H89 Reverses Multidrug Resistance in Colorectal Cancer by Inhibiting the ATPase Activity of ABCB1
Abstract
1. Introduction
2. Materials and Methods
2.1. ReagentsandCell Culture
2.2. Cytotoxicity Assay
2.3. Cell Cycle Assay
2.4. Drug Accumulation Assay
2.5. Western Blot Assay
2.6. ABCB1 ATPase Activity Assay
2.7. Molecular Docking Analysis
2.8. Statistical Analysis
3. Results
3.1. H89 Reverses ABCB1-Mediated MDR in CRC Cells
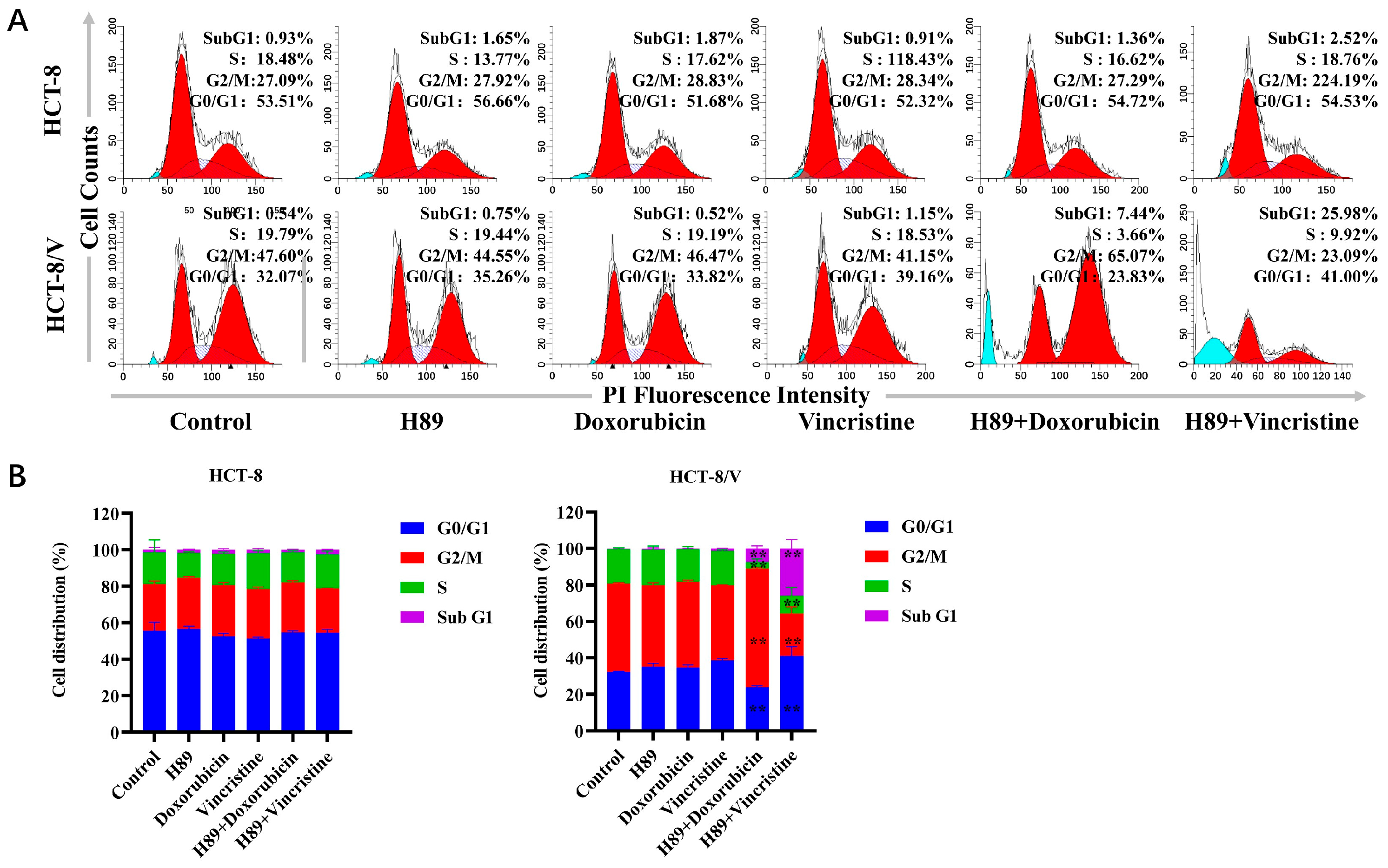
3.2. The Combination of H89 with Doxorubicin or Vincristine Induces Cell Cycle Arrest
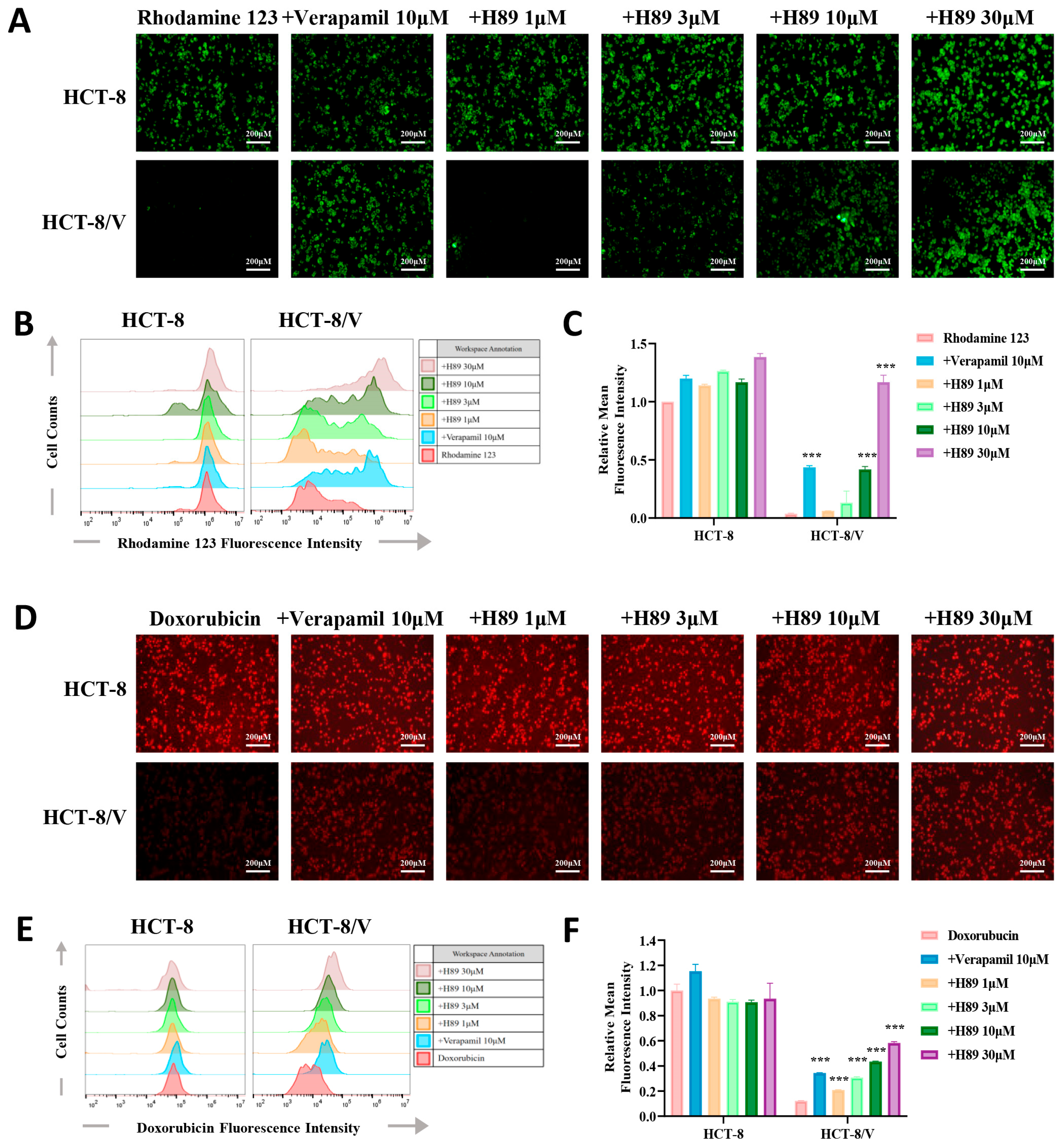
3.3. H89 Enhances theIntracellular Accumulation of Substrate Drugs in HCT-8/V Cells
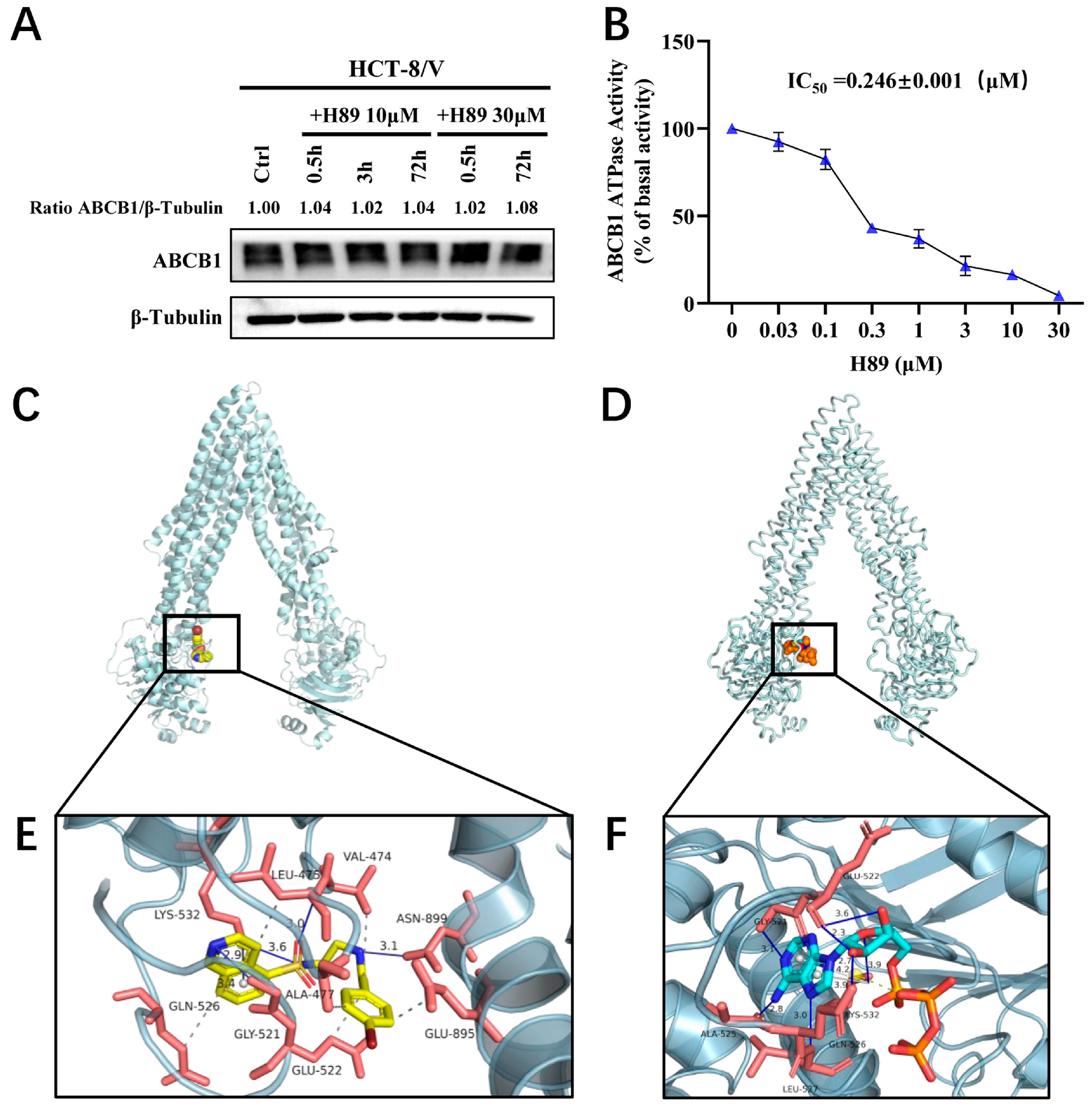
3.4. H89 Inhibits the ATPase Activityof ABCB1 and Occupies Its ATP-Binding Pocket
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Murphy, C.C.; Zaki, T.A. Changing epidemiology of colorectal cancer—Birth cohort effects and emerging risk factors. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 25–34. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Deng, Z.F.; Wu, X.T.; Lu, Y.; Zhu, Z.Y.; Wen, Q.; Zhang, W.; Zhang, H.Y.; Chen, X.Z.; Wu, Y.S.; et al. Low miR-224-5p in exosomes confers colorectal cancer 5-FU resistance by upregulating S100A4. Drug Resist. Updat. 2025, 79, 101211. [Google Scholar] [CrossRef] [PubMed]
- Alkharsan, A.; Safaralizadeh, R.; Khalaj-Kondori, M.; HosseinpourFeizi, M. Examination of the effects of capecitabine treatment on the HT-29 colorectal cancer cell line and HCG 11, HCG 15, and HCG 18 lncRNAs in CRC patients before and after chemotherapy. Naunyn Schmiedeb. Arch. Pharmacol. 2025, 398, 6929–6940. [Google Scholar] [CrossRef]
- Zeng, K.; Li, W.; Wang, Y.; Zhang, Z.; Zhang, L.; Zhang, W.; Xing, Y.; Zhou, C. Inhibition of CDK1 Overcomes Oxaliplatin Resistance by Regulating ACSL4-mediated Ferroptosis in Colorectal Cancer. Adv. Sci. 2023, 10, e2301088. [Google Scholar] [CrossRef]
- Kopetz, S.; Guthrie, K.A.; Morris, V.K.; Lenz, H.J.; Magliocco, A.M.; Maru, D.; Yan, Y.; Lanman, R.; Manyam, G.; Hong, D.S.; et al. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J. Clin. Oncol. 2021, 39, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Baguley, B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef]
