1. Introduction
Hospitalized adults with lower urinary tract infection (UTI) represent a clinically diverse population in whom outcomes vary with host factors, device exposure, and local resistance patterns. Catheter-associated UTI (CAUTI) contributes substantially to hospital-acquired infection (HAI) burden and prolonged recovery, while community-onset cases are increasingly complicated by prior antibiotic exposure and comorbidities [
1,
2]. Pragmatic, early indicators that flag patients unlikely to respond to initial therapy are needed to direct stewardship and source-control decisions in real time.
Across Europe, antimicrobial resistance (AMR) among
Enterobacterales remains high, with
E. coli and
Klebsiella pneumoniae driving the majority of bloodstream infections involving resistance; more than half of invasive
E. coli isolates in 2023 were resistant to ≥1 key antimicrobial group, and combined resistance remains frequent [
3]. Beyond bloodstream infections, recent reviews highlight high and rising resistance rates in uropathogenic
K. pneumoniae, including the contribution of siderophore-mediated virulence and resistance mechanisms. Likewise, contemporary syntheses of antimicrobial resistance in
E. coli underscore widespread resistance to key oral agents and the increasing prevalence of extended-spectrum β-lactamase (ESBL)-producing strains, particularly in urinary isolates [
3,
4,
5]. Globally,
E. coli and
K. pneumoniae remain the predominant causes of both community- and hospital-acquired UTI, as illustrated by laboratory-based surveillance from Lebanon and other regions reporting these species as the most frequent uropathogens and key drivers of resistance profiles [
6,
7]. Similar resistance patterns have been reported in surveillance studies from the Middle East, Africa, and Asia, where urinary
E. coli and
Klebsiella spp. frequently exhibit high rates of resistance to fluoroquinolones and third-generation cephalosporins, underscoring the global relevance of timely active therapy and structured early response assessment [
6,
7,
8,
9,
10].
In Western Romania, recent cohort work delineated differences between CAUTI and non-catheter UTIs and highlighted the interplay among pathogen profiles, inflammatory response, and hospital trajectory. That analysis showed longer stays and more resistant flora in catheterized patients, motivating a shift from static admission variables to dynamic, early response assessment during hospitalization [
4].
Contemporary stewardship frameworks explicitly promote a 48–72 h “antibiotic time-out” to reassess diagnosis, cultures, route, and opportunities to de-escalate or discontinue therapy [
5]. Embedding such a checkpoint within a disease-specific early-response tool for UTI could harmonize bedside decision-making with stewardship goals and reduce unnecessary intravenous (IV) days [
5].
Biomarker trajectories—particularly C-reactive protein (CRP) decline—capture treatment–pathogen–host interactions more completely than single time-point values. In acute pyelonephritis, failure to defervesce by 72 h and persistent systemic signs have been associated with early clinical failure [
6], while steeper early CRP decreases correlate with favorable response [
7,
8,
9]. Such kinetics, together with symptom improvement, can be operationalized into an early-response composite suitable for routine wards [
6,
7,
8,
9]. Beyond CRP, several other early markers have been proposed to evaluate response in complicated UTI and CAUTI, including time to defervescence, resolution of lower urinary symptoms, and organ dysfunction measures, such as the Sequential Organ Failure Assessment (SOFA) score in septic presentations. Procalcitonin and other inflammatory biomarkers have also been explored as adjuncts to guide the duration of therapy. However, many of these indices require intensive monitoring, are not routinely available on general wards, or lack standardized thresholds in lower UTI [
6,
7,
8,
9].
Time-to-effective therapy is a second, modifiable driver of outcome. Delays in active treatment for infections caused by resistant Gram-negatives—particularly carbapenemase-producing strains—have been linked to increased mortality and prolonged hospitalization, reinforcing the need for rapid reassessment when initial empiric coverage is inactive [
6,
10].
Concurrently, source control for CAUTI—prompt catheter removal or exchange—is fundamental but inconsistently executed within the first 48 h. Long-standing guidelines and implementation studies show that early removal via nurse-driven protocols reduces catheter days and lowers CAUTI rates, and large multicenter prevention programs emphasize catheter stewardship as a cornerstone of quality care [
1,
2,
11,
12].
