Significance of Initial Serum Phosphate Imbalance in Traumatic Brain Injury with and Without Concomitant Spinal Injuries: Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Electrolyte Imbalance
3.3. TBI Severity by GCS
3.4. Electrolyte–Phosphate Correlations
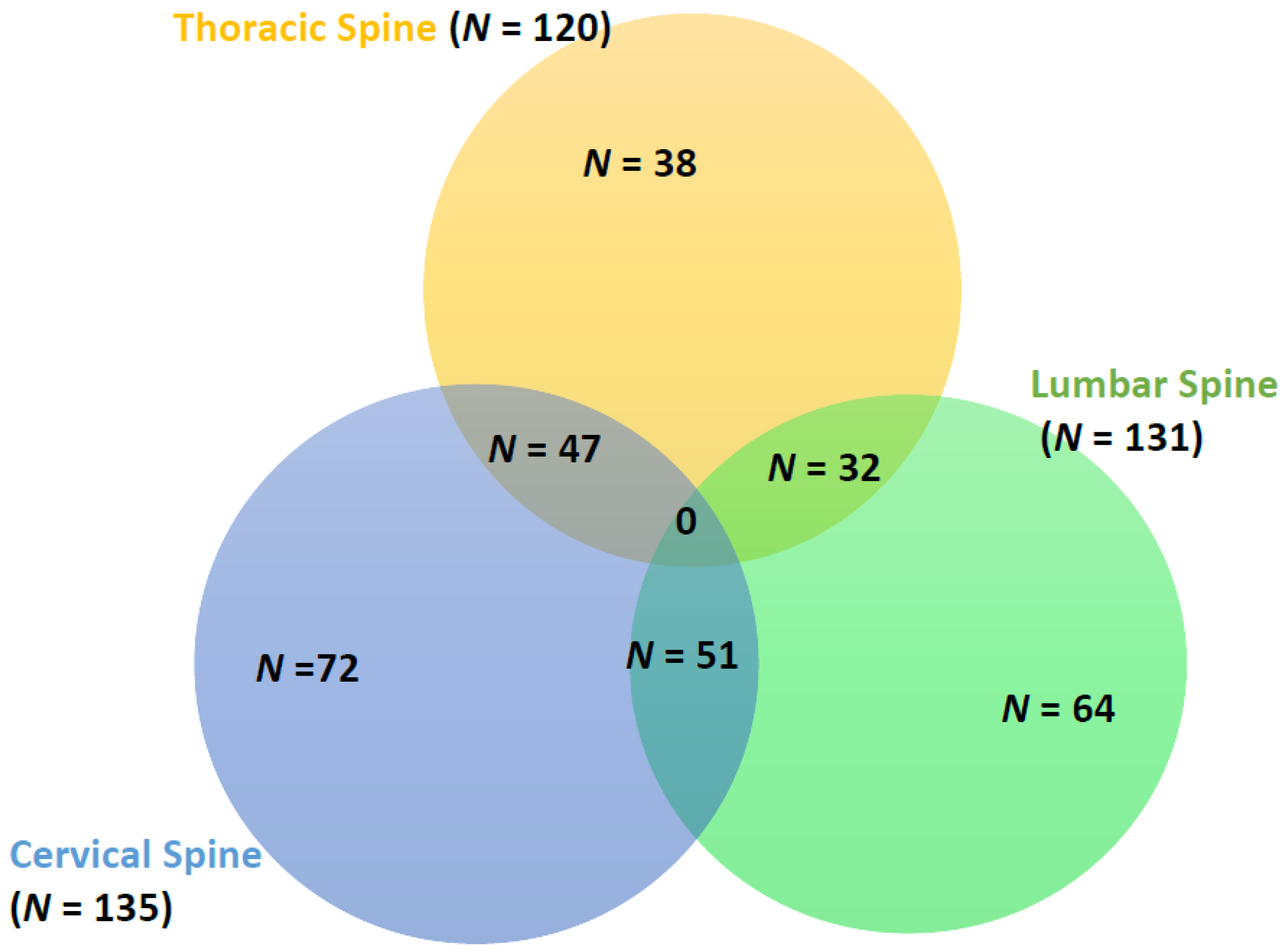
3.5. Neurological Deficits and Spinal Injury Levels
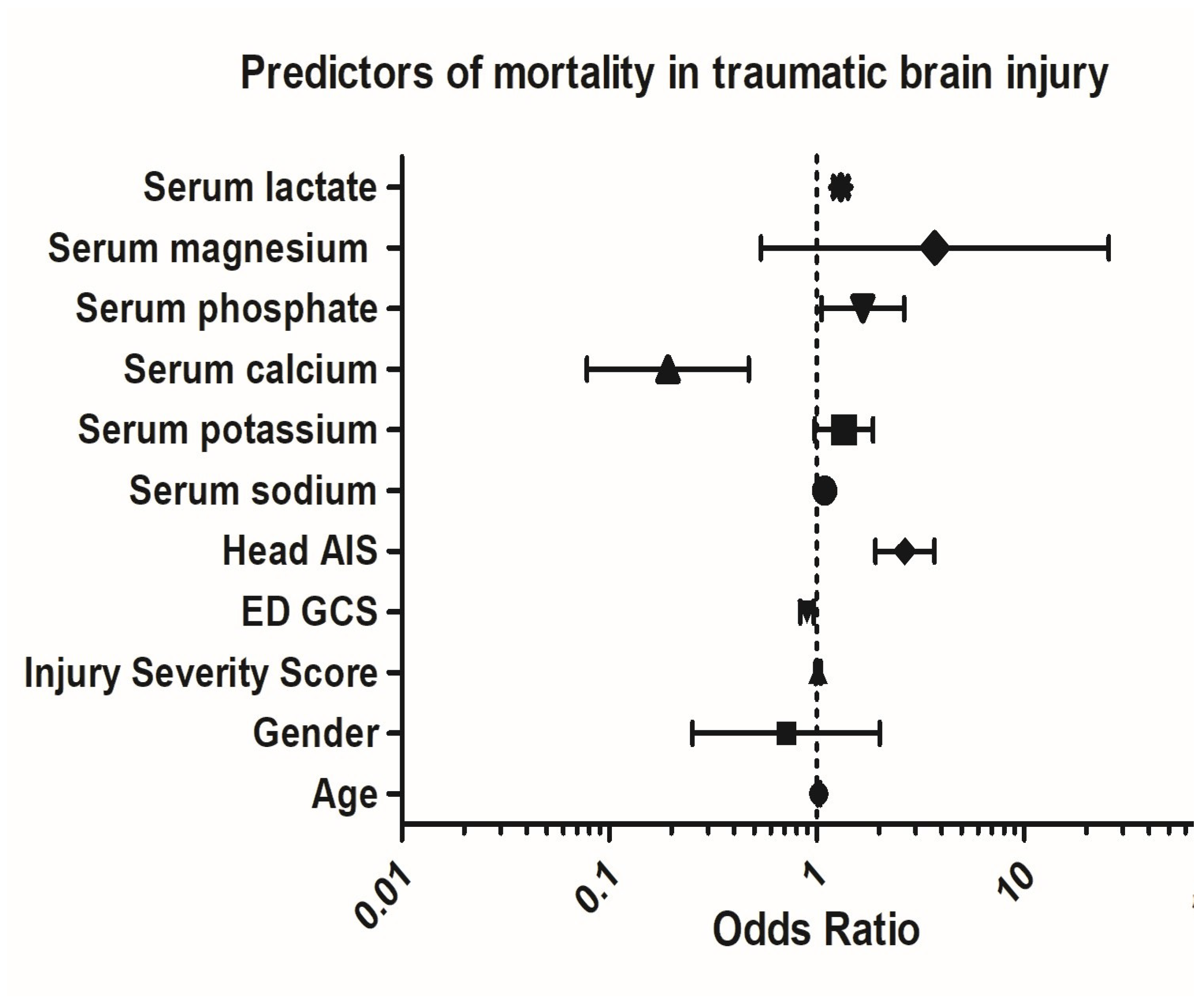
3.6. Mortality and Phosphate Association
3.7. Multivariable Regression Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hills, M.W.; Deane, S.A. Head injury and facial injury: Is there an increased risk of cervical spine injury? J. Trauma 1993, 34, 549–553; discussion 553–554. [Google Scholar] [CrossRef]
- Mulligan, R.P.; Friedman, J.A.; Mahabir, R.C. A nationwide review of the associations among cervical spine injuries, head injuries, and facial fractures. J. Trauma 2010, 68, 587–592. [Google Scholar] [CrossRef]
- Nazir, M.; Khan, S.A.; Raja, R.A.; Bhatti, S.N.; Ahmed, E. Cervical spinal injuries in moderate to severe head injuries. J. Ayub Med. Coll. Abbottabad 2012, 24, 100–102. [Google Scholar]
- O’Malley, K.F.; Ross, S.E. The incidence of injury to the cervical spine in patients with craniocerebral injury. J. Trauma 1988, 28, 1476–1478. [Google Scholar] [CrossRef]
- Piatt, J.H., Jr. Detected and overlooked cervical spine injury among comatose trauma patients: From the Pennsylvania Trauma Outcomes Study. Neurosurg. Focus 2005, 19, E6. [Google Scholar] [CrossRef]
- Fujii, T.; Faul, M.; Sasser, S. Risk factors for cervical spine injury among patients with traumatic brain injury. J. Emerg. Trauma Shock 2013, 6, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Pandrich, M.J.; Demetriades, A.K. Prevalence of concomitant traumatic cranio-spinal injury: A systematic review and meta-analysis. Neurosurg. Rev. 2020, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hlwatika, P.; Hardcastle, T.C. Concurrent cranial and cervical spine injuries by associated injury mechanisms in traumatic brain injury patients. SA J. Radiol. 2022, 26, 2321. [Google Scholar]
