Long COVID Prevalence and Risk Factors: A Systematic Review and Meta-Analysis of Prospective Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Research Framework and Eligibility Criteria (PICO)
2.3. Inclusion and Exclusion Criteria
2.4. Information Sources and Search Strategy
2.5. Study Selection Process
2.6. Data Extraction and Management
2.7. Risk of Bias and Quality Assessment
2.8. Statistical Analysis
2.8.1. Heterogeneity Assessment
2.8.2. Subgroup and Sensitivity Analyses
2.8.3. Risk Factor Meta-Analysis
2.8.4. Publication Bias and Small-Study Effects
2.8.5. Significance Threshold
3. Results
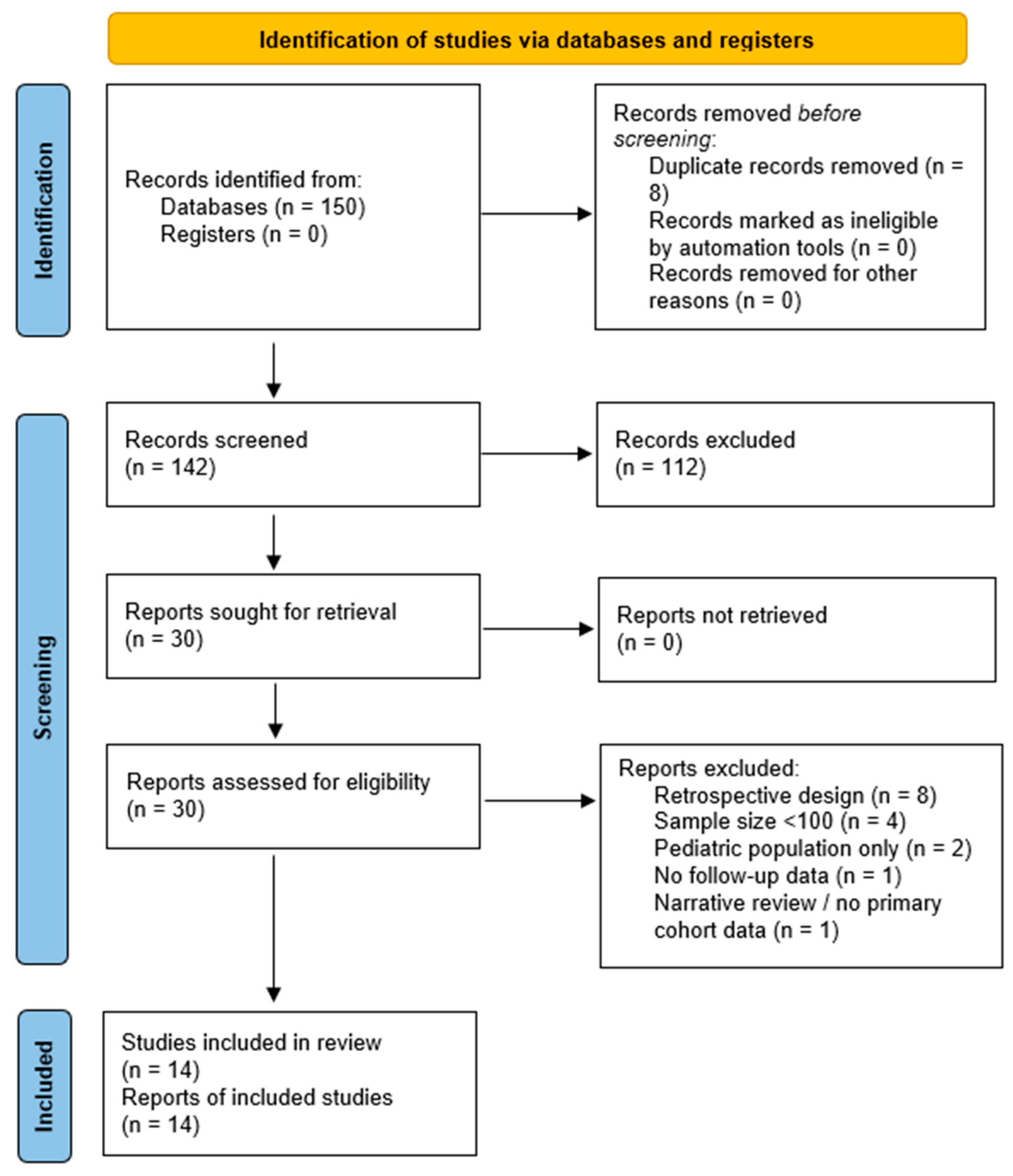
3.1. Study Selection
3.2. Study Characteristics
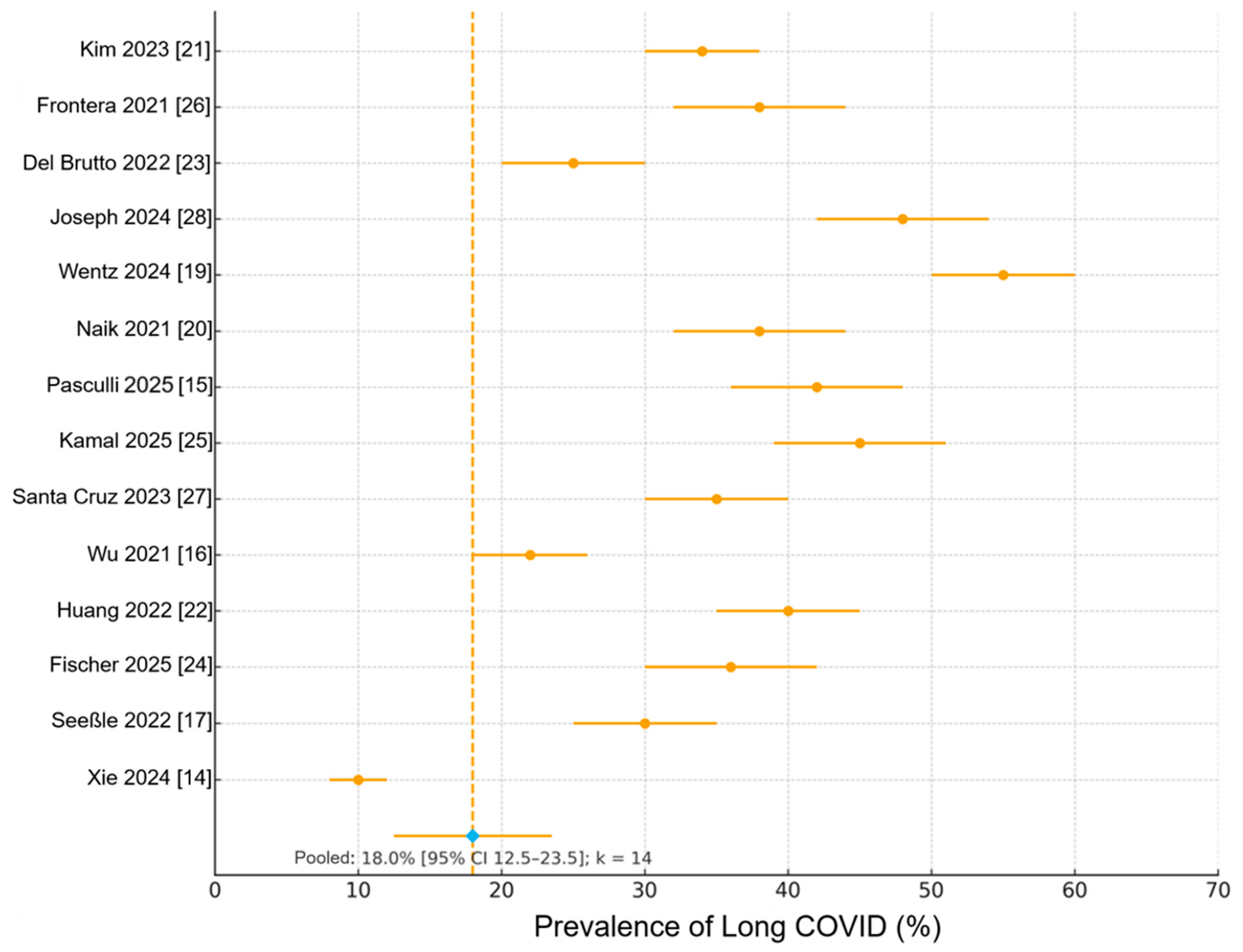
3.3. Pooled Prevalence of Long COVID and Core Symptoms
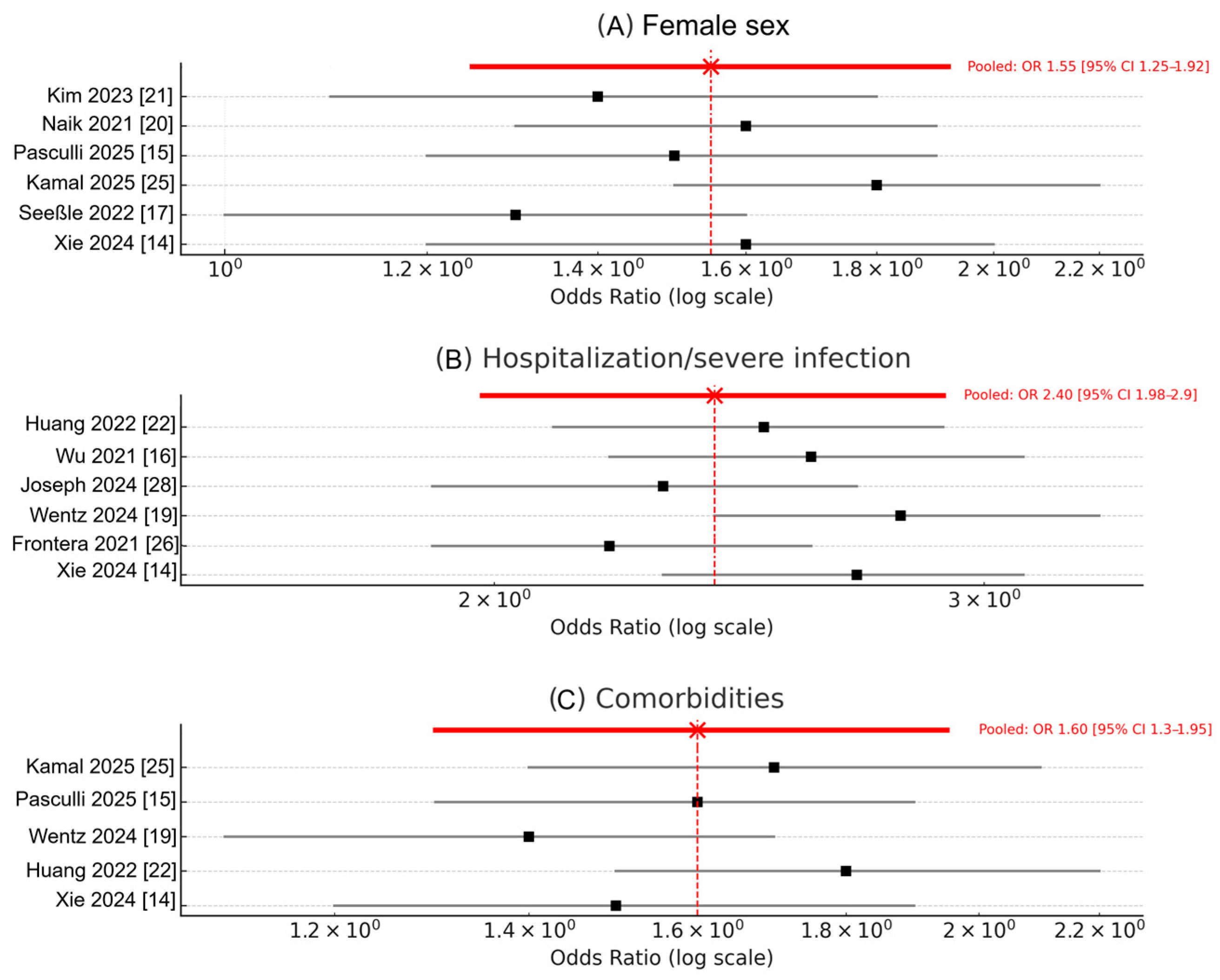
3.4. Risk Factors for Long COVID
3.5. Heterogeneity, Sensitivity, and Publication Bias
3.6. Summary of Findings
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Literature
4.3. Heterogeneity and Quality Considerations
4.4. Novelty and Contribution Beyond Existing Literature
4.5. Clinical and Research Implications
4.6. Strengths and Limitations
4.7. Overall Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| PASC | Post-Acute Sequelae of COVID-19 (Long COVID) |
| WHO | World Health Organization |
| OR | Odds Ratio |
| CI | Confidence Interval |
| NOS | Newcastle–Ottawa Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| QoL | Quality of Life |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| CD8+ | Cluster of Differentiation 8 Positive T Lymphocytes |
| PEM | Post-Exertional Malaise |
| REML | Restricted Maximum Likelihood |
| IPD | Individual Participant Data |
| BMJ | British Medical Journal |