Finally, early IV-to-oral switch after clinical stabilization is safe for many patients with Gram-negative bacteremia of urinary origin and is associated with shorter length of stay—supporting a standardized 72 h checkpoint that includes route optimization when an active oral option exists [
13,
14].
Therefore, the current study was designed to evaluate a 72 h composite—REACT-UTI (Response Evaluation After Catheter/antibiotic Timing for UTI)—that merges CRP clearance, defervescence, and symptom improvement. The objectives were to (1) validate REACT-UTI against 72 h non-response and LOS; (2) quantify independent effects of CAUTI, time-to-effective therapy, early catheter removal (≤48 h), and IV-to-oral switch (≤72 h) on early response; and (3) describe pathogen and resistance patterns in relation to early response. REACT-UTI is intended to provide a pragmatic, disease-specific 72 h checkpoint that couples a simple physiologic–inflammatory composite with clearly actionable stewardship levers in routine ward care, extending concepts from acute pyelonephritis and sepsis to hospitalized lower UTI.
2. Materials and Methods
2.1. Study Design and Setting
This prospective observational study enrolled consecutive adults admitted with lower UTI to Victor Babeș Hospital for Infectious Diseases, Timișoara, a tertiary referral center serving Western Romania. Enrollment spanned from December 2023 to August 2025. Clinical care followed hospital protocols; no interventions were mandated by the study. Participants were followed prospectively from hospital admission through the end of the index hospitalization (discharge or in-hospital death); no post-discharge follow-up was performed.
The ethics committee approved the protocol and waived individual consent, given the minimal risk and use of routinely collected data. Patient confidentiality was preserved through de-identification and secure data handling, consistent with national regulations and the Declaration of Helsinki.
2.2. PICO Statement
In a prospective, single-center cohort of adults (≥18 years) hospitalized with culture-confirmed lower urinary tract infection at Victor Babeș Hospital, Timișoara (n = 126), the exposures (I) of interest were modifiable, early care processes: time-to-effective antimicrobial therapy (measured in hours from admission), early catheter removal/exchange among catheterized patients (≤48 h), and early IV-to-oral antibiotic switch (≤72 h), with catheter-associated UTI (CAUTI) status treated as a contextual exposure. These were compared (C) with usual care counterparts—longer time-to-effective therapy, no early catheter removal, no early IV-to-oral switch, and non-catheter UTI—to estimate their association with early recovery. The primary outcome (O) was 72 h early clinical response defined by the REACT-UTI composite (CRP clearance ≥35%, temperature < 37.5 °C, and ≥2-point improvement on a 0–10 symptom scale), while secondary outcomes included hospital length of stay (days) and ICU transfer during the index admission; organism profiles and resistance (e.g., ESBL, fluoroquinolone resistance) were evaluated as explanatory endpoints. The time horizon (T) for the primary assessment was 72 h after treatment initiation, with secondary outcomes measured through discharge.
2.3. Population, Definitions, and Microbiology
Inclusion criteria were age ≥ 18 years; dysuria/urgency/frequency/suprapubic pain with no flank pain; positive urine culture (≥105 CFU/mL, or ≥104 CFU/mL in symptomatic patients) with ≤2 uropathogens; and antibiotic therapy initiated during admission. Exclusion criteria were pregnancy/postpartum ≤6 weeks; polymicrobial cultures >2 organisms; upper UTI/pyelonephritis; and concurrent non-urinary infection driving systemic signs.
CAUTI was defined as an indwelling urethral catheter in place for ≥48 h prior to symptom onset. Time-to-effective therapy (hours) measured from admission to the first dose of an antibiotic with in vitro activity against the index isolate. Early catheter removal denoted removal or exchange ≤48 h after admission among catheterized patients. Early IV-to-oral switch was any transition to a bioavailable oral agent ≤72 h, guided by hemodynamic stability and oral tolerance.
Urine specimens were collected aseptically and processed according to internal standard operating procedures. Quantitative cultures were performed on routine urinary media with incubation at 35–37 °C for 18–24 h, and colony counts were interpreted using conventional thresholds (≥105 colony-forming units [CFUs]/mL, or ≥104 CFUs/mL in symptomatic patients). Species identification relied on conventional biochemical tests and/or an automated identification system, and antimicrobial susceptibility testing was performed by disk diffusion and/or broth microdilution, interpreted according to contemporary Clinical and Laboratory Standards Institute (CLSI) breakpoints. The ESBL phenotype among Enterobacterales was assessed using the CLSI-recommended screening and confirmatory combination-disk approach, and suspected carbapenemase producers underwent additional phenotypic testing in line with national algorithms.
