273. [Google Scholar] [CrossRef]
- Tolstova, A.P.; Adzhubei, A.A.; Mitkevich, V.A.; Petrushanko, I.Y.; Makarov, A.A. Docking and Molecular Dynamics-Based Identification of Interaction between Various Beta-Amyloid Isoforms and RAGE Receptor. Int. J. Mol. Sci. 2022, 23, 11816. [Google Scholar] [CrossRef]
- Mocanu, A.-I.; Mocanu, H.; Moldovan, C.; Soare, I.; Niculet, E.; Tatu, A.L.; Vasile, C.I.; Diculencu, D.; Postolache, P.A.; Nechifor, A. Some Manifestations of Tuberculosis in Otorhinolaryngology—Case Series and a Short Review of Related Data from South-Eastern Europe. Infect. Drug Resist. 2022, 15, 2753–2762. [Google Scholar] [CrossRef]
- Park, J.; Ji, I.J.; An, H.J.; Kang, M.; Kang, S.; Kim, D.; Yoon, S. Disease-Associated Mutations of TREM2 Alter the Processing of N-Linked Oligosaccharides in the Golgi Apparatus. Traffic 2015, 16, 510–518. [Google Scholar] [CrossRef]
- Shirotani, K.; Hatta, D.; Wakita, N.; Watanabe, K.; Iwata, N. The Role of TREM2 N-Glycans in Trafficking to the Cell Surface and Signal Transduction of TREM2. J. Biochem. 2022, 172, 347–353. [Google Scholar] [CrossRef]
- Tang, X.; Schindler, R.L.; Di Lucente, J.; Oloumi, A.; Tena, J.; Harvey, D.; Lebrilla, C.B.; Zivkovic, A.M.; Jin, L.-W.; Maezawa, I. Unique N-Glycosylation Signatures in Human iPSC Derived Microglia Activated by Aβ Oligomer and Lipopolysaccharide. Sci. Rep. 2025, 15, 12348. [Google Scholar] [CrossRef]
- Gizaw, S.T.; Ohashi, T.; Tanaka, M.; Hinou, H.; Nishimura, S.-I. Glycoblotting Method Allows for Rapid and Efficient Glycome Profiling of Human Alzheimer’s Disease Brain, Serum and Cerebrospinal Fluid towards Potential Biomarker Discovery. Biochim. Biophys. Acta BBA—Gen. Subj. 2016, 1860, 1716–1727. [Google Scholar] [CrossRef]
- Kumar, A.; Karuppagounder, S.S.; Chen, Y.; Corona, C.; Kawaguchi, R.; Cheng, Y.; Balkaya, M.; Sagdullaev, B.T.; Wen, Z.; Stuart, C.; et al. 2-Deoxyglucose Drives Plasticity via an Adaptive ER Stress-ATF4 Pathway and Elicits Stroke Recovery and Alzheimer’s Resilience. Neuron 2023, 111, 2831–2846.e10. [Google Scholar] [CrossRef]
- Iordache, M.P.; Buliman, A.; Costea-Firan, C.; Gligore, T.C.I.; Cazacu, I.S.; Stoian, M.; Teoibaș-Şerban, D.; Blendea, C.-D.; Protosevici, M.G.-I.; Tanase, C.; et al. Immunological and Inflammatory Biomarkers in the Prognosis, Prevention, and Treatment of Ischemic Stroke: A Review of a Decade of Advancement. Int. J. Mol. Sci. 2025, 26, 7928. [Google Scholar] [CrossRef]
- Ramos-Martinez, I.; Martínez-Loustalot, P.; Lozano, L.; Issad, T.; Limón, D.; Díaz, A.; Perez-Torres, A.; Guevara, J.; Zenteno, E. Neuroinflammation Induced by Amyloid Β25–35 Modifies Mucin-Type O-Glycosylation in the Rat’s Hippocampus. Neuropeptides 2018, 67, 56–62. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Q.; Yu, Q.; Johnson, J.; Shipman, R.; Zhong, X.; Huang, J.; Asthana, S.; Carlsson, C.; Okonkwo, O.; et al. In-Depth Site-Specific Analysis of N-Glycoproteome in Human Cerebrospinal Fluid and Glycosylation Landscape Changes in Alzheimer’s Disease. Mol. Cell. Proteom. 2021, 20, 100081. [Google Scholar] [CrossRef]
- Kobeissy, F.; Kobaisi, A.; Peng, W.; Barsa, C.; Goli, M.; Sibahi, A.; El Hayek, S.; Abdelhady, S.; Ali Haidar, M.; Sabra, M.; et al. Glycomic and Glycoproteomic Techniques in Neurodegenerative Disorders and Neurotrauma: Towards Personalized Markers. Cells 2022, 11, 581. [Google Scholar] [CrossRef]
- Schloss, J.V. Is Dolichol Pathway Dysfunction a Significant Factor in Alzheimer’s Disease? Inflammopharmacology 2025, 33, 4651–4658. [Google Scholar] [CrossRef]
- Huang, C.-W.; Rust, N.; Wu, H.-F.; Hart, G. Altered O-GlcNAcylation and Mitochondrial Dysfunction, a Molecular Link between Brain Glucose Dysregulation and Sporadic Alzheimer’s Disease. Neural Regen. Res. 2023, 18, 779. [Google Scholar] [CrossRef]
- Medina, M.; Hernández, F.; Avila, J. New Features about Tau Function and Dysfunction. Biomolecules 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, C.; Li, L.; Chin, L.-S. Differential Analysis of N-Glycopeptide Abundance and N-Glycosylation Site Occupancy for Studying Protein N-Glycosylation Dysregulation in Human Disease. BIO-Protoc. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Ibrahim, M.; Alkorbi, N.; Alkuwari, S.; Pedersen, S.; Rathore, H.A. Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer’s Disease and Other Neurological Disorders: A Comprehensive Review. Biomedicines 2025, 13, 2073. [Google Scholar] [CrossRef] [PubMed]
- Demirev, A.V.; Song, H.-L.; Cho, M.-H.; Cho, K.; Peak, J.-J.; Yoo, H.J.; Kim, D.-H.; Yoon, S.-Y. V232M Substitution Restricts a Distinct O-Glycosylation of PLD3 and Its Neuroprotective Function. Neurobiol. Dis. 2019, 129, 182–194. [Google Scholar] [CrossRef]
- Fang, P.; Yu, X.; Ding, M.; Qifei, C.; Jiang, H.; Shi, Q.; Zhao, W.; Zheng, W.; Li, Y.; Ling, Z.; et al. Ultradeep N-Glycoproteome Atlas of Mouse Reveals Spatiotemporal Signatures of Brain Aging and Neurodegenerative Diseases. Nat. Commun. 2025, 16, 5568. [Google Scholar] [CrossRef] [PubMed]
- Gaunitz, S.; Tjernberg, L.O.; Schedin-Weiss, S. What Can N-Glycomics and N-Glycoproteomics of Cerebrospinal Fluid Tell Us about Alzheimer Disease? Biomolecules 2021, 11, 858. [Google Scholar] [CrossRef]