- Tian, H.L.; Guo, Y.; Hu, J.; Rong, B.Y.; Wang, G.; Gao, W.W.; Chen, S.W.; Chen, H. Clinical characterization of comatose patients with cervical spine injury and traumatic brain injury. J. Trauma 2009, 67, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Drainer, E.K.; Graham, C.A.; Munro, P.T. Blunt cervical spine injuries in Scotland 1995–2000. Injury 2003, 34, 330–333. [Google Scholar] [CrossRef]
- Anthony, D.C.; Couch, Y. The systemic response to CNS injury. Exp. Neurol. 2014, 258, 105–111. [Google Scholar] [CrossRef]
- Jain, A.; Gouda, B.; Gajjar, R.; Gupta, P.B. Correlation between the severity of head injury and electrolytes in patients with traumatic brain injury. IOSR-JDMS 2017, 16, 55–58. [Google Scholar]
- Gupta, S.K.; Ahuja, J.; Sharma, A. Electrolytes imbalance in traumatic brain injury patients. Int. J. Med. Sci. Ed. 2014, 1, 1. [Google Scholar]
- Adiga, U.S.; Vickneshwaran, V.; Sen, S.K. Electrolyte derangements in traumatic brain injury. Basic Res. J. Med. Clin. Sci. 2012, 12, 15–18. [Google Scholar]
- Dey, S.; Kumar, R.; Tarat, A. Evaluation of Electrolyte Imbalance in Patients with Traumatic Brain Injury Admitted in the Central ICU of a Tertiary Care Centre: A Prospective Observational Study. Cureus 2021, 13, e17517. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Kumar, N.; Singh, Y.; Kumar, V.; Yadav, G.; Gupta, B.K.; Pandey, A.R.; Pandey, S. Evaluation of serum electrolytes in traumatic brain injury patients: Prospective randomized observational study. J. Anesth. Crit. Care Open Access 2016, 5, 00184. [Google Scholar]
- Mekkodathil, A.; El-Menyar, A.; Hakim, S.; Al Jogol, H.; Parchani, A.; Peralta, R.; Rizoli, S.; Al-Thani, H. Initial Serum Levels of Magnesium and Calcium as Predictors of Mortality in Traumatic Brain Injury Patients: A Retrospective Study. Diagnostics 2023, 13, 1172. [Google Scholar] [CrossRef]