Because several individual uropathogen species were infrequent, resistance analyses were prespecified at the Enterobacterales level (ESBL, fluoroquinolone resistance) rather than by individual species to preserve statistical power.
2.4. Outcomes and the 72 h REACT-UTI Composite
The primary outcome was 72 h early clinical response (ECR) versus non-response (ECR−). REACT-UTI is an a priori composite developed by the authors, informed by prior data on CRP kinetics and 72 h clinical response in acute pyelonephritis and sepsis cohorts [
6,
7,
8,
9,
15,
16]. ECR (responders) required all three at 72 h: (i) CRP clearance ≥35% from baseline, (ii) temperature < 37.5 °C, and (iii) ≥2-point improvement on a 0–10 UTI symptom scale (dysuria/urgency composite). This study represents the first prospective validation of REACT-UTI in hospitalized adults with lower UTI.
The CRP clearance threshold of ≥35% over 72 h was chosen a priori based on prior reports suggesting that approximately 30–40% of early CRP reductions distinguish favorable from unfavorable trajectories in acute urinary and other bacterial infections [
6,
7,
8].
2.5. Sample Size Considerations
This was a pragmatic, single-center, prospective observational study. No formal a priori sample size calculation was performed; instead, we planned to enroll all consecutive eligible patients with lower UTI over the 21-month study period to ensure adequate precision around early response estimates. The resulting sample of 126 patients provided sufficient events for multivariable modeling with the prespecified covariates, as reflected by reasonably narrow confidence intervals around the main adjusted odds ratios. We acknowledge that the study was not powered to detect small differences in less frequent outcomes or rare resistance phenotypes.
2.6. Statistical Analysis
Normality was evaluated with the Shapiro–Wilk test. Continuous variables were summarized as mean ± SD (or median [IQR]) and compared by Welch’s t-test or the Mann–Whitney U test, as appropriate. Categorical variables used χ2 or Fisher’s exact tests. Correlations used Spearman’s ρ. A multivariable logistic regression modeled the odds of 72 h non-response, including clinically relevant covariates (CAUTI; time-to-effective therapy per 6 h; baseline CRP per 20 mg/L; diabetes; early catheter removal; early IV-to-oral switch). Model calibration used the Hosmer–Lemeshow test; performance used Nagelkerke R2. Two-sided α = 0.05. Analyses were performed in R 4.3.1.
3. Results
Overall, 126 adults with culture-confirmed lower UTI were enrolled. The mean age was 61.8 ± 14.1 years, and 67/126 (53.2%) were male. Diabetes was present in 36/126 (28.6%), and chronic kidney disease was present in 20/126 (15.9%). Just over half of infections were hospital-acquired (66/126, 52.4%), and 57/126 (45.2%) met the criteria for CAUTI. Baseline CRP averaged 83.9 ± 32.4 mg/L, and the mean length of stay for the cohort was 11.6 ± 3.7 days (
Table 1).
In
Table 2, catheter status delineated expected microbiologic patterns.
E. coli was less common with catheters (28.1%) than without (47.8%;
p = 0.024), reflecting biofilm ecology and prior antibiotic exposure that select for non-fermenters and more resistant
Enterobacterales among CAUTI. Although
P. aeruginosa showed a numerically higher frequency in CAUTI (14.0% vs. 5.8%), this difference did not reach significance (
p = 0.117), likely due to sample size.
K. pneumoniae and
Enterococcus spp. proportions were comparable across strata. Mixed/other isolates clustered slightly in CAUTI (19.3% vs. 14.5%,
p = 0.472), consistent with device-related polymicrobial colonization. Among
Enterococcus isolates, no vancomycin-resistant enterococci (VRE) were detected.
Table 3 links early non-response to resistance phenotype among
Enterobacterales. ESBL positivity was nearly twice as common in ECR− (44.7%) as ECR+ (22.2%;
p = 0.022), implying that initial empirical therapy in ECR− was more likely inactive or suboptimal before the susceptibility results arrived. Fluoroquinolone resistance followed the same pattern (47.4% vs. 25.9%;
p = 0.033). Carbapenem resistance was uncommon in both groups (≤10.5%;
p = 0.376).