- Rajabally, Y.A. Chronic Inflammatory Demyelinating Polyneuropathy with Positive Anti-myelin Associated Glycoprotein Antibodies: Back to Clinical Basics. Eur. J. Neurol. 2023, 30, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Stempler, S.; Tal-Mazaki, S.; Losev, Y.; Singh-Anand, A.; Escobar-Álvarez, D.; Lezmy, J.; Gazit, E.; Ruppin, E.; Segal, D. Altered Protein Glycosylation Predicts Alzheimer’s Disease and Modulates Its Pathology in Disease Model Drosophila. Neurobiol. Aging 2017, 56, 159–171. [Google Scholar] [CrossRef]
- Rusu, E.; Necula, L.G.; Neagu, A.I.; Alecu, M.; Stan, C.; Albulescu, R.; Tanase, C.P. Current Status of Stem Cell Therapy: Opportunities and Limitations. Turk. J. Biol. 2016, 40, 955–967. [Google Scholar] [CrossRef]
- Ahmad, W.; Shabbiri, K.; Ahmad, I. Prediction of Human Tau 3D Structure, and Interplay between O-β-GlcNAc and Phosphorylation Modifications in Alzheimer’s Disease: C. Elegans as a Suitable Model to Study These Interactions in Vivo. Biochem. Biophys. Res. Commun. 2020, 528, 466–472. [Google Scholar] [CrossRef]
- Meuret, C.J.; Hu, Y.; Smadi, S.; Bantugan, M.A.; Xian, H.; Martinez, A.E.; Krauss, R.M.; Ma, Q.-L.; Nedelkov, D.; Yassine, H.N. An Association of CSF Apolipoprotein E Glycosylation and Amyloid-Beta 42 in Individuals Who Carry the APOE4 Allele. Alzheimers Res. Ther. 2023, 15, 96. [Google Scholar] [CrossRef]
- Popa, M.-L.; Popa, A.; Tanase, C.; Gheorghisan-Galateanu, A.-A. Acanthosis Nigricans: To Be or Not to Be Afraid (Review). Oncol. Lett. 2019, 17, 4133–4138. [Google Scholar] [CrossRef]
- Tena, J.; Tang, X.; Zhou, Q.; Harvey, D.; Barajas-Mendoza, M.; Jin, L.; Maezawa, I.; Zivkovic, A.M.; Lebrilla, C.B. Glycosylation Alterations in Serum of Alzheimer’s Disease Patients Show Widespread Changes in N-glycosylation of Proteins Related to Immune Function, Inflammation, and Lipoprotein Metabolism. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12309. [Google Scholar] [CrossRef]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau Post-Translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef]
- Ercan, E.; Eid, S.; Weber, C.; Kowalski, A.; Bichmann, M.; Behrendt, A.; Matthes, F.; Krauss, S.; Reinhardt, P.; Fulle, S.; et al. A Validated Antibody Panel for the Characterization of Tau Post-Translational Modifications. Mol. Neurodegener. 2017, 12, 87. [Google Scholar] [CrossRef]
- Thummel, R.; Li, L.; Tanase, C.; Sarras, M.P.; Godwin, A.R. Differences in Expression Pattern and Function between Zebrafish Hoxc13 Orthologs: Recruitment of Hoxc13b into an Early Embryonic Role. Dev. Biol. 2004, 274, 318–333. [Google Scholar] [CrossRef]
- Okła, E.; Hołota, M.; Michlewska, S.; Zawadzki, S.; Miłowska, K.; Sánchez-Nieves, J.; Gómez, R.; De La Mata, F.J.; Bryszewska, M.; Ionov, M. Crossing Barriers: PEGylated Gold Nanoparticles as Promising Delivery Vehicles for siRNA Delivery in Alzheimer’s Disease. Biomedicines 2025, 13, 2108. [Google Scholar] [CrossRef]
- Park, E.; He, C.; Abbasi, A.Z.; Tian, M.; Huang, S.; Wang, L.; Georgiou, J.; Collingridge, G.L.; Fraser, P.E.; Henderson, J.T.; et al. Brain Microenvironment-Remodeling Nanomedicine Improves Cerebral Glucose Metabolism, Mitochondrial Activity and Synaptic Function in a Mouse Model of Alzheimer’s Disease. Biomaterials 2025, 318, 123142. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, K.; Xu, Z.; De Las Rivas, M.; Wang, C.; Li, X.; Lu, J.; Zhou, Y.; Delso, I.; Merino, P.; et al. The Small Molecule Luteolin Inhibits N-Acetyl-α-Galactosaminyltransferases and Reduces Mucin-Type O-Glycosylation of Amyloid Precursor Protein. J. Biol. Chem. 2017, 292, 21304–21319. [Google Scholar] [CrossRef] [PubMed]
- Bukke, V.N.; Villani, R.; Archana, M.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Ferraro, L.; Serviddio, G.; Cassano, T. The Glucose Metabolic Pathway as A Potential Target for Therapeutics: Crucial Role of Glycosylation in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 7739. [Google Scholar] [CrossRef]
- Hoshi, K.; Ito, H.; Abe, E.; Fuwa, T.J.; Kanno, M.; Murakami, Y.; Abe, M.; Murakami, T.; Yoshihara, A.; Ugawa, Y.; et al. Transferrin Biosynthesized in the Brain Is a Novel Biomarker for Alzheimer’s Disease. Metabolites 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Constantinoiu, S.; Cochior, D. Severe Acute Pancreatitis—Determinant Factors and Current Therapeutic Conduct. Chirurgia 2018, 113, 385. [Google Scholar] [CrossRef]
- Pan, K.; Chen, S.; Wang, Y.; Yao, W.; Gao, X. MicroRNA-23b Attenuates Tau Pathology and Inhibits Oxidative Stress by Targeting GnT-III in Alzheimer’s Disease. Neuropharmacology 2021, 196, 108671. [Google Scholar] [CrossRef]
- Rosewood, T.J.; Nho, K.; Risacher, S.L.; Liu, S.; Gao, S.; Shen, L.; Foroud, T.; Saykin, A.J.; for the Alzheimer’s Disease Neuroimaging Initiative. Pathway Enrichment in Genome-wide Analysis of Longitudinal Alzheimer’s Disease Biomarker Endophenotypes. Alzheimer’s Dement. 2024, 20, 8639–8650. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Huang, H.; Xue, Y.; Chen, S.; Gao, X. DHEC Mesylate Attenuates Pathologies and Aberrant Bisecting N-Glycosylation in Alzheimer’s Disease Models. Neuropharmacology 2024, 248, 109863. [Google Scholar] [CrossRef]