| SD | Standard Deviation |
| SE | Standard Error |
References
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 30 September 2025).
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological FactorsThat May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological Dysfunction Persists for 8 Months Following Initial Mild-to-Moderate SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of Long Covid Symptoms After COVID-19 Vaccination: Community Based Cohort Study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Postacute Sequelae of SARS-CoV-2 Infection in the Pre-Delta, Delta, and Omicron Eras. N. Engl. J. Med. 2024, 391, 515–525. [Google Scholar] [CrossRef]
- Pasculli, P.; Zingaropoli, M.A.; Dominelli, F.; Solimini, A.G.; Masci, G.M.; Birtolo, L.I.; Pasquariello, L.; Paribeni, F.; Iafrate, F.; Panebianco, V.; et al. Insights into Long COVID: Unraveling Risk Factors, Clinical Features, Radiological Findings, Functional Sequelae and Correlations: A Retrospective Cohort Study. Am. J. Med. 2025, 138, 721–731. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. 2013. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 September 2025).
- Wentz, E.; Ni, Z.; Yenokyan, K.; Vergara, C.; Pahwa, J.; Kammerling, T.; Xiao, P.; Duggal, P.; Lau, B.; Mehta, S.H. Cohort profile: The Johns Hopkins COVID Long Study (JHCLS)—A US nationwide prospective cohort study. BMJ Open 2024, 14, e077742. [Google Scholar] [CrossRef]
- Naik, S.; Haldar, S.N.; Soneja, M.; Mundadan, N.G.; Garg, P.; Mittal, A.; Desai, D.; Trilangi, P.K.; Chakraborty, S.; Begam, N.N.; et al. Post COVID-19 sequelae: A prospective observational study from Northern India. Drug Discov. Ther. 2021, 15, 254–260. [Google Scholar] [CrossRef]