Table 4 operationalizes REACT-UTI’s core premise: what changes by 72 h matters most. Responders achieved robust CRP clearance (46.3%) versus minimal decline in non-responders (12.7%;
p < 0.001), normalized temperature (36.9 °C vs. 37.7 °C;
p < 0.001), and greater symptom improvement (+3.6 vs. +1.1 points;
p < 0.001). These physiologic gains aligned with earlier active therapy (10.7 vs. 22.9 h;
p < 0.001), confirming that timeliness of correct coverage drives the early biological signal. Process metrics distinguished groups: early IV-to-oral step-down (≤72 h) occurred in 55.3% of ECR+ vs. 12.0% of ECR− (
p < 0.001), while early catheter removal among catheterized patients was 68.0% vs. 31.0% (
p = 0.005).
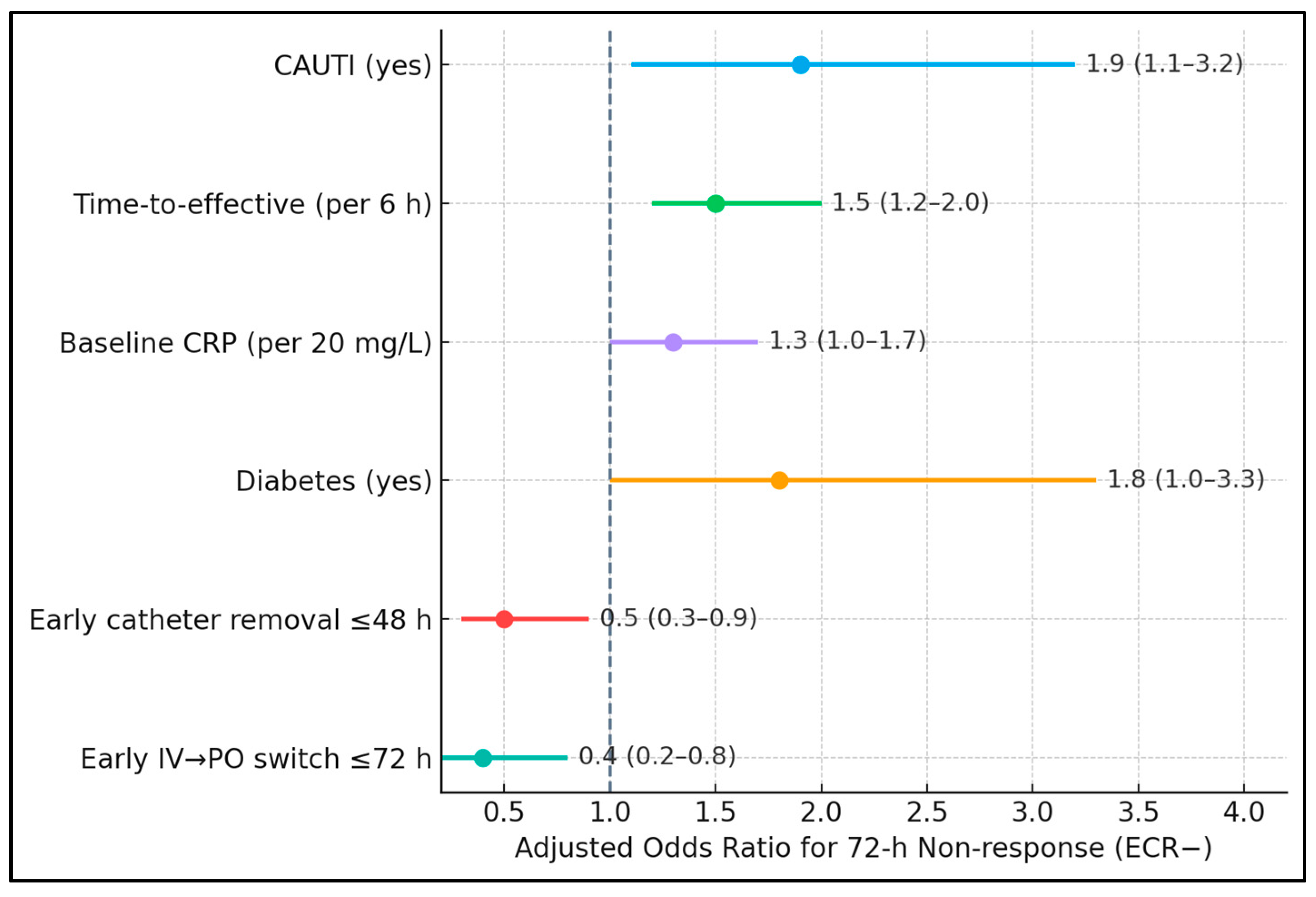
Table 5 confirms that both exposure and timing independently shape the 72 h trajectory. After adjustment, CAUTI nearly doubled the odds of non-response (aOR 1.9), while each 6 h delay in effective therapy raised the odds by 50% (aOR 1.5). Higher baseline CRP modestly increased risk (aOR 1.3 per 20 mg/L), consistent with larger inflammatory burdens requiring more time to resolve. Diabetes retained significance (aOR 1.8), plausibly via impaired neutrophil function and vascular compromise. Crucially, early catheter removal was protective (aOR 0.5), as was early IV-to-oral switch (aOR 0.4).
Non-response odds were higher with CAUTI (aOR 1.9, 95% CI 1.1–3.2), longer time-to-effective therapy per 6 h (aOR 1.5, 1.2–2.0), baseline CRP per 20 mg/L (aOR 1.3, 1.0–1.7), and diabetes (aOR 1.8, 1.0–3.3). Protective associations included early catheter removal ≤48 h (aOR 0.5, 0.3–0.9) and early IV → PO switch ≤72 h (aOR 0.4, 0.2–0.8). Visually, all risk factors plotted to the right of the null (OR = 1), while protective ones lay to the left, with non-overlapping confidence intervals for the most influential variables (time-to-effective therapy and early IV → PO), as presented in
Figure 1.
Table 6 quantifies relationships underpinning REACT-UTI. Greater CRP clearance strongly correlated with shorter LOS (ρ = −0.52;
p < 0.001), reinforcing the construct’s biological validity: faster dampening of systemic inflammation aligns with accelerated clinical recovery and discharge readiness. Conversely, longer time-to-effective therapy correlated with longer LOS (ρ = +0.41;
p < 0.001), capturing the downstream impact of initial regimen mismatches when resistant organisms are involved. Baseline CRP related modestly to LOS (ρ = +0.28;
p = 0.002), consistent with
Table 5, while age had a weaker association (ρ = +0.18;
p = 0.048), suggesting that what happens early in treatment (coverage timing and response) outweighs fixed demographics. Importantly, time-to-effective therapy correlated inversely with CRP clearance (ρ = −0.36;
p < 0.001).
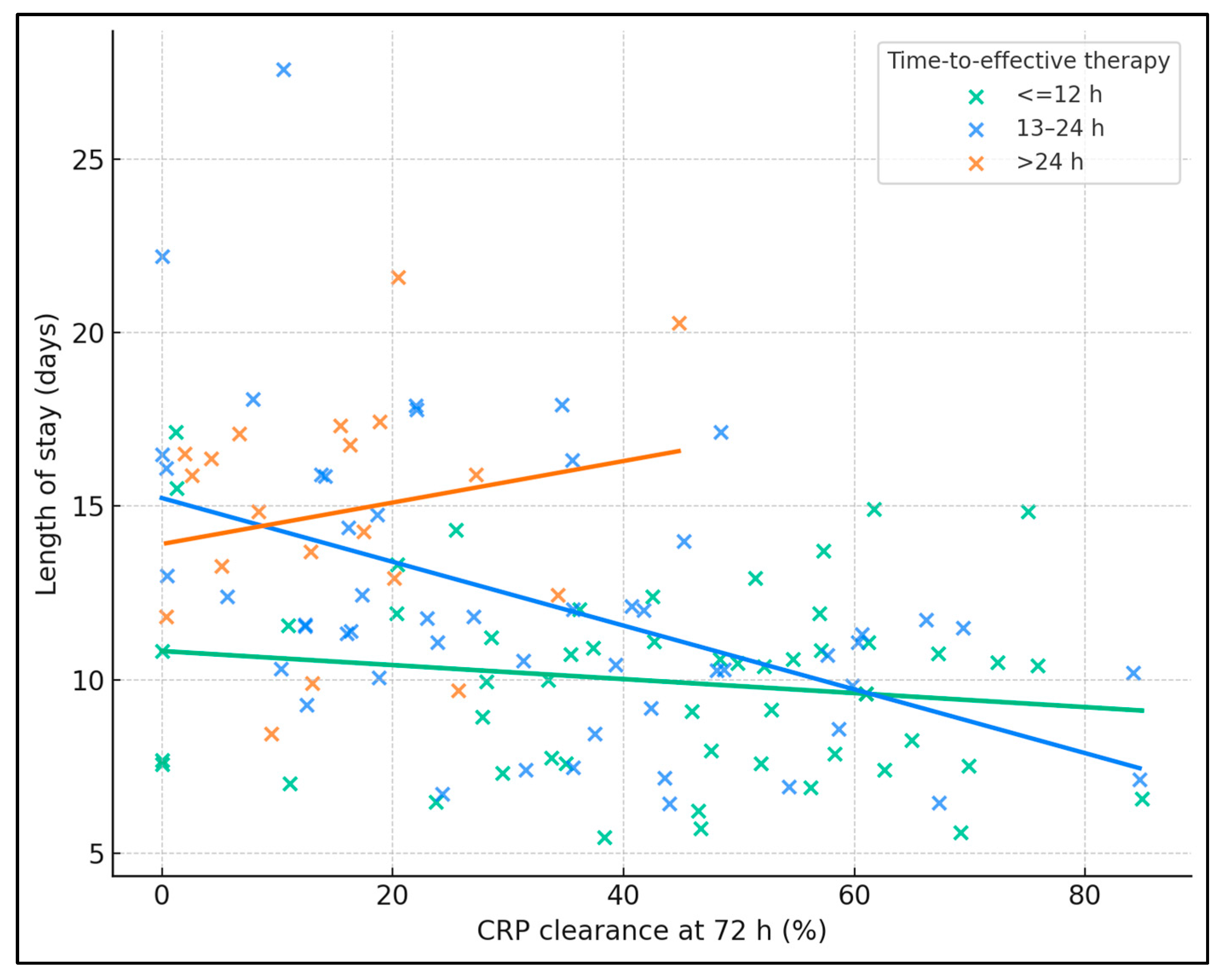
Across the cohort, higher 72 h CRP clearance correlated with shorter LOS (Spearman’s ρ −0.44,
p < 0.001). The 13–24 h stratum showed a clear inverse relationship (ρ −0.53,
p < 0.001), with regression slope indicating progressively shorter stays as clearance rose, while the ≤12 h group showed a flatter but still negative trend (ρ −0.11,
p = 0.44), consistent with already low LOS when therapy was timely. In contrast, the >24 h group displayed a weak, non-significant pattern (ρ 0.14,
p = 0.55) and higher LOS values overall, illustrating that delays blunt the benefit of biomarker improvement (
Figure 2).
Table 7 examines how delays to active therapy are mapped onto early response and length of stay. Patients who received an active agent within 12 h had an ECR+ rate of 84.6% and a mean LOS of 10.2 days, whereas those who started after 24 h had an ECR+ rate of 25.0% and stayed 13.8 days on average. The graded pattern was statistically robust: ECR proportions differed markedly across strata (χ
2 p < 0.001), and LOS increased stepwise (Kruskal–Wallis
p = 0.007). Pairwise testing confirmed that ≤12 h and >24 h strata diverged significantly in LOS (
p = 0.0069), while intermediate delays (13–24 h) showed numerically longer stays versus ≤12 h but without multiplicity-adjusted significance.
Within the catheterized subset, early device management was strongly associated with better 72 h outcomes. Two-thirds (67.9%) of patients who had removal/exchange within 48 h achieved ECR+, compared with 31.0% without early source control. The effect was statistically significant (Fisher
p = 0.008) and clinically meaningful (RR 2.19; 95% CI 1.20–3.98), indicating that timely catheter action nearly doubled the chance of early response, as presented in
Table 8.
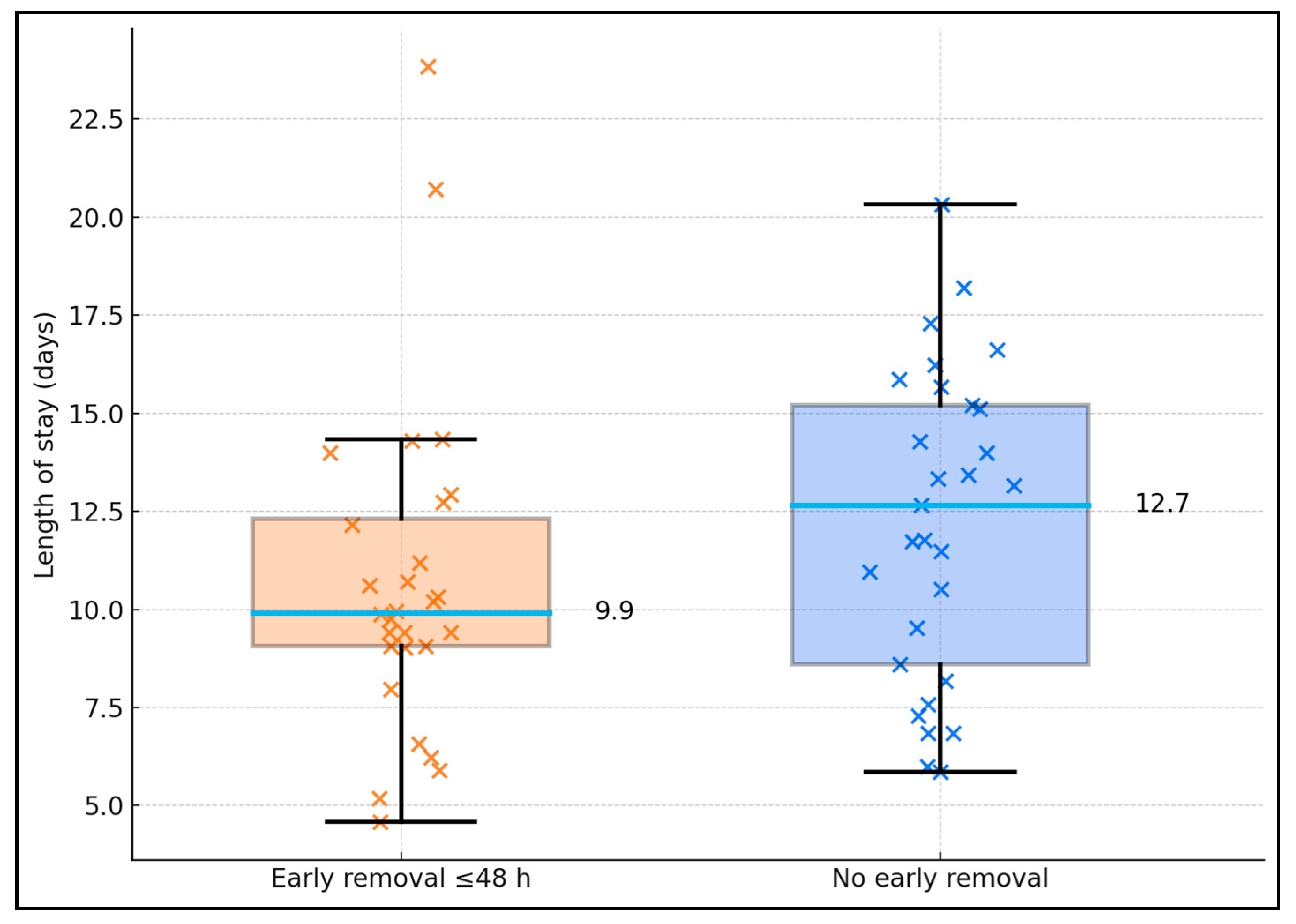
Among CAUTI (n = 57), early catheter removal was associated with shorter stays. LOS averaged 9.9 ± 3.8 days with early removal (n = 28) vs. 12.7 ± 3.1 days without (n = 29); Mann–Whitney
p = 0.0038. Distribution spread demonstrating fewer long-stay outliers when the catheter was removed/exchanged by 48 h—aligning with the CAUTI aOR 0.5 (non-response) in the adjusted model (
Figure 3).