- Finke, J.M.; Ayres, K.R.; Brisbin, R.P.; Hill, H.A.; Wing, E.E.; Banks, W.A. Antibody Blood-Brain Barrier Efflux Is Modulated by Glycan Modification. Biochim. Biophys. Acta BBA—Gen. Subj. 2017, 1861, 2228–2239. [Google Scholar] [CrossRef]
- Blendea, C.-D.; Khan, M.T.; Stoian, M.; Gligore, T.C.I.; Cuculici, Ș.; Stanciu, I.L.; Protosevici, M.G.-I.; Iordache, M.; Buliman, A.; Costea-Firan, C.; et al. Advances in Minimally Invasive Treatments for Prostate Cancer: A Review of the Role of Ultrasound Therapy and Laser Therapy. Balneo PRM Res. J. 2025, 16, 827. [Google Scholar] [CrossRef]
- Gaunitz, S.; Tjernberg, L.O.; Schedin-Weiss, S. The N-glycan Profile in Cortex and Hippocampus Is Altered in Alzheimer Disease. J. Neurochem. 2021, 159, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Nyarko, J.N.K.; Quartey, M.O.; Heistad, R.M.; Pennington, P.R.; Poon, L.J.; Knudsen, K.J.; Allonby, O.; El Zawily, A.M.; Freywald, A.; Rauw, G.; et al. Glycosylation States of Pre- and Post-Synaptic Markers of 5-HT Neurons Differ with Sex and 5-HTTLPR Genotype in Cortical Autopsy Samples. Front. Neurosci. 2018, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, Y.; Huang, H.; Cao, Y.; Pan, K.; Zhou, Y.; He, J.; Yao, W.; Chen, S.; Gao, X. Targeting Aberrant Glycosylation to Modulate Microglial Response and Improve Cognition in Models of Alzheimer’s Disease. Pharmacol. Res. 2024, 202, 107133. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Marcus, J.; Pearson, M.; Song, L.; Smith, K.; Terracina, G.; Lee, J.; Hong, K.-L.K.; Lu, S.X.; et al. MK-8719, a Novel and Selective O-GlcNAcase Inhibitor That Reduces the Formation of Pathological Tau and Ameliorates Neurodegeneration in a Mouse Model of Tauopathy. J. Pharmacol. Exp. Ther. 2020, 374, 252–263. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Bardakci, F.; Surti, M.; Badraoui, R.; Patel, M. NEU1-Mediated Extracellular Vesicle Glycosylation in Alzheimer’s Disease: Mechanistic Insights into Intercellular Communication and Therapeutic Targeting. Pharmaceuticals 2025, 18, 921. [Google Scholar] [CrossRef]
- Du, L.; Su, Z.; Wang, S.; Meng, Y.; Xiao, F.; Xu, D.; Li, X.; Qian, X.; Lee, S.B.; Lee, J.; et al. EGFR-Induced and c-Src-Mediated CD47 Phosphorylation Inhibits TRIM21-Dependent Polyubiquitylation and Degradation of CD47 to Promote Tumor Immune Evasion. Adv. Sci. 2023, 10, 2206380. [Google Scholar] [CrossRef]
- Barbu, L.A.; Vasile, L.; Cercelaru, L.; Șurlin, V.; Mogoantă, S.-Ș.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Iordache, M.P.; Buliman, A. Aggressiveness in Well-Differentiated Small Intestinal Neuroendocrine Tumors: A Rare Case and Narrative Literature Review. J. Clin. Med. 2025, 14, 5821. [Google Scholar] [CrossRef]
- Tanase, C.; Enciu, A.M.; Codrici, E.; Popescu, I.D.; Dudau, M.; Dobri, A.M.; Pop, S.; Mihai, S.; Gheorghișan-Gălățeanu, A.-A.; Hinescu, M.E. Fatty Acids, CD36, Thrombospondin-1, and CD47 in Glioblastoma: Together and/or Separately? Int. J. Mol. Sci. 2022, 23, 604. [Google Scholar] [CrossRef]
- Dong, L.; Shen, S.; Xu, Y.; Wang, L.; Feng, R.; Zhang, J.; Lu, H. Computational Studies on the Potency and Selectivity of PUGNAc Derivatives Against GH3, GH20, and GH84 β-N-Acetyl-D-Hexosaminidases. Front. Chem. 2019, 7, 235. [Google Scholar] [CrossRef]
- Krüger, L.; Biskup, K.; Schipke, C.G.; Kochnowsky, B.; Schneider, L.-S.; Peters, O.; Blanchard, V. The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease. Biomolecules 2024, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.Z.; Gaunitz, S.; Kirsebom, B.-E.; Lundin, B.; Hellström, M.; Jejcic, A.; Sköldunger, A.; Wimo, A.; Winblad, B.; Fladby, T.; et al. Blood N-Glycomics Reveals Individuals at Risk for Cognitive Decline and Alzheimer’s Disease. eBioMedicine 2025, 113, 105598. [Google Scholar] [CrossRef]
- Zhou, R.Z.; Vetrano, D.L.; Grande, G.; Duell, F.; Jönsson, L.; Laukka, E.J.; Fredolini, C.; Winblad, B.; Tjernberg, L.; Schedin-Weiss, S. A Glycan Epitope Correlates with Tau in Serum and Predicts Progression to Alzheimer’s Disease in Combination with APOE4 Allele Status. Alzheimer’s Dement. 2023, 19, 3244–3249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.Z.; Duell, F.; Axenhus, M.; Jönsson, L.; Winblad, B.; Tjernberg, L.O.; Schedin-Weiss, S. A Glycan Biomarker Predicts Cognitive Decline in Amyloid- and Tau-Negative Patients. Brain Commun. 2024, 6, fcae371. [Google Scholar] [CrossRef]
- Chun, Y.S.; Kwon, O.-H.; Chung, S. O-GlcNAcylation of Amyloid-β Precursor Protein at Threonine 576 Residue Regulates Trafficking and Processing. Biochem. Biophys. Res. Commun. 2017, 490, 486–491. [Google Scholar] [CrossRef]
- Moldovan, C.; Cochior, D.; Gorecki, G.; Rusu, E.; Ungureanu, F.-D. Clinical and Surgical Algorithm for Managing Iatrogenic Bile Duct Injuries during Laparoscopic Cholecystectomy: A Multicenter Study. Exp. Ther. Med. 2021, 22, 1385. [Google Scholar] [CrossRef]
- Fabiano, M.; Oikawa, N.; Kerksiek, A.; Furukawa, J.; Yagi, H.; Kato, K.; Schweizer, U.; Annaert, W.; Kang, J.; Shen, J.; et al. Presenilin Deficiency Results in Cellular Cholesterol Accumulation by Impairment of Protein Glycosylation and NPC1 Function. Int. J. Mol. Sci. 2024, 25, 5417. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Kizuka, Y.; Takata, M.; Nakano, M.; Ito, E.; Mishra, S.; Akatsuka, H.; Harada, Y.; Taniguchi, N. Peptide Sequence Mapping around Bisecting GlcNAc-Bearing N-Glycans in Mouse Brain. Int. J. Mol. Sci. 2021, 22, 8579. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef]
- Reyes, C.D.G.; Hakim, M.A.; Atashi, M.; Goli, M.; Gautam, S.; Wang, J.; Bennett, A.I.; Zhu, J.; Lubman, D.M.; Mechref, Y. LC-MS/MS Isomeric Profiling of N-Glycans Derived from Low-Abundant Serum Glycoproteins in Mild Cognitive Impairment Patients. Biomolecules 2022, 12, 1657. [Google Scholar] [CrossRef]
- Shao, Y.; Yi, L.; Fu, M.; Feng, Q.; Mao, X.; Mao, H.; Yan, Y.; Ding, C.-F. Anti-Nonspecific Hydrophilic Hydrogel for Efficient Capture of N-Glycopeptides from Alzheimer’s Disease Patient’s Serum. Talanta 2023, 253, 124068. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Z.; Chen, X.; Li, W. The Significance of Sialylation on the Pathogenesis of Alzheimer’s Disease. Brain Res. Bull. 2021, 173, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Caramello, A.; Fancy, N.; Tournerie, C.; Eklund, M.; Chau, V.; Adair, E.; Papageorgopoulou, M.; Willumsen, N.; Jackson, J.S.; Hardy, J.; et al. Intracellular Accumulation of Amyloid-ß Is a Marker of Selective Neuronal Vulnerability in Alzheimer’s Disease. Nat. Commun. 2025, 16, 5189. [Google Scholar] [CrossRef]
- Didonna, A.; Benetti, F. 1 Department of Neurology, University of California San Francisco, San Francisco, CA 94158, USA Post-Translational Modifications in Neurodegeneration. AIMS Biophys. 2015, 3, 27–49. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Gill, D.; Rannikmäe, K.; Traylor, M.; Anderson, C.D.; MEGASTROKE Consortium of the International Stroke Genetics Consortium (ISGC); Lee, J.-M.; Kamatani, Y.; Hopewell, J.C.; Worrall, B.B.; et al. Genetically Determined Levels of Circulating Cytokines and Risk of Stroke: Role of Monocyte Chemoattractant Protein-1. Circulation 2019, 139, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Jin, Z.; Yu, T.; Guo, C.; He, Y.; Kan, Y.; Yan, L.; Wu, L. Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides. Molecules 2025, 30, 441. [Google Scholar] [CrossRef]
- Tang, X.; Tena, J.; Di Lucente, J.; Maezawa, I.; Harvey, D.J.; Jin, L.-W.; Lebrilla, C.B.; Zivkovic, A.M. Transcriptomic and Glycomic Analyses Highlight Pathway-Specific Glycosylation Alterations Unique to Alzheimer’s Disease. Sci. Rep. 2023, 13, 7816. [Google Scholar] [CrossRef]
- Alkuhlani, A.; Gad, W.; Roushdy, M.; Salem, A.-B.M. PUStackNGly: Positive-Unlabeled and Stacking Learning for N-Linked Glycosylation Site Prediction. IEEE Access 2022, 10, 12702–12713. [Google Scholar] [CrossRef]
- Alkuhlani, A.; Gad, W.; Roushdy, M.; Voskoglou, M.G.; Salem, A.M. PTG-PLM: Predicting Post-Translational Glycosylation and Glycation Sites Using Protein Language Models and Deep Learning. Axioms 2022, 11, 469. [Google Scholar] [CrossRef]
- Lebart, M.-C.; Trousse, F.; Valette, G.; Torrent, J.; Denus, M.; Mestre-Frances, N.; Marcilhac, A. Reg-1α, a New Substrate of Calpain-2 Depending on Its Glycosylation Status. Int. J. Mol. Sci. 2022, 23, 8591. [Google Scholar] [CrossRef]
- Liang, N.; Nho, K.; Newman, J.W.; Arnold, M.; Huynh, K.; Meikle, P.J.; Borkowski, K.; Kaddurah-Daouk, R.; the Alzheimer’s Disease Metabolomics Consortium; Kueider-Paisley, A.; et al. Peripheral Inflammation Is Associated with Brain Atrophy and Cognitive Decline Linked to Mild Cognitive Impairment and Alzheimer’s Disease. Sci. Rep. 2024, 14, 17423. [Google Scholar] [CrossRef] [PubMed]
- Losev, Y.; Paul, A.; Frenkel-Pinter, M.; Abu-Hussein, M.; Khalaila, I.; Gazit, E.; Segal, D. Novel Model of Secreted Human Tau Protein Reveals the Impact of the Abnormal N-Glycosylation of Tau on Its Aggregation Propensity. Sci. Rep. 2019, 9, 2254. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Grant, O.C.; Woods, R.J.; Rebeck, G.W. O-Glycosylation on Cerebrospinal Fluid and Plasma Apolipoprotein E Differs in the Lipid-Binding Domain. Glycobiology 2020, 30, 74–85. [Google Scholar] [CrossRef]
- Hu, Y.; Meuret, C.; Go, S.; Yassine, H.N.; Nedelkov, D. Simple and Fast Assay for Apolipoprotein E Phenotyping and Glycotyping: Discovering Isoform-Specific Glycosylation in Plasma and Cerebrospinal Fluid. J. Alzheimer’s Dis. 2020, 76, 883–893. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, J.; Zhao, D.; Ruan, C.; Zhou, J.; Tan, H.; Bao, Y. Netrin-1 Upregulates GPX4 and Prevents Ferroptosis after Traumatic Brain Injury via the UNC5B/Nrf2 Signaling Pathway. CNS Neurosci. Ther. 2023, 29, 216–227. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, F.; Fan, J.; Li, X.; Liu, Y.; Chen, W.; Sun, H.; Ma, T.; Wang, Q.; Maihaiti, Y.; et al. Identification and Immune Characteristics of Molecular Subtypes Related to Protein Glycosylation in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 968190. [Google Scholar] [CrossRef]
- Kronimus, Y.; Albus, A.; Hasenberg, M.; Walkenfort, B.; Seifert, M.; Budeus, B.; Gronewold, J.; Hermann, D.M.; Ross, J.A.; Lochnit, G.; et al. Fc N-glycosylation of Autoreactive Aβ Antibodies as a Blood-based Biomarker for Alzheimer’s Disease. Alzheimer’s Dement. 2023, 19, 5563–5572. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.E.; Bollinger, J.G.; Schindler, S.E.; Hodge, C.R.; Iglesias, N.J.; Krishnan, V.; Coulton, J.B.; Li, Y.; Holtzman, D.M.; Bateman, R.J. Apolipoprotein E O-Glycosylation Is Associated with Amyloid Plaques and APOE Genotype. Anal. Biochem. 2023, 672, 115156. [Google Scholar] [CrossRef]
- Ephrame, S.J.; Cork, G.K.; Marshall, V.; Johnston, M.A.; Shawa, J.; Alghusen, I.; Qiang, A.; Denson, A.R.; Carman, M.S.; Fedosyuk, H.; et al. O-GlcNAcylation Regulates Extracellular Signal-Regulated Kinase (ERK) Activation in Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1155630. [Google Scholar] [CrossRef]
- Pinho, T.S.; Correia, S.C.; Perry, G.; Ambrósio, A.F.; Moreira, P.I. Diminished O-GlcNAcylation in Alzheimer’s Disease Is Strongly Correlated with Mitochondrial Anomalies. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019, 1865, 2048–2059. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Shmueli, M.D.; Raz, C.; Yanku, M.; Zilberzwige, S.; Gazit, E.; Segal, D. Interplay between Protein Glycosylation Pathways in Alzheimer’s Disease. Sci. Adv. 2017, 3, e1601576. [Google Scholar] [CrossRef]
- Boix, C.P.; Lopez-Font, I.; Cuchillo-Ibañez, I.; Sáez-Valero, J. Amyloid Precursor Protein Glycosylation Is Altered in the Brain of Patients with Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Haukedal, H.; Freude, K.K. Implications of Glycosylation in Alzheimer’s Disease. Front. Neurosci. 2021, 14, 625348. [Google Scholar] [CrossRef] [PubMed]
- Helmfors, L.; Boman, A.; Civitelli, L.; Nath, S.; Sandin, L.; Janefjord, C.; McCann, H.; Zetterberg, H.; Blennow, K.; Halliday, G.; et al. Protective Properties of Lysozyme on β-Amyloid Pathology: Implications for Alzheimer Disease. Neurobiol. Dis. 2015, 83, 122–133. [Google Scholar] [CrossRef]
- Vela Navarro, N.; De Nadai Mundim, G.; Cudic, M. Implications of Mucin-Type O-Glycosylation in Alzheimer’s Disease. Molecules 2025, 30, 1895. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, Q.; Xu, S.; Yu, Y. The Alteration and Role of Glycoconjugates in Alzheimer’s Disease. Front. Aging Neurosci. 2024, 16, 1398641. [Google Scholar] [CrossRef]
- Hong, X.; Huang, L.; Lei, F.; Li, T.; Luo, Y.; Zeng, M.; Wang, Z. The Role and Pathogenesis of Tau Protein in Alzheimer’s Disease. Biomolecules 2025, 15, 824. [Google Scholar] [CrossRef]
- Nedelkov, D.; Tsokolas, Z.E.; Rodrigues, M.S.; Sible, I.; Han, S.D.; Kerman, B.E.; Renteln, M.; Mack, W.J.; Pascoal, T.A.; Yassine, H.N.; et al. Increased Cerebrospinal Fluid and Plasma apoE Glycosylation Is Associated with Reduced Levels of Alzheimer’s Disease Biomarkers. Alzheimer’s Res. Ther. 2025, 17, 151. [Google Scholar] [CrossRef]
- Olejnik, B.; Ferens-Sieczkowska, M. Lectins and Neurodegeneration: A Glycobiologist’s Perspective. Adv. Clin. Exp. Med. 2025, 34, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Pająk, B.; Kania, E.; Orzechowski, A. Killing Me Softly: Connotations to Unfolded Protein Response and Oxidative Stress in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2016, 2016, 1805304. [Google Scholar] [CrossRef] [PubMed]
- Pimenova, A.A.; Goate, A.M. Novel Presenilin 1 and 2 Double Knock-out Cell Line for in Vitro Validation of PSEN1 and PSEN2 Mutations. Neurobiol. Dis. 2020, 138, 104785. [Google Scholar] [CrossRef]
- Qiu, G.; Cao, L.; Chen, Y.-J. Novel Heterozygous Mutation in Alpha-2-Macroglobulin (A2M) Suppressing the Binding of Amyloid-β (Aβ). Front. Neurol. 2023, 13, 1090900. [Google Scholar] [CrossRef]
- Piscitelli, E.; Abeni, E.; Balbino, C.; Angeli, E.; Cocola, C.; Pelucchi, P.; Palizban, M.; Diaspro, A.; Götte, M.; Zucchi, I.; et al. Glycosylation Regulation by TMEM230 in Aging and Autoimmunity. Int. J. Mol. Sci. 2025, 26, 2412. [Google Scholar] [CrossRef]
- Ercan-Herbst, E.; Ehrig, J.; Schöndorf, D.C.; Behrendt, A.; Klaus, B.; Gomez Ramos, B.; Prat Oriol, N.; Weber, C.; Ehrnhoefer, D.E. A Post-Translational Modification Signature Defines Changes in Soluble Tau Correlating with Oligomerization in Early Stage Alzheimer’s Disease Brain. Acta Neuropathol. Commun. 2019, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. 3D Structure and Function of Glycosyltransferases Involved in N-Glycan Maturation. Int. J. Mol. Sci. 2020, 21, 437. [Google Scholar] [CrossRef]
- Ramazi, S.; Dadzadi, M.; Darvazi, M.; Seddigh, N.; Allahverdi, A. Protein Modification in Neurodegenerative Diseases. MedComm 2024, 5, e674. [Google Scholar] [CrossRef]
- Saha, A.; Fernández-Tejada, A. Chemical Biology Tools to Interrogate the Roles of O-GlcNAc in Immunity. Front. Immunol. 2023, 13, 1089824. [Google Scholar] [CrossRef]
- Shi, J.; Ku, X.; Zou, X.; Hou, J.; Yan, W.; Zhang, Y. Comprehensive Analysis of O-Glycosylation of Amyloid Precursor Protein (APP) Using Targeted and Multi-Fragmentation MS Strategy. Biochim. Biophys. Acta BBA—Gen. Subj. 2021, 1865, 129954. [Google Scholar] [CrossRef]
- Singh, Y.; Regmi, D.; Ormaza, D.; Ayyalasomayajula, R.; Vela, N.; Mundim, G.; Du, D.; Minond, D.; Cudic, M. Mucin-Type O-Glycosylation Proximal to β-Secretase Cleavage Site Affects APP Processing and Aggregation Fate. Front. Chem. 2022, 10, 859822. [Google Scholar] [CrossRef] [PubMed]
- Suttapitugsakul, S.; Stavenhagen, K.; Donskaya, S.; Bennett, D.A.; Mealer, R.G.; Seyfried, N.T.; Cummings, R.D. Glycoproteomics Landscape of Asymptomatic and Symptomatic Human Alzheimer’s Disease Brain. Mol. Cell. Proteom. 2022, 21, 100433. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Ohkawa, Y.; Maeda, K.; Kanto, N.; Johnson, E.L.; Harada, Y. N-Glycan Branching Enzymes Involved in Cancer, Alzheimer’s Disease and COPD and Future Perspectives. Biochem. Biophys. Res. Commun. 2022, 633, 68–71. [Google Scholar] [CrossRef]
- Tena, J.; Maezawa, I.; Barboza, M.; Wong, M.; Zhu, C.; Alvarez, M.R.; Jin, L.-W.; Zivkovic, A.M.; Lebrilla, C.B. Regio-Specific N-Glycome and N-Glycoproteome Map of the Elderly Human Brain with and Without Alzheimer’s Disease. Mol. Cell. Proteom. 2022, 21, 100427. [Google Scholar] [CrossRef]
- Urano, Y.; Takahachi, M.; Higashiura, R.; Fujiwara, H.; Funamoto, S.; Imai, S.; Futai, E.; Okuda, M.; Sugimoto, H.; Noguchi, N. Curcumin Derivative GT863 Inhibits Amyloid-Beta Production via Inhibition of Protein N-Glycosylation. Cells 2020, 9, 349. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, X.; Zeng, J.; Yuan, J.; Wang, Z.; Zhou, W.; Zhang, Y. LW-AFC Effects on N-Glycan Profile in Senescence-Accelerated Mouse Prone 8 Strain, a Mouse Model of Alzheimer’s Disease. Aging Dis. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gopal, S.; Pocock, R.; Xiao, Z. Glycan Mimetics from Natural Products: New Therapeutic Opportunities for Neurodegenerative Disease. Molecules 2019, 24, 4604. [Google Scholar] [CrossRef]
- Wani, W.Y.; Chatham, J.C.; Darley-Usmar, V.; McMahon, L.L.; Zhang, J. O-GlcNAcylation and Neurodegeneration. Brain Res. Bull. 2017, 133, 80–87. [Google Scholar] [CrossRef]
- Weber, P.; Bojarová, P.; Brouzdová, J.; Křen, V.; Kulik, N.; Stütz, A.E.; Thonhofer, M.; Wrodnigg, T.M. Diaminocyclopentane—l-Lysine Adducts: Potent and Selective Inhibitors of Human O-GlcNAcase. Bioorganic Chem. 2024, 148, 107452. [Google Scholar] [CrossRef]
- Wheatley, E.G.; Albarran, E.; White, C.W.; Bieri, G.; Sanchez-Diaz, C.; Pratt, K.; Snethlage, C.E.; Ding, J.B.; Villeda, S.A. Neuronal O-GlcNAcylation Improves Cognitive Function in the Aged Mouse Brain. Curr. Biol. 2019, 29, 3359–3369.e4. [Google Scholar] [CrossRef]
- Wu, F.; Li, W.; Lu, H.; Li, L. Recent Advances in Mass Spectrometry-Based Studies of Post-Translational Modifications in Alzheimer’s Disease. Mol. Cell. Proteom. 2025, 24, 101003. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wen, R.; Yang, N.; Zhang, T.-N.; Liu, C.-F. Roles of Protein Post-Translational Modifications in Glucose and Lipid Metabolism: Mechanisms and Perspectives. Mol. Med. 2023, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, T.; Zhao, H.; Ren, S. Glycosylation in Aging and Neurodegenerative Diseases. Acta Biochim. Biophys. Sin. 2024, 56, 1208–1220. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lang, M. New Insight into Protein Glycosylation in the Development of Alzheimer’s Disease. Cell Death Discov. 2023, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Moll, T.; Shaw, P.J.; Cooper-Knock, J. Disrupted Glycosylation of Lipids and Proteins Is a Cause of Neurodegeneration. Brain 2020, 143, 1332–1340. [Google Scholar] [CrossRef]
- Pradeep, P.; Kang, H.; Lee, B. Glycosylation and Behavioral Symptoms in Neurological Disorders. Transl. Psychiatry 2023, 13, 154. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Chin, L.-S.; Pan, S.; Li, L. Human Brain Glycoform Coregulation Network and Glycan Modification Alterations in Alzheimer’s Disease. Sci. Adv. 2024, 10, eadk6911. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Chin, L.-S.; Li, L. Integrative Glycoproteomics Reveals Protein N-Glycosylation Aberrations and Glycoproteomic Network Alterations in Alzheimer’s Disease. Sci. Adv. 2020, 6, eabc5802. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Menéndez-González, M.; Schreiner, O.D.; Ciobanu, R.C. Intrathecal Therapies for Neurodegenerative Diseases: A Review of Current Approaches and the Urgent Need for Advanced Delivery Systems. Biomedicines 2025, 13, 2167. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Y.; Wan, B.; Ren, X.; Guo, L.-H. Label-Free Electrochemical Biosensing of Small-Molecule Inhibition on O-GlcNAc Glycosylation. Biosens. Bioelectron. 2017, 95, 94–99. [Google Scholar] [CrossRef]
- Lin, T.; Van Husen, L.S.; Yu, Y.; Tjernberg, L.O.; Schedin-Weiss, S. Lack of N-Glycosylation Increases Amyloidogenic Processing of the Amyloid Precursor Protein. Glycobiology 2022, 32, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Buliman, A.; Calin, M.A.; Iordache, M.P. Targeting Anxiety with Light: Mechanistic and Clinical Insights into Photobiomodulation Therapy: A Mini Narrative Review. Balneo PRM Res. J. 2025, 16, 846. [Google Scholar] [CrossRef]
- Adam, R.; Moldovan, C.; Tudorache, S.; Hârșovescu, T.; Orban, C.; Pogărășteanu, M.; Rusu, E. Patellar Resurfacing in Total Knee Arthroplasty, a Never-Ending Controversy; Case Report and Literature Review. Diagnostics 2023, 13, 383. [Google Scholar] [CrossRef] [PubMed]


| Analytical Limitation | Description | References |
|---|---|---|
| 1. Glycoform and glycan isomer complexity | Single glycosites carry multiple glycoforms; many glycans are isomeric, complicating confident assignment. | [1] |
| 2. Fragmentation limitations | Collision-based fragmentation preferentially cleaves labile glycosidic bonds → good glycan info but poor peptide backbone coverage; limits site localization on multiply modified peptides. | [3] |
| 3. Enrichment biases | Lectin/HILIC enrichment favors only subsets of glycans; under-represents low-abundance or highly sialylated/fucosylated species; co-purified non-glycopeptides reduce dynamic range. | [1,3] |
| 4. Semi-quantitative datasets | Lack of isotopically labeled standards; matrix effects in CSF/plasma; batch effects; inter-laboratory variability limit absolute quantification. | [3,59] |
| 5. Proteoform search-space inflation | Assigning spectra to specific glycoproteoforms increases FDR; peptide-centric pipelines struggle with co-existing PTMs (O-GlcNAc, phosphorylation) and site-occupancy reporting. | [59] |
| 6. Sample-handling artifacts | Partial desialylation or de-N-glycosylation during preparation distorts native profiles; limited biofluid material restricts replication. | [1,3] |
| 7. Limited spatial resolution | Bulk proteomics lacks regional and cell-type resolution needed to map glycosylation to tauopathy topography. | [1,3,59] |
| Solution Category | Description/Technologies | References |
|---|---|---|
| 1. Hybrid fragmentation for improved site localization | ETD, EThcD, AI-ETD, stepped-HCD provide complementary backbone plus glycan information, improving site resolution. | [61] |
| 2. Ion mobility for isomer separation and sensitivity | FAIMS and TIMS-PASEF separate isomeric/isobaric glycopeptides, increasing sensitivity and reducing chemical noise in CSF. | [62] |
| 3. Orthogonal structural decoding | Exoglycosidase arrays, permethylation, linkage-specific sialic acid chemistry constrain structural assignments. | [63] |
| 4. Targeted quantitative workflows | PRM, DIA-SWATH, and stable-isotope internal standards enable reproducible multi-center-ready quantification. | [61,62] |
| 5. Top-down and native MS | Resolve intact proteoforms containing combined glyco- and phospho-states, clarifying PTM crosstalk on tau. | [62,63] |
| 6. Spatial glycoproteomics | MALDI imaging, lectin-guided IMS, laser capture microdissection + LC-MS provide anatomical resolution aligned with Braak staging. | [63] |
| 7. Advanced bioinformatics and standardization | Open/offset searches, glycan-aware FDR control, site-occupancy reporting; improved metadata standards and enzyme-toggle controls enhance transparency. | [61,62,63] |
| Disorder | Tau Isoform/Filament | Tau N-Glycosylation (Trend/Examples) | Tau O-GlcNAcylation (Trend) | Phosphorylation Pattern (Illustrative) | Predominant Regions/Phenotype | CSF/Serum Glyco-Biomarker Notes |
|---|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Mixed 3R/4R; PHFs | Aberrant N-glycosylation detected in NFTs; contributes to aggregation and primes hyperphosphorylation (e.g., Asn sites reported). | Reduced O-GlcNAcylation vs. controls; loss of protective competition with phosphorylation. | Broad phospho-tau engagement (e.g., S202/T205, T231, S396/404). | Medial temporal → associative neocortex; progressive cognitive decline. | CSF shows disease-linked glycoform shifts; glycoproteomics distinguishes AD from controls and supports diagnostic potential. |
| Progressive supranuclear palsy (PSP) | Predominantly 4R; straight filaments | Enrichment of high-mannose N-glycans on tau reported relative to AD, indicating distinct enzymatic dysregulation. | O-GlcNAc changes reported but generally less reduced than in AD (comparatively). | Phospho-site usage differs from AD; strong 4R context. | Brainstem, basal ganglia; axial rigidity, vertical gaze palsy. | Distinct glycan patterns vs. AD suggest differential-diagnosis utility when combined with phospho-tau. |
| Corticobasal degeneration (CBD) | Predominantly 4R; straight filaments | Relative enrichment of complex-type N-glycans vs. PSP; disease-specific N-glycan profile. | O-GlcNAc alterations present; comparative magnitude uncertain. | 4R-biased phospho-signature; corticobasal distribution. | Asymmetric cortical and basal ganglia involvement; apraxia, dystonia. | Glyco-profiling may separate CBD from PSP/AD in research cohorts. |
| FTLD-tau (e.g., Pick’s disease/other subtypes) | Often 3R (Pick’s); mixed in others; diverse inclusion types | Abnormal tau glycosylation reported; pattern varies by subtype/glial vs. neuronal predominance. | O-GlcNAc deficits also observed in FTLD tissue, linking metabolism to tau pathology. | Subtype-specific phospho-maps; Pick bodies in 3R disease. | FTLD; behavioral/language syndromes. | Distinct glyco-signatures vs. AD/PSP/CBD are emerging in glycoproteomics studies. |
| Process/Target | Type of Glycosylation | Pathological Consequence | Biomarker/Therapeutic Relevance | Key References |
|---|---|---|---|---|
| Tau protein | N-glycosylation | Promotes hyperphosphorylation and aggregation | Detected in NFTs; biomarker potential | [12,82] |
| Tau protein | O-GlcNAcylation | Protective, reduces phosphorylation and aggregation | Reduced in AD brains; OGA inhibitors in trials | [20,59,68] |
| APP | N-glycosylation | Alters trafficking; increases amyloidogenic cleavage | Potential target for secretase regulation | [5,8,26] |
| BACE1 (β-secretase) | N-glycosylation | Stabilizes enzyme, promotes Aβ production | Inhibition reduces Aβ levels | [17,24,32,47,77,85] |
| Nicastrin (γ-secretase) | N-glycosylation | Modulates substrate binding and Aβ species ratio | Glycan-targeting therapies under exploration | [18,55,64,80] |
| Synaptic receptors (NMDA, AMPA) | N-glycosylation | Controls receptor trafficking and function | Aberrant glycosylation increases Aβ vulnerability | [38,61] |
| NCAM (adhesion) | Polysialylation | Regulates neurite outgrowth and synaptic plasticity | Reduced in AD hippocampus | [32,64,68] |
| Immune receptors (TREM2, CD33) | N-glycosylation | Controls stability and microglial response | Mutations affect AD risk | [27,59,67] |
| Cytokines (IL-6, TNF-α) | N-glycosylation | Regulates secretion and signaling | Altered profiles detected in CSF | [28,41] |
| Complement proteins (C1q, C3) | Sialylation | Regulates activation and synaptic pruning | Aberrant glycosylation enhances synapse loss | [9,12,54,60] |
| Enzymes (OGT, OGA) | Glycosylation enzymes | Balances O-GlcNAcylation/phosphorylation | Biomarker and therapeutic target | [83,88] |
| Category | Item | Description | References |
|---|---|---|---|
| A. Minimum reporting set (per study) | Tau isoform and filament context | Report tau isoform composition (3R, 4R, mixed) and filament type (paired helical filaments vs. straight filaments), together with sampled brain regions. | [4,26] |
| Site-resolved PTM map | Provide integrated PTM map of the same specimens: (i) N-glycosylation (Asn), (ii) O-GlcNAc (Ser/Thr), and (iii) phosphorylation at canonical AD sites (T231, S202/T205, S396/404). | [1,2,72] | |
| Co-occupancy or competition metrics | Quantify O-GlcNAc vs. phospho-tau co-occupancy or competition at adjacent residues using MS or orthogonal immunoassays (e.g., antibody–lectin hybrids). | [74,75,76,77,78,79,80,81,82,83,84,85,86] | |
| Specimen matrix and assay performance | Report matrix (brain region, CSF, plasma) and assay characteristics (LoD, LoQ, spike-recovery, matrix effects) to allow cross-study comparison. | [99,100] | |
| B. Disease-specific signatures to report | Alzheimer’s disease | Mixed 3R/4R tau, PHF filaments; increased N-glycosylation with reduced O-GlcNAc; broad phospho-site engagement. | [4,104] |
| PSP/CBD | Predominantly 4R tau, straight filaments; restricted N-glycan complexity; distinct phospho-site profile; evaluate whether O-GlcNAc loss is less pronounced than in AD. | [7,26,51] | |
| Pick’s disease and others | Specify 3R tau (e.g., Pick’s) vs. mixed isoforms; document glial vs. neuronal glycosylation patterns. | [52,53,54] | |
| C. Assay and antibody validation rules | Phospho-tau antibodies | Validate antibodies such as AT8 (pS202/pT205), PHF-1 (pS396/pS404), CP13 (pS202) for IHC and CSF. Include epitope-blocking peptide and phosphatase-treatment controls. | [1,2,8,56] |
| Glyco-tau antibodies | Because glyco-tau antibodies remain research-grade, use: (i) enzymatic toggling (PNGase F; OGA/OGT modulation), (ii) lectin co-capture, and (iii) MS confirmation in the same samples (especially for CSF/serum). | [69,70,71] | |
| Fluid-based assays | Prefer antibody–lectin hybrid ELISA or IP→LC-MS/MS for glyco-site certainty in fluids; interpret IHC glyco-signal cautiously unless enzymatic controls are demonstrated. | [84] | |
| D. Decision framework for clinical translation | Tier 1—Exploratory | MS-centric site discovery in brain tissue with matched phospho- and O-GlcNAc-tau data. | [115] |
| Tier 2—Verification | Antibody–lectin or IP-MS assays in CSF; report full analytical validation parameters. | [112] | |
| Tier 3—Qualification | Multi-center CSF and plasma biomarker panels combining phospho-tau and tau glyco-epitopes with APP-related and immune glyco-markers for differential diagnosis (AD vs. PSP/CBD). | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondar, A.-C.; Iordache, M.P.; Coroescu, M.; Buliman, A.; Rusu, E.; Budișteanu, M.; Tanase, C. Unlocking the Sugar Code: Implications and Consequences of Glycosylation in Alzheimer’s Disease and Other Tauopathies. Biomedicines 2025, 13, 2884. https://doi.org/10.3390/biomedicines13122884
Bondar A-C, Iordache MP, Coroescu M, Buliman A, Rusu E, Budișteanu M, Tanase C. Unlocking the Sugar Code: Implications and Consequences of Glycosylation in Alzheimer’s Disease and Other Tauopathies. Biomedicines. 2025; 13(12):2884. https://doi.org/10.3390/biomedicines13122884
Chicago/Turabian StyleBondar, Andrei-Cristian, Marius P. Iordache, Mirela Coroescu, Anca Buliman, Elena Rusu, Magdalena Budișteanu, and Cristiana Tanase. 2025. "Unlocking the Sugar Code: Implications and Consequences of Glycosylation in Alzheimer’s Disease and Other Tauopathies" Biomedicines 13, no. 12: 2884. https://doi.org/10.3390/biomedicines13122884
APA StyleBondar, A.-C., Iordache, M. P., Coroescu, M., Buliman, A., Rusu, E., Budișteanu, M., & Tanase, C. (2025). Unlocking the Sugar Code: Implications and Consequences of Glycosylation in Alzheimer’s Disease and Other Tauopathies. Biomedicines, 13(12), 2884. https://doi.org/10.3390/biomedicines13122884