- Ko, H.Y. Electrolyte Disorders in Spinal Cord Injuries. In Management and Rehabilitation of Spinal Cord Injuries.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Kraft, M.D.; Btaiche, I.F.; Sacks, G.S. Review of the refeeding syndrome. Nutr. Clin. Pract. 2005, 20, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Sousse, L.E.; Irick, R.; Schryver, E.; Klein, G.L. Interactions of Phosphate Metabolism with Serious Injury, Including Burns. JBMR Plus 2017, 1, 59–65. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, W.J.; Lee, D.K.; Lee, K.J.; Choi, H.J. Association between the initial serum phosphate level and 30-day mortality in blunt trauma patients. J. Trauma Acute Care Surg. 2021, 91, 507–513. [Google Scholar] [CrossRef]
- Al-Thani, H.; El-Menyar, A.; Khan, N.A.; Consunji, R.; Mendez, G.; Abulkhair, T.S.; Mollazehi, M.; Peralta, R.; Abdelrahman, H.; Chughtai, T.; et al. Trauma Quality Improvement Program: A Retrospective Analysis from A Middle Eastern National Trauma Center. Healthcare 2023, 11, 2865. [Google Scholar] [CrossRef]
- Mekkodathil, A.; El-Menyar, A.; Kanbar, A.; Hakim, S.; Ahmed, K.; Siddiqui, T.; Al-Thani, H. Epidemiological and clinical characteristics of fall-related injuries: A retrospective study. BMC Public Health 2020, 20, 1186. [Google Scholar] [CrossRef]
- Im, C.; Jang, D.H.; Jung, W.J.; Park, S.M.; Lee, D.K. The Magnitude of Change in Serum Phosphate Concentration Is Associated with Mortality in Patients with Severe Trauma. Yonsei Med. J. 2024, 65, 181–188. [Google Scholar] [CrossRef]
- Polderman, K.H.; Bloemers, F.W.; Peerdeman, S.M.; Girbes, A.R. Hypomagnesemia and hypophosphatemia at admission in patients with severe head injury. Crit. Care Med. 2000, 28, 2022–2025. [Google Scholar] [CrossRef]
- Qiu, S.Z.; Zheng, G.R.; Chen, B.; Huang, J.J.; Shen, J.; Mao, W. Prognostic value of admission serum glucose-phosphate ratio in predicting the 6-month outcome of patients with severe traumatic brain injury: A retrospective study. Clin. Chim. Acta 2020, 510, 659–664. [Google Scholar] [CrossRef]
- Ngatuvai, M.; Martinez, B.; Sauder, M.; Beeton, G.; Andrade, R.; Maka, P.; Smith, C.P.; Kornblith, L.; Elkbuli, A. Traumatic Brain Injury, Electrolyte Levels, and Associated Outcomes: A Systematic Review. J. Surg. Res. 2023, 289, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chavasiri, C.; Suriyachat, N.; Luksanapruksa, P.; Wilartratsami, S.; Chavasