Preoperative Differentiation of Non-Subungual Glomus Tumors from Other Superficial Soft Tissue Tumors Using a Clinical and Ultrasound-Based Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
2.3. Ultrasonography Examinations
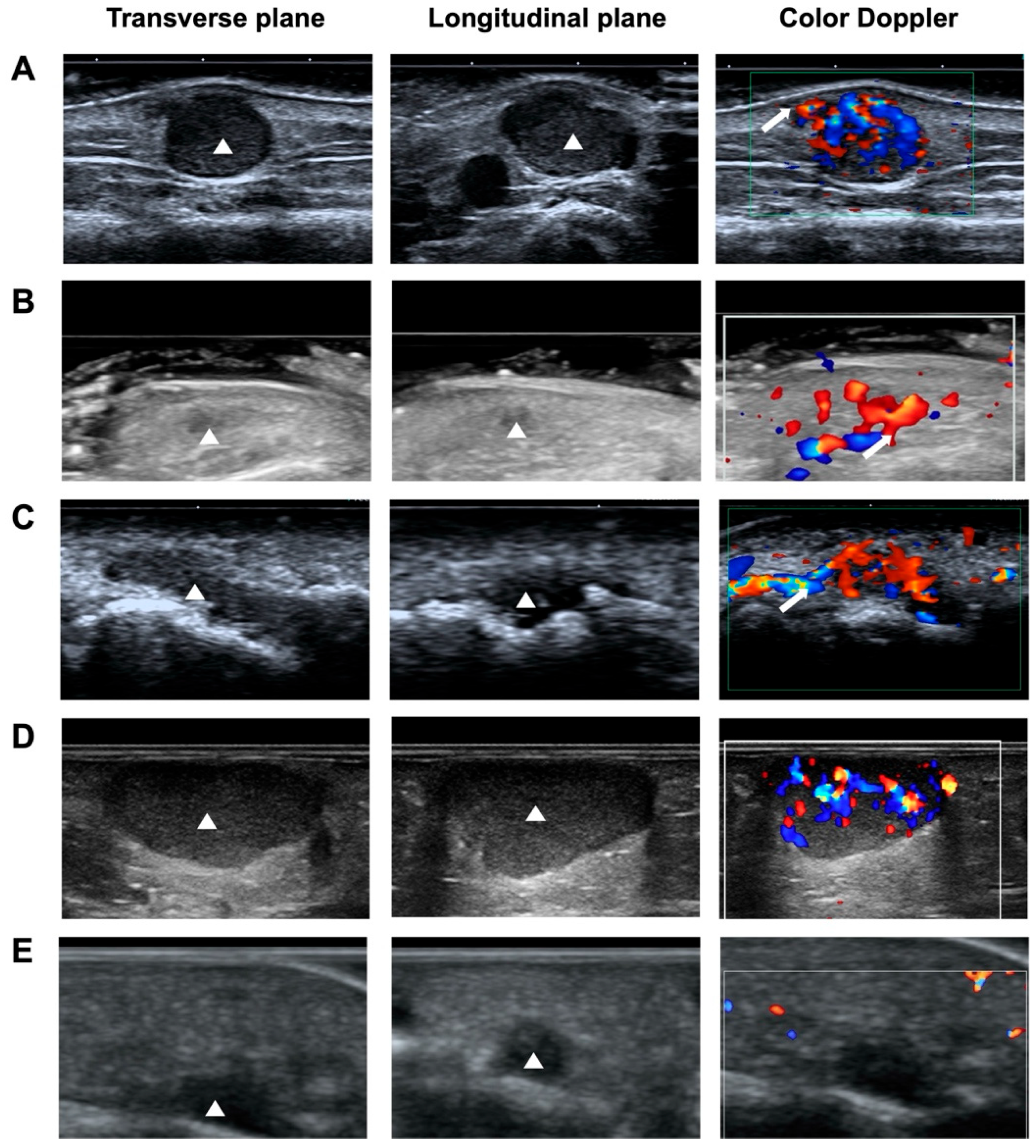
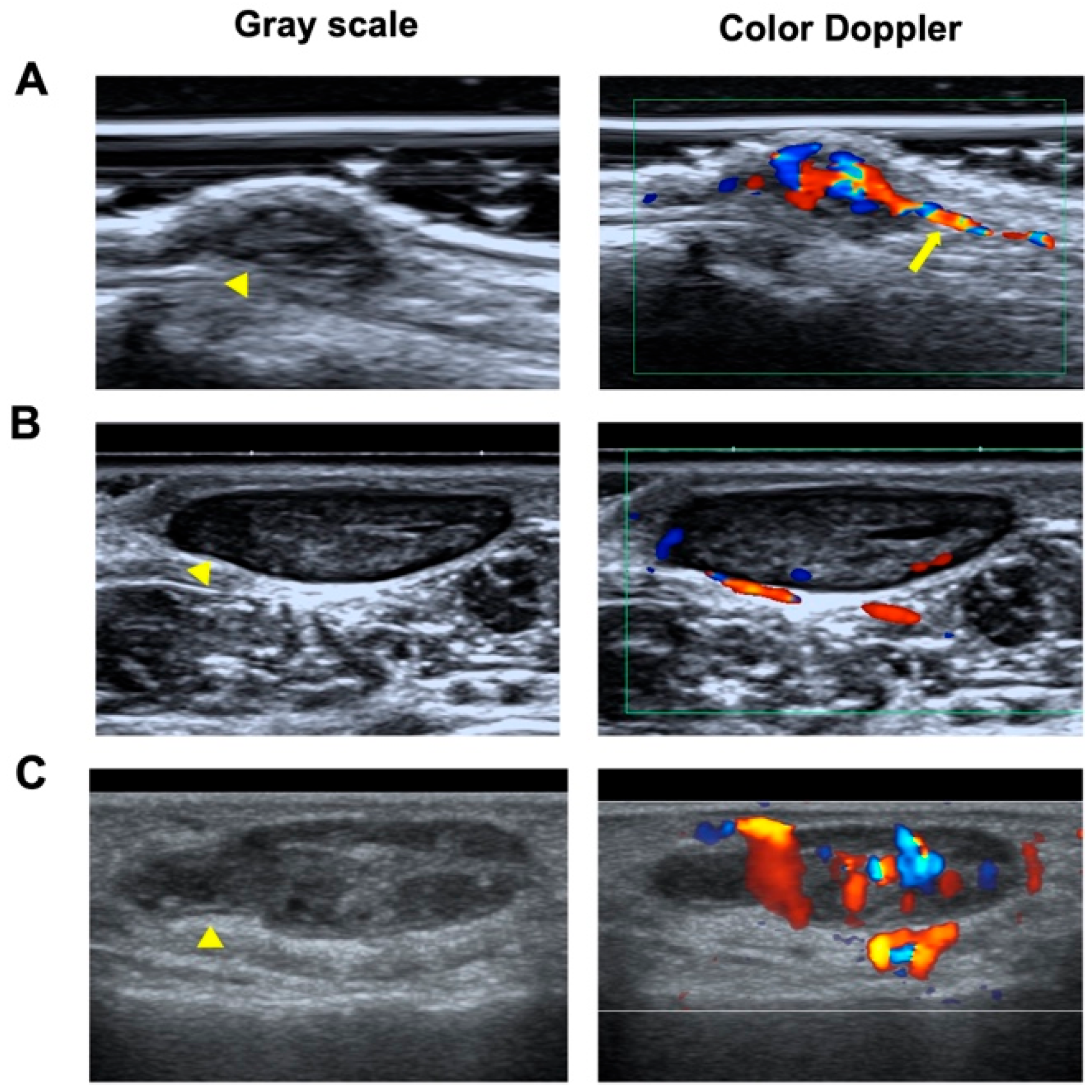
2.4. Images Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Mass Site
3.3. Mass Size
3.4. Sonographic Findings
3.4.1. Shape and Orientation
3.4.2. Margin
3.4.3. Echogenicity and Echotexture
3.4.4. Vascularity
3.4.5. Logistic Regression Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GTs | Glomus tumors |
| NSGTs | Non-subungual glomus tumors |
| ALMs | Angioleiomyomas |
| ROC | Receiver operating characteristic |
| AUC | Area under curve |
Appendix A
| NSGT (n = 58) | ALM (n = 68) | Hemangioma (n = 67) | |
|---|---|---|---|
| Mass location | Finger (39) | Ear (1) | Neck (5) |
| Hand (5) | Finger (5) | Forehead (3) | |
| Arm (3) | Hand (2) | Face (3) | |
| Shoulder (1) | Wrist (2) | Eyelid (1) | |
| Teo (3) | Elbow (1) | Nose (1) | |
| Thigh (2) | Foot (21) | Lip (1) | |
| Knee (2) | Calf (11) | Head (1) | |
| Foot (1) | Knee (11) | Finger (12) | |
| Buttock (1) | Thigh (3) | Arm (11) | |
| Chest wall (1) | Ankle (6) | Hand (5) | |
| Teo (3) | Wrist (3) | ||
| Epididymis (1) | Elbow (1) | ||
| Buttock (1) | Shoulder (1) | ||
| Axilla (1) | |||
| Thigh (2) | |||
| Knee (2) | |||
| Popliteal fossa (1) | |||
| Calf (1) | |||
| Foot (3) | |||
| Teo (1) | |||
| Chest wall (1) | |||
| Abdominal wall (1) | |||
| Back (1) | |||
| Waist (1) | |||
| Buttock (1) | |||
| Vulvae (3) | |||
| Ectopectoralis (1) |
| p | Exp(B) | EXP(B) 95% IC | |||
|---|---|---|---|---|---|
| Upper Bound | Lower Bound | ||||
| Step 1 | Pain (1) | 0.003 | 4.923 | 1.694 | 14.305 |
| Tenderness (1) | 0.082 | 2.534 | 0.887 | 7.239 | |
| Location (1) | <0.001 | 10.415 | 3.672 | 29.547 | |
| Diameter (1) | <0.001 | 6.534 | 2.296 | 18.590 | |
| Length/Thickness ratio (1) | 0.443 | 1.482 | 0.542 | 4.055 | |
| Shape (1) | 0.567 | 0.679 | 0.181 | 2.551 | |
| Margin (1) | 0.053 | 5.124 | 0.977 | 26.878 | |
| Vascular stalk sign (1) | 0.003 | 4.098 | 1.603 | 10.477 | |
| Constant | <0.001 | 0.023 | |||
| Step 2 | Pain (1) | 0.003 | 4.875 | 1.686 | 14.096 |
| Tenderness (1) | 0.084 | 2.514 | 0.885 | 7.139 | |
| Location (1) | <0.001 | 10.733 | 3.805 | 30.271 | |
| Diameter (1) | <0.001 | 6.267 | 2.230 | 17.611 | |
| Length/Thickness (1) | 0.408 | 1.525 | 0.561 | 4.144 | |
| Margin (1) | 0.057 | 4.128 | 0.961 | 17.721 | |
| Vascular stalk sign (1) | 0.004 | 4.004 | 1.576 | 10.172 | |
| Constant | <0.001 | 0.023 | |||
| Step 3 | Pain (1) | 0.002 | 5.135 | 1.804 | 14.618 |
| Tenderness (1) | 0.091 | 2.452 | 0.867 | 6.934 | |
| Location (1) | <0.001 | 10.195 | 3.653 | 28.448 | |
| Diameter (1) | <0.001 | 7.133 | 2.658 | 19.146 | |
| Margin (1) | 0.072 | 3.711 | 0.888 | 15.505 | |
| Vascular stalk sign (1) | 0.001 | 4.381 | 1.765 | 10.878 | |
| Constant | <0.001 | 0.026 | |||
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and Perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Wolter, N.E.; Adil, E.; Irace, A.L.; Werger, A.; Perez-Atayde, A.R.; Weldon, C.; Orbach, D.B.; Rodriguez-Galindo, C.; Rahbar, R. Malignant Glomus Tumors of the Head and Neck in Children and Adults: Evaluation and Management. Laryngoscope 2017, 127, 2873–2882. [Google Scholar] [CrossRef]
- Zanjani, L.O.; Nia, B.S.; Vosoughi, F.; Mirzaian, E.; Aghaghazvini, L.; Arabzadeh, A. An Unusual Case of Chest Wall Glomus Tumor Presenting with Axillary Pain: A Case Report and Literature Review. Eur. J. Med. Res. 2021, 26, 49. [Google Scholar] [CrossRef]
- Yadav, S.K.; Rajnish, R.K.; Prakash, V.; Verma, V. Glomus Tumour Located in the Volar Aspect of Distal Phalanx of Thumb. BMJ Case Rep. 2024, 17, e260130. [Google Scholar] [CrossRef]
- She, C.; Peng, J.; Li, Y.; Ma, F. Endoscopic-Guided Therapy for Tracheal Glomus Tumor: A Case Report on Radiofrequency Plasma-Assisted Therapy. Ear Nose Throat J. 2024, 1455613241272640. [Google Scholar] [CrossRef]
- Liu, X.-J.; Hu, Y.-T.; Zhao, L.-Y. Rapidly Progressive Malignant Glomus Tumor of the Breast: A Case Report and Review of the Literature. J. Int. Med. Res. 2024, 52, 3000605241272609. [Google Scholar] [CrossRef]
- Engle, J.A.; Dibb, J.T.; Kundu, S.; Jakob, J.A. Glomus Tumor of the Chest Wall With Metastases to Lung. Cureus 2024, 16, e69122. [Google Scholar] [CrossRef] [PubMed]
- Sandrasegaran, K.; Shah, A.; Thompson, C.; Chen, L.; Silva, A. Imaging Findings of Gastric Glomus Tumors. Abdom. Radiol. 2025, 50, 1099–1104. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Juan, Y.-S.; Chen, Y.-T. Renal Glomus Tumor: A Case Report and Literature Review. Urol. Case Rep. 2024, 56, 102813. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, J.; Wang, Y.; Ai, C.; Wang, H.; Jin, Y.; Zhang, H.; Li, M.; Zhang, Y. Benign Glomus Tumor of Prostate: A Case Report. BMC Urol. 2024, 24, 204. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Alfageme Roldän, F.; Solivetti, F.M.; Scotto di Santolo, M.; Bouer, M.; Wortsman, X. Color Doppler Sonography of Extradigital Glomus Tumors. J. Ultrasound Med. 2017, 36, 231–238. [Google Scholar] [CrossRef]
- Falcone, M.-O.; Asmar, G.; Chassat, R. Subungual Glomus Tumor. Hand Surg. Rehabil. 2024, 43, 101607. [Google Scholar] [CrossRef]
- Chou, T.; Pan, S.C.; Shieh, S.J.; Lee, J.W.; Chiu, H.Y.; Ho, C.L. Glomus Tumor: Twenty-Year Experience and Literature Review. Ann. Plast. Surg. 2016, 76, S35–S40. [Google Scholar] [CrossRef]
- Giugale, J.M.; Fowler, J.R. Glomus Tumors: A Review of Preoperative Magnetic Resonance Imaging to Detect Satellite Lesions. Orthopedics 2015, 38, e888–e890. [Google Scholar] [CrossRef]
- Huang, H.-P.; Tsai, M.-C.; Hong, K.-T.; Chang, S.-C.; Wang, C.-H.; Li, C.-C.; Chiu, W.-K.; Chen, S.-G. Outcome of Microscopic Excision of a Subungual Glomus Tumor: A 12-Year Evaluation. Dermatol. Surg. 2015, 41, 487–492. [Google Scholar] [CrossRef]
- Schiefer, T.K.; Parker, W.L.; Anakwenze, O.A.; Amadio, P.C.; Inwards, C.Y.; Spinner, R.J. Extradigital Glomus Tumors: A 20-Year Experience. Mayo Clin. Proc. 2006, 81, 1337–1344. [Google Scholar] [CrossRef]
- Lee, D.K.; Hill, R.C.; Desai, A.D.; Lipner, S.R. Utility of Imaging in Diagnosing Subungual Glomus Tumors: A Single-Center Retrospective Study. J. Am. Acad. Dermatol. 2024, 91, 348–350. [Google Scholar] [CrossRef]
- Matos, M.J.; Soares, S.; Schwab, J.M.; Tannast, M.; Seidel, A. Foot and Ankle Angioleiomyoma: A Systematic Review. BMC Musculoskelet. Disord. 2025, 26, 246. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Wang, Z.; Zhou, H.; Alhaskawi, A.; Dong, Y.; Lu, H. Ten Years’ Experience with Diagnosis and Treatment of Non-Subungual Glomus Tumor in the Fingers. Technol. Cancer Res. Treat. 2024, 23, 15330338241287087. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Middleton, W.D.; Allison, S.J.; Dahiya, N.; Lee, K.S.; Levine, B.D.; Lucas, D.R.; Murphey, M.D.; Nazarian, L.N.; Siegel, G.W.; et al. Ultrasonography of Superficial Soft-Tissue Masses: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2022, 304, 18–30. [Google Scholar] [CrossRef]
- Takanashi, N.; Asai, S.; Ogase, Y.; Fujii, A.; Atsumi, H.; Doi, M.; Kumaki, N.; Mabuchi, T.; Miyachi, H. Ultrasonographic Characteristics in the Fingers and Other Superficial Glomus Tumours. Dermatol. Res. Pract. 2023, 2023, 7126799. [Google Scholar] [CrossRef]
- Park, H.-J.; Jeon, Y.H.; Kim, S.S.; Lee, S.-M.; Kim, W.-T.; Park, N.-H.; Park, S.-I.; Hong, H.-P.; Rho, M.-H. Gray-Scale and Color Doppler Sonographic Appearances of Nonsubungual Soft-Tissue Glomus Tumors. J. Clin. Ultrasound 2011, 39, 305–309. [Google Scholar] [CrossRef]
- Lee, D.; Yang, J.; Chang, S.; Won, C.; Lee, M.; Choi, J.; Moon, K. Clinical and Pathological Characteristics of Extradigital and Digital Glomus Tumours: A Retrospective Comparative Study. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1392–1397. [Google Scholar] [CrossRef]
- Mravic, M.; LaChaud, G.; Nguyen, A.; Scott, M.A.; Dry, S.M.; James, A.W. Clinical and Histopathological Diagnosis of Glomus Tumor: An Institutional Experience of 138 Cases. Int. J. Surg. Pathol. 2015, 23, 181–188. [Google Scholar] [CrossRef]
- Del Carpio, G.S.; Burgos, E.M.P.; Kreilinger, J.J.P.; Taboada, D.B.; Pensado, M.P.; Viñé, M.T. Case Series of Extradigital Glomus Tumors: Imaging Findings, Differential Diagnosis and Radiologic–Pathologic Correlation. Egypt. J. Radiol. Nucl. Med. 2024, 55, 10. [Google Scholar] [CrossRef]
- Gómez-Sánchez, M.E.; Alfageme-Roldán, F.; Roustán-Gullón, G.; Segurado-Rodríguez, M.A. The Usefulness of Ultrasound Imaging in Digital and Extradigital Glomus Tumors. Actas Dermosifiliogr. 2014, 105, e45–e49. [Google Scholar] [CrossRef]
- Kransdorf, M.J.; Larsen, B.T.; Fox, M.G.; Murphey, M.D.; Long, J.R. Musculoskeletal Glomus Tumor a Review of 218 Lesions in 176 Patients. Skeletal Radiol. 2025, 54, 457–479. [Google Scholar] [CrossRef]

| NSGT (n = 58) | ALM (n = 68) | a p | Hemangioma (n = 67) | b p | |
|---|---|---|---|---|---|
| Age (year) | 40.98 ± 13.48 | 45.13 ± 13.23 | 0.11 | 38.99 ± 14.47 | 0.42 |
| Sex (n, %) | 0.66 | 0.98 | |||
| Female | 33 (56.90%) | 36 (52.94%) | 38 (56.72%) | ||
| Male | 25 (43.1%) | 32 (47.06%) | 29 (43.28%) | ||
| Symptom (n, %) | <0.001 | <0.001 | |||
| Pain | 31 (53.45%) | 13 (19.12%) | 3 (4.48%) | ||
| Tenderness | 24 (41.38%) | 21 (30.88%) | 11 (39.40%) | ||
| Mass site | <0.001 | <0.001 | |||
| Head and neck | 0 (0%) | 1 (1.47%) | 15 (22.39%) | ||
| Upper extremity (n, %) | 48 (82.76%) | 10 (14.71%) | 34 (50.75%) | ||
| Lower extremity (n, %) | 8 (13.79%) | 55 (80.88%) | 10 (14.92%) | ||
| Trunk (n, %) | 2 (3.45%) | 2 (2.94%) | 8 (11.94%) | ||
| Mass size (median, IQR) | |||||
| Length (mm) | 5 (3, 8.75) | 10 (7.10, 17) | <0.001 | 15 (10, 31) | <0.001 |
| Thickness (mm) | 3 (2, 4) | 5.65 (4, 7) | <0.001 | 6 (3, 10) | <0.001 |
| Length/Thickness ratio | 1.78 (1.33, 2) | 2.23 (1.67, 3.29) | <0.001 | 2.75 (2, 3.71) | <0.001 |
| Sonographic Appearance | NSGT | ALM | a p | Hemangioma | bp |
|---|---|---|---|---|---|
| Shape (n, %) | <0.001 | <0.001 | |||
| Round | 6 (10.34%) | 1 (1.47%) | 0 | ||
| Oval | 44 (75.86%) | 64 (94.12%) | 34 (50.75%) | ||
| Irregular | 8 (13.80%) | 3 (4.41%) | 33 (49.25%) | ||
| Orientation (n, %) | >0.99 | 0.46 | |||
| Horizontal | 57 (98.28%) | 67 (98.53%) | 67 (100%) | ||
| Vertical | 1 (1.72%) | 1 (1.47%) | 0 (0%) | ||
| Margin (n, %) | 0.51 | <0.001 | |||
| Well-circumscribed | 52 (89.66%) | 64 (94.12%) | 33 (49.25%) | ||
| Ill-circumscribed | 6 (10.34%) | 4 (5.88%) | 34 (50.75%) | ||
| Echogenicity (n, %) | >0.99 | 0.25 | |||
| Hypoechoic | 58 (100%) | 67 (98.53%) | 62 (92.53%) | ||
| Isoechoic | 0 (0%) | 1 (1.47%) | 3 (4.48%) | ||
| Hyperechoic | 0 (0%) | 0 (0%) | 2 (2.99%) | ||
| Echotexture (n, %) | 0.14 | <0.001 | |||
| Homogeneous | 51 (87.93%) | 53 (77.94%) | 16 (23.88%) | ||
| Heterogeneous | 7 (12.07%) | 15 (22.06%) | 51 (76.12%) | ||
| Vascularity (n, %) | 0.80 | 0.26 | |||
| Absent | 11 (18.97%) | 18 (26.47%) | 7 (10.45%) | ||
| Minimal | 11 (18.97%) | 12 (17.65%) | 18 (26.87%) | ||
| Moderate | 18 (31.03%) | 19 (27.94%) | 27 (40.30%) | ||
| Marked | 18 (31.03%) | 19 (27.94%) | 15 (22.38%) | ||
| Vascular stalk (n, %) | <0.001 | <0.001 | |||
| Absent | 20 (34.48%) | 49 (72.06%) | 56 (83.58%) | ||
| Present | 38 (65.52%) | 19 (27.94%) | 11 (16.42%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Dan, Q.; Zhai, Y.; Guo, A.; Shen, Y.; Wang, R.; Sun, D.; Chen, X. Preoperative Differentiation of Non-Subungual Glomus Tumors from Other Superficial Soft Tissue Tumors Using a Clinical and Ultrasound-Based Model. Biomedicines 2025, 13, 2883. https://doi.org/10.3390/biomedicines13122883
Xiang H, Dan Q, Zhai Y, Guo A, Shen Y, Wang R, Sun D, Chen X. Preoperative Differentiation of Non-Subungual Glomus Tumors from Other Superficial Soft Tissue Tumors Using a Clinical and Ultrasound-Based Model. Biomedicines. 2025; 13(12):2883. https://doi.org/10.3390/biomedicines13122883
Chicago/Turabian StyleXiang, Hongjin, Qing Dan, Yue Zhai, Anran Guo, Yuzhou Shen, Run Wang, Desheng Sun, and Xiangmei Chen. 2025. "Preoperative Differentiation of Non-Subungual Glomus Tumors from Other Superficial Soft Tissue Tumors Using a Clinical and Ultrasound-Based Model" Biomedicines 13, no. 12: 2883. https://doi.org/10.3390/biomedicines13122883
APA StyleXiang, H., Dan, Q., Zhai, Y., Guo, A., Shen, Y., Wang, R., Sun, D., & Chen, X. (2025). Preoperative Differentiation of Non-Subungual Glomus Tumors from Other Superficial Soft Tissue Tumors Using a Clinical and Ultrasound-Based Model. Biomedicines, 13(12), 2883. https://doi.org/10.3390/biomedicines13122883