- Fojo, T.; Menefee, M. Mechanisms of multidrug resistance: The potential role of microtubule-stabilizing agents. Ann. Oncol. 2007, 18 (Suppl. S5), v3–v8. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, B.; Chen, Z.; Chen, Z.S. Progress in the studies on the molecular mechanisms associated with multidrug resistance in cancers. Acta Pharm. Sin. B 2023, 13, 982–997. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Xu, B.Q.; Wang, Y.Y.; Zhang, P.W.; Shu, Y.B.; Shi, Z. GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs. Biomedicines 2023, 11, 3103. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Teng, Q.X.; Yang, Y.; Acharekar, N.; Wang, J.Q.; He, M.; Yoganathan, S.; Lin, J.; Wang, J.; Chen, Z.S. MET inhibitor tepotinib antagonizes multidrug resistance mediated by ABCG2 transporter: In vitro and in vivo study. Acta Pharm. Sin. B 2022, 12, 2609–2618. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Lelong, I.H.; Zhang, J.; Cardarelli, C. Purification and reconstitution of human P-glycoprotein. Methods Enzymol. 1998, 292, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Clark, D.; Ueda, K.; Pastan, I.; Gottesman, M.M.; Roninson, I.B. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. J. Biol. Chem. 1990, 265, 506–514. [Google Scholar] [CrossRef]
- Raguz, S.; Randle, R.A.; Sharpe, E.R.; Foekens, J.A.; Sieuwerts, A.M.; Gelder, M.E.M.-V.; Melo, J.V.; Higgins, C.F.; Yagüe, E. Production of P-glycoprotein from the MDR1 upstream promoter is insufficient to affect the response to first-line chemotherapy in advanced breast cancer. Int. J. Cancer 2008, 122, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Raguz, S.; De Bella, M.T.; Tripuraneni, G.; Slade, M.J.; Higgins, C.F.; Coombes, R.C.; Yagüe, E. Activation of the MDR1 upstream promoter in breast carcinoma as a surrogate for metastatic invasion. Clin. Cancer Res. 2004, 10, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Mora Lagares, L.; Pérez-Castillo, Y.; Minovski, N.; Novič, M. Structure-Function Relationships in the Human P-Glycoprotein (ABCB1): Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2021, 23, 362. [Google Scholar] [CrossRef]