- Kim, Y.; Bae, S.; Chang, H.H.; Kim, S.W. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci. Rep. 2023, 13, 11207. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Rumbea, D.A.; Recalde, B.Y.; Mera, R.M. Cognitive sequelae of long COVID may not be permanent: A prospective study. Eur. J. Neurol. 2022, 29, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Zhang, L.; Elbéji, A.; Wilmes, P.; Snoeck, C.J.; Larché, J.; Oustric, P.; Ollert, M.; Fagherazzi, G. Trajectories of persisting COVID-19 symptoms up to 24 months after acute infection: Findings from the Predi-Covid cohort study. BMC Infect. Dis. 2025, 25, 603. [Google Scholar] [CrossRef]
- Kamal, S.M.; Al Qahtani, M.S.; Al Aseeri, A.; Naghib, M.E.M.; Al Mazroua, A.M.M.; Alshamrani, A.M.M.; Al Mazroua, M.M.; AlHarbi, F.S. Long COVID-19: A Four-Year prospective cohort study of risk factors, recovery, and quality of life. BMC Infect. Dis. 2025, 25, 1082. [Google Scholar] [CrossRef]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef]
- Santa Cruz, A.; Mendes-Frias, A.; Azarias-da-Silva, M.; André, S.; Oliveira, A.I.; Pires, O.; Mendes, M.; Oliveira, B.; Braga, M.; Lopes, J.R.; et al. Post-acute sequelae of COVID-19 is characterized by diminished peripheral CD8+β7 integrin+ T cells and anti-SARS-CoV-2 IgA response. Nat. Commun. 2023, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.; Margalit, I.; Weiss-Ottolenghi, Y.; Rubin, C.; Murad, H.; Gardner, R.C.; Barda, N.; Ben-Shachar, E.; Indenbaum, V.; Gilboa, M.; et al. Persistence of Long COVID Symptoms Two Years After SARS-CoV-2 Infection: A Prospective Longitudinal Cohort Study. Viruses 2024, 16, 1955. [Google Scholar] [CrossRef] [PubMed]
| Nr. | First Author (Year) | Country/Setting | Study Design & Focus | N (Participants) | Follow-Up Duration | Key Reported Symptoms/Focus | Main Risk Factors Identified | NOS Score | Population Type: Hospitalized/Community/Mixed | Participation Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Naik S (2021) [20] | India | Prospective post-discharge cohort | 254 | 3–6 months | Myalgia (10.9%), fatigue (5.5%), shortness of breath (6.1%), cough (2.1%), insomnia (1.4%) | Hypoxia, hypothyroidism | 8 | Hospitalized (post-discharge cohort) | NR |
| 2 | Kim Y (2023) [21] | South Korea | Online longitudinal survey | 132 | 24 months | Fatigue (34.8%), amnesia (30.3%), concentration difficulties (24.2%), insomnia (20.5%), depression (19.7%) | Female sex | 7 | Community (online national survey) | 16.7% |
| 3 | Frontera JA (2021) [26] | USA | Prospective hospital cohort with neurologic evaluation | 382 | 6 months | Limited ADLs (56%), impaired cognition (50%), cannot return to work (47%), anxiety/depression, sleep disorders | Acute neurologic complications | 8 | Hospitalized (neurology-focused cohort) | 49.6% |
| 4 | Del Brutto OH (2022) [23] | Ecuador | Community-based prospective cognitive study | 78 | 3–6 months | Decreased MoCA scores; reversible cognitive deficits | Age, low education | 7 | Community (population-based cognitive study) | 100% |
| 5 | Joseph G (2024) [28] | Israel | Longitudinal 2-year cohort | 323 | 24 months | Fatigue (57%), PEM (46%), dyspnoea | Female gender, smoking, severity of acute COVID-19 | 9 | Mixed (hospitalized + community adults, national cohort) | 25.7% |
| 6 | Wentz E (2024) [19] | USA (Johns Hopkins) | National online cohort (JHCLS) | 16,764 | 24+ months | 63% Long COVID per WHO definition; fatigue, cognitive issues | Female sex, unvaccinated status | 9 | Community (national online registry, JHCLS) | NR |
| 7 | Pasculli P (2025) [15] | Italy | Retrospective cohort (included due to prospective follow-up and standardized post-acute assessments) | 364 | 6–12 months | Abnormal CT (20–30%), fatigue (50%) | Residual lung changes | 7 | Mixed (hospital and ambulatory participants) | NR |
| 8 | Kamal SM (2025) [25] | Saudi Arabia | 4-year prospective cohort | 816 | 48 months | Fatigue (57.1%), post-exertional malaise (45.8%), cough (41.2%), cognitive dysfunction (30.7%) | Diabetes, reinfection | 9 | Community (national follow-up registry) | 53.6% |
| 9 | Santa Cruz A (2023) [27] | Brazil | Prospective immunophenotypic cohort | 215 | 6 months | Immunological dysfunction (↑ IL-6/IL-8, ↓ CD8+ β7 integrin + T cells) | Severe acute infection | 8 | Hospitalized (post-acute immunophenotypic cohort) | NR |
| 10 | Wu X (2021) [16] | China (Wuhan) | Respiratory follow-up cohort | 83 | 12 months | Dyspnoea (24%), ↓ lung function | Disease severity | 8 | Hospitalized (Wuhan respiratory follow-up) | 89.2% |
| 11 | Huang L (2022) [22] | China (multicentric) | Longitudinal hospital cohort | 1192 | 24 months | Fatigue (52%), anxiety (26%), ↓ QoL | Hospitalization, comorbidities | 9 | Hospitalized (multicenter, China) | 75.2% |
| 12 | Fischer A (2025) [24] | Luxembourg | National Predi-COVID cohort | 555 | 24 months | Fatigue (30–40%), persistent symptoms | Female sex, obesity | 8 | Community (Predi-COVID national cohort, Luxembourg) | NR |
| 13 | Seeßle J (2022) [17] | Germany | University cohort, non-severe adults | 96 | 12 months | Persistent symptoms (40%), neurocognitive | Female sex | 7 | Community (university employees, non-severe infection) | 50.0% |
| 14 | Xie Y (2024) [14] | USA (Veterans Affairs) | Variant-based prospective cohort | 441,583 | 12–18 months | Multi-organ sequelae (OR >2.0) | Variant era, hospitalization | 9 | Mixed (Veterans Affairs cohort, hospitalized + outpatient) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halas, R.-G.; Berceanu Vaduva, D.M.; Radulescu, M.; Bredicean, A.-C.; Mateescu, D.-M.; Toma, A.-O.; Cotet, I.-G.; Guse, C.-E.; Marginean, A.; Margan, M.-M.; et al. Long COVID Prevalence and Risk Factors: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Biomedicines 2025, 13, 2859. https://doi.org/10.3390/biomedicines13122859
Halas R-G, Berceanu Vaduva DM, Radulescu M, Bredicean A-C, Mateescu D-M, Toma A-O, Cotet I-G, Guse C-E, Marginean A, Margan M-M, et al. Long COVID Prevalence and Risk Factors: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Biomedicines. 2025; 13(12):2859. https://doi.org/10.3390/biomedicines13122859
Chicago/Turabian StyleHalas, Ramona-Georgiana, Delia Mira Berceanu Vaduva, Matilda Radulescu, Ana-Cristina Bredicean, Diana-Maria Mateescu, Ana-Olivia Toma, Ioana-Georgiana Cotet, Cristina-Elena Guse, Andrei Marginean, Madalin-Marius Margan, and et al. 2025. "Long COVID Prevalence and Risk Factors: A Systematic Review and Meta-Analysis of Prospective Cohort Studies" Biomedicines 13, no. 12: 2859. https://doi.org/10.3390/biomedicines13122859
APA StyleHalas, R.-G., Berceanu Vaduva, D. M., Radulescu, M., Bredicean, A.-C., Mateescu, D.-M., Toma, A.-O., Cotet, I.-G., Guse, C.-E., Marginean, A., Margan, M.-M., & Lazureanu, V. E. (2025). Long COVID Prevalence and Risk Factors: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Biomedicines, 13(12), 2859. https://doi.org/10.3390/biomedicines13122859