- Chufan, E.E.; Kapoor, K.; Ambudkar, S.V. Drug-protein hydrogen bonds govern the inhibition of the ATP hydrolysis of the multidrug transporter P-glycoprotein. Biochem. Pharmacol. 2016, 101, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Guo, Y.; Ashrafizadeh, M.; Tambuwala, M.M.; Ren, J.; Orive, G.; Yu, G. P-glycoprotein (P-gp)-driven cancer drug resistance: Biological profile, non-coding RNAs, drugs and nanomodulators. Drug Discov. Today 2024, 29, 104161. [Google Scholar] [CrossRef]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef]
- Meschini, S.; Calcabrini, A.; Monti, E.; Del Bufalo, D.; Stringaro, A.; Dolfini, E.; Arancia, G. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int. J. Cancer 2000, 87, 615–628. [Google Scholar] [CrossRef]
- Besse, A.; Stolze, S.C.; Rasche, L.; Weinhold, N.; Morgan, G.J.; Kraus, M.; Bader, J.; Overkleeft, H.S.; Besse, L.; Driessen, C. Carfilzomib resistance due to ABCB1/MDR1 overexpression is overcome by nelfinavir and lopinavir in multiple myeloma. Leukemia 2018, 32, 391–401. [Google Scholar] [CrossRef]
- Sadeghi, M.R.; Jeddi, F.; Soozangar, N.; Somi, M.H.; Shirmohamadi, M.; Khaze, V.; Samadi, N. Nrf2/P-glycoprotein axis is associated with clinicopathological characteristics in colorectal cancer. Biomed. Pharmacother. 2018, 104, 458–464. [Google Scholar] [CrossRef]
- Lei, Z.N.; Teng, Q.X.; Wu, Z.X.; Ping, F.F.; Song, P.; Wurpel, J.N.D.; Chen, Z.S. Overcoming multidrug resistance by knockout of ABCB1 gene using CRISPR/Cas9 system in SW620/Ad300 colorectal cancer cells. MedComm 2021, 2, 765–777. [Google Scholar] [CrossRef]
- Dahlmann, M.; Werner, R.; Kortüm, B.; Kobelt, D.; Walther, W.; Stein, U. Restoring Treatment Response in Colorectal Cancer Cells by Targeting MACC1-Dependent ABCB1 Expression in Combination Therapy. Front. Oncol. 2020, 10, 599. [Google Scholar] [CrossRef]
- Ye, P.; Xing, H.; Lou, F.; Wang, K.; Pan, Q.; Zhou, X.; Gong, L.; Li, D. Histone deacetylase 2 regulates doxorubicin (Dox) sensitivity of colorectal cancer cells by targeting ABCB1 transcription. Cancer Chemother. Pharmacol. 2016, 77, 613–621. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Wang, F.; Moyer, M.P.; Wei, Q.; Zhang, P.; Yang, Z.; Liu, W.; Zhang, H.; Chen, N.; et al. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signalling pathway via suppression of activator protein 2α. Gut 2016, 65, 1494–1504. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, F.; Gu, X.; Feng, L.; Xu, M.; Li, T.; Liu, X.; Zhang, X. Binding of RNA m6A by IGF2BP3 triggers chemoresistance of HCT8 cells via upregulation of ABCB1. Am. J. Cancer Res. 2021, 11, 1428–1445. [Google Scholar]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.J. Strategies to overcome cancer multidrug resistance (MDR) through targeting P-glycoprotein (ABCB1): An updated review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef]
- Wang, S.Q.; Teng, Q.X.; Wang, S.; Lei, Z.N.; Hu, H.H.; Lv, H.F.; Chen, B.B.; Wang, J.Z.; Shi, X.J.; Xu, W.F.; et al. Preclinical studies of the triazolo[1,5-a]pyrimidine derivative WS-716 as a highly potent, specific and orally active P-glycoprotein (P-gp) inhibitor. Acta Pharm. Sin. B 2022, 12, 3263–3280. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Błaszczyk, M.; Środa-Pomianek, K.; Wesołowska, O. Isobavachalcone as an Active Membrane Perturbing Agent and Inhibitor of ABCB1 Multidrug Transporter. Molecules 2021, 26, 4637. [Google Scholar] [CrossRef] [PubMed]
- Nosol, K.; Romane, K.; Irobalieva, R.N.; Alam, A.; Kowal, J.; Fujita, N.; Locher, K.P. Cryo-EM structures reveal distinct mechanisms of inhibition of the human multidrug transporter ABCB1. Proc. Natl. Acad. Sci. USA 2020, 117, 26245–26253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Liu, H.M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med. Res. Rev. 2021, 41, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, J.G.; Li, Y.; Di, J.M.; Zhang, W.J.; Jiang, Q.W.; Zheng, D.W.; Chen, Y.; Wei, M.N.; Huang, J.R.; et al. Targeting ABCB1-mediated tumor multidrug resistance by CRISPR/Cas9-based genome editing. Am. J. Transl. Res. 2016, 8, 3986–3994. [Google Scholar]
- Gao, H.L.; Gupta, P.; Cui, Q.; Ashar, Y.V.; Wu, Z.X.; Zeng, L.; Lei, Z.N.; Teng, Q.X.; Ashby, C.R., Jr.; Guan, Y.; et al. Sapitinib Reverses Anticancer Drug Resistance in Colon Cancer Cells Overexpressing the ABCB1 Transporter. Front. Oncol. 2020, 10, 574861. [Google Scholar] [CrossRef]
- Wu, Z.X.; Teng, Q.X.; Cai, C.Y.; Wang, J.Q.; Lei, Z.N.; Yang, Y.; Fan, Y.F.; Zhang, J.Y.; Li, J.; Chen, Z.S. Tepotinib reverses ABCB1-mediated multidrug resistance in cancer cells. Biochem. Pharmacol. 2019, 166, 120–127. [Google Scholar] [CrossRef]
- Boichuk, S.; Dunaev, P.; Mustafin, I.; Mani, S.; Syuzov, K.; Valeeva, E.; Bikinieva, F.; Galembikova, A. Infigratinib (BGJ 398), a Pan-FGFR Inhibitor, Targets P-Glycoprotein and Increases Chemotherapeutic-Induced Mortality of Multidrug-Resistant Tumor Cells. Biomedicines 2022, 10, 601. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, J.Q.; Yang, Y.; Li, J.; Chen, Z.S. Natural Product as Substrates of ABC Transporters: A Review. Recent Pat. Anticancer Drug Discov. 2021, 16, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Chijiwa, T.; Mishima, A.; Hagiwara, M.; Sano, M.; Hayashi, K.; Inoue, T.; Naito, K.; Toshioka, T.; Hidaka, H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990, 265, 5267–5272. [Google Scholar] [PubMed]
- Wu, K.; Kuang, J.; Huang, N.; Sheng, L.; Li, J.; Li, R.; Gong, L.; Lu, Q.; Liu, R.; Sun, R. Shouhui Tongbian Capsule ameliorates obesity by enhancing energy consumption and promoting lipolysis via cAMP-PKA pathway. Phytomedicine 2025, 138, 156375. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Shawlot, W.; Homanics, G.E.; Osterndorff-Kahanek, E.A.; Mason, S.; Mayfield, J.; Smalley, J.L.; Moss, S.J.; Messing, R.O. The PDE4 inhibitor apremilast modulates ethanol responses in Gabrb1-S409A knock-in mice via PKA-dependent and independent mechanisms. Neuropharmacology 2024, 257, 110035. [Google Scholar] [CrossRef]
- Cortier, M.; Boina-Ali, R.; Racoeur, C.; Paul, C.; Solary, E.; Jeannin, J.F.; Bettaieb, A. H89 enhances the sensitivity of cancer cells to glyceryl trinitrate through a purinergic receptor-dependent pathway. Oncotarget 2015, 6, 6877–6886. [Google Scholar] [CrossRef]
- Inoue, H.; Hase, K.; Segawa, A.; Takita, T. H89 (N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulphonamide) induces autophagy independently of protein kinase A inhibition. Eur. J. Pharmacol. 2013, 714, 170–177. [Google Scholar] [CrossRef]
- Ghione, S.; Racoeur, C.; Mabrouk, N.; Shan, J.; Groetz, E.; Ballot, E.; Truntzer, C.; Chouchane, L.; Végran, F.; Paul, C.; et al. Protein Kinase Inhibitor-Mediated Immunoprophylactic and Immunotherapeutic Control of Colon Cancer. Front. Immunol. 2022, 13, 875764. [Google Scholar] [CrossRef]
- Li, Y.C.; Xiong, Y.M.; Long, Z.P.; Huang, Y.P.; Shu, Y.B.; He, K.; Sun, H.Y.; Shi, Z. ML210 Antagonizes ABCB1- Not ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Biomedicines 2025, 13, 1245. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559–84571. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updat. 2020, 50, 100682. [Google Scholar] [CrossRef]
- Huo, Q.; Yuan, J.; Zhu, T.; Li, Z.; Xie, N. A Combined Bioinformatic and Nanoparticle-Based Study Reveal the Role of ABCG2 in the Drug Resistant Breast Cancer. Recent Pat. Anticancer Drug Discov. 2021, 16, 393–406. [Google Scholar] [CrossRef]
- Yu, X.; Shang, Q.; Yang, M.; Wang, J.; Zhang, W.; Wang, X.; Yao, Y.; Zhuang, J.; Sun, C. ABC Transporter Protein-mediated Multidrug Resistance in Breast Cancer and Development of Targeting Strategies. Recent Pat. Anticancer Drug Discov. 2025. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Cox, J.; Weinman, S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat. Oncol. 2016, 3, 57–59. [Google Scholar] [CrossRef]
- Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Cornelison, R.; Llaneza, D.C.; Landen, C.N. Emerging Therapeutics to Overcome Chemoresistance in Epithelial Ovarian Cancer: A Mini-Review. Int. J. Mol. Sci. 2017, 18, 2171. [Google Scholar] [CrossRef]
- Lochner, A.; Moolman, J.A. The many faces of H89: A review. Cardiovasc. Drug Rev. 2006, 24, 261–274. [Google Scholar] [CrossRef]
- Jiang, Y.; Puliyappadamba, V.T.; Zhang, L.; Wu, W.; Wali, A.; Yaffe, M.B.; Fontana, J.A.; Rishi, A.K. A novel mechanism of cell growth regulation by Cell Cycle and Apoptosis Regulatory Protein (CARP)-1. J. Mol. Signal 2010, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, T.; Chen, Y.; Li, Y.; Li, W. Combination therapy with protein kinase inhibitor H89 and Tetrandrine elicits enhanced synergistic antitumor efficacy. J. Exp. Clin. Cancer Res. 2018, 37, 114. [Google Scholar] [CrossRef]
- Farrow, B.; Rychahou, P.; Murillo, C.; O’Connor, K.L.; Iwamura, T.; Evers, B.M. Inhibition of pancreatic cancer cell growth and induction of apoptosis with novel therapies directed against protein kinase A. Surgery 2003, 134, 197–205. [Google Scholar] [CrossRef]
- Liu, X.; Müller, F.; Wayne, A.S.; Pastan, I. Protein Kinase Inhibitor H89 Enhances the Activity of Pseudomonas Exotoxin A-Based Immunotoxins. Mol. Cancer Ther. 2016, 15, 1053–1062. [Google Scholar] [CrossRef]
- Budworth, J.; Davies, R.; Malkhandi, J.; Gant, T.W.; Ferry, D.R.; Gescher, A. Comparison of staurosporine and four analogues: Their effects on growth, rhodamine 123 retention and binding to P-glycoprotein in multidrug-resistant MCF-7/Adr cells. Br. J. Cancer 1996, 73, 1063–1068. [Google Scholar] [CrossRef]
- Beltran, P.J.; Fan, D.; Fidler, I.J.; O’Brian, C.A. Chemosensitization of cancer cells by the staurosporine derivative CGP 41251 in association with decreased P-glycoprotein phosphorylation. Biochem. Pharmacol. 1997, 53, 245–247. [Google Scholar] [CrossRef]
- Ganeshaguru, K.; Wickremasinghe, R.G.; Jones, D.T.; Gordon, M.; Hart, S.M.; Virchis, A.E.; Prentice, H.G.; Hoffbrand, A.V.; Man, A.; Champain, K.; et al. Actions of the selective protein kinase C inhibitor PKC412 on B-chronic lymphocytic leukemia cells in vitro. Haematologica 2002, 87, 167–176. [Google Scholar] [PubMed]
- Zhang, W.J.; Li, Y.; Wei, M.N.; Chen, Y.; Qiu, J.G.; Jiang, Q.W.; Yang, Y.; Zheng, D.W.; Qin, W.M.; Huang, J.R.; et al. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017, 386, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhang, Y.K.; Zhang, G.N.; Al Rihani, S.B.; Wei, M.N.; Gupta, P.; Zhang, X.Y.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study. Cancer Lett. 2017, 396, 145–154. [Google Scholar] [CrossRef] [PubMed]
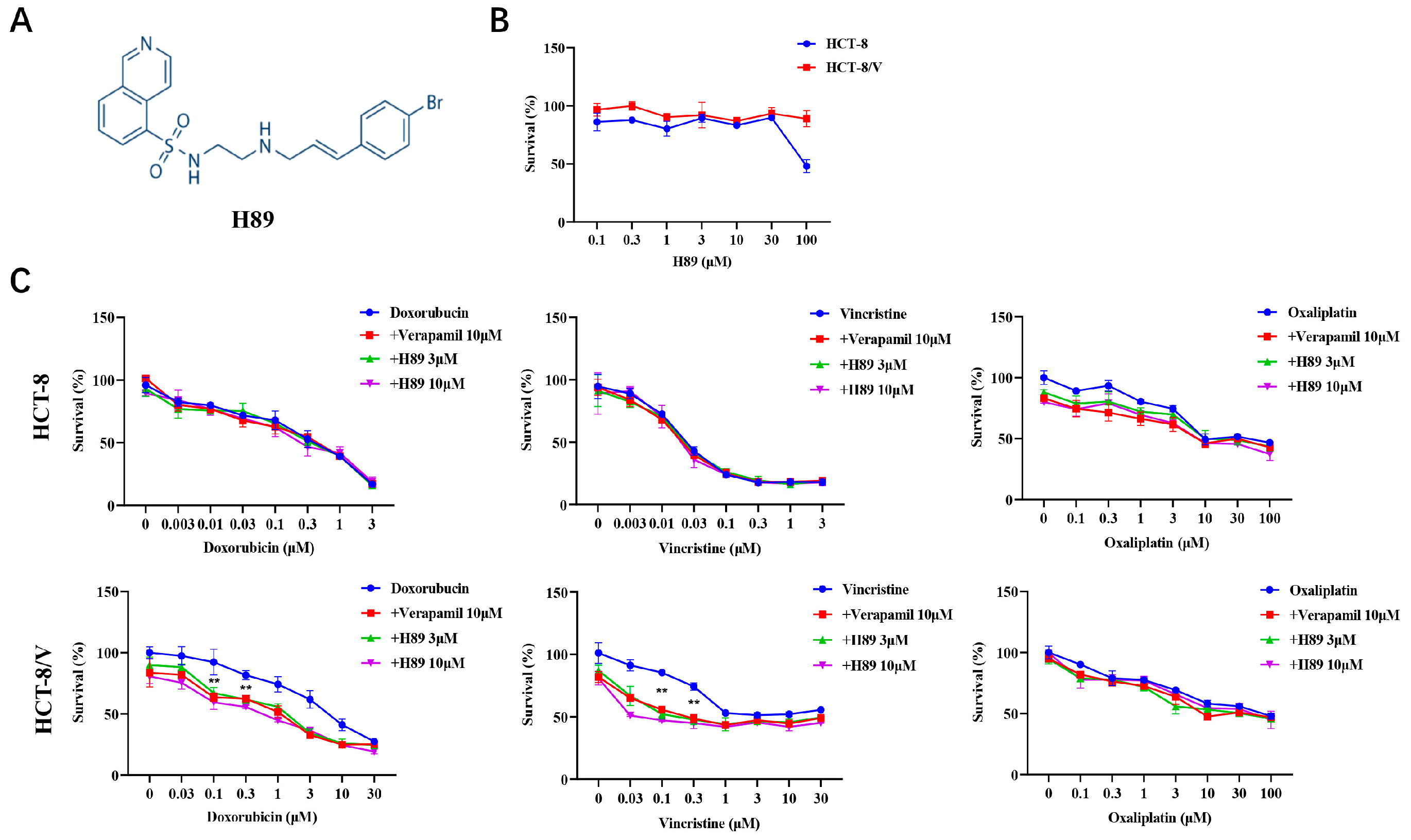
| Compounds | IC50 (μM) ± SD (FoldResistance) | |
|---|---|---|
| HCT-8 | HCT-8/V | |
| Doxorubicin | 0.350 ± 0.081 (1.0) | 6.978 ± 0.444 (19.94) |
| +Verapamil 10 μM | 0.374 ± 0.002 (1.06) | 0.934 ± 0.026 (2.67) ** |
| +H89 3 μM | 0.372 ± 0.160 (1.06) | 1.586 ± 0.177 (4.53) ** |
| +H89 10 μM | 0.369 ± 0.183 (1.05) | 0.648 ± 0.012 (1.85) ** |
| Vincristine | 0.025 ± 0.002 (1.0) | >30 (1200.0) |
| +Verapamil 10 μM | 0.023 ± 0.001 (0.92) | 0.238 ± 0.022 (9.52) ** |
| +H89 3 μM | 0.024 ± 0.001 (0.96) | 0.140 ± 0.055 (5.60) ** |
| +H89 10 μM | 0.022 ± 0.001 (0.88) | 0.047 ± 0.007 (1.88) ** |
| Oxaliplatin | 9.303 ± 0.549 (1.0) | 10.101 ± 0.090 (1.08) |
| +Verapamil 10 μM | 8.730 ± 0.441 (0.93) | 7.892 ± 0.149 (0.84) |
| +H89 3 μM | 8.924 ± 0.797 (0.96) | 8.288 ± 0.092 (0.89) |
| +H89 10 μM | 8.478 ± 0.475 (0.91) | 7.351 ± 0.186 (0.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-J.; Shan, Y.-Y.; Wang, H.; Xiong, Y.-M.; Shi, L.-Y.; Song, X.-P.; Li, M.; He, K.; Huang, J.; Shi, Z. H89 Reverses Multidrug Resistance in Colorectal Cancer by Inhibiting the ATPase Activity of ABCB1. Biomedicines 2025, 13, 2869. https://doi.org/10.3390/biomedicines13122869
Liu W-J, Shan Y-Y, Wang H, Xiong Y-M, Shi L-Y, Song X-P, Li M, He K, Huang J, Shi Z. H89 Reverses Multidrug Resistance in Colorectal Cancer by Inhibiting the ATPase Activity of ABCB1. Biomedicines. 2025; 13(12):2869. https://doi.org/10.3390/biomedicines13122869
Chicago/Turabian StyleLiu, Wei-Jing, Yi-Yao Shan, Huan Wang, Yu-Meng Xiong, Le-Yao Shi, Xiao-Peng Song, Min Li, Ke He, Jia Huang, and Zhi Shi. 2025. "H89 Reverses Multidrug Resistance in Colorectal Cancer by Inhibiting the ATPase Activity of ABCB1" Biomedicines 13, no. 12: 2869. https://doi.org/10.3390/biomedicines13122869
APA StyleLiu, W.-J., Shan, Y.-Y., Wang, H., Xiong, Y.-M., Shi, L.-Y., Song, X.-P., Li, M., He, K., Huang, J., & Shi, Z. (2025). H89 Reverses Multidrug Resistance in Colorectal Cancer by Inhibiting the ATPase Activity of ABCB1. Biomedicines, 13(12), 2869. https://doi.org/10.3390/biomedicines13122869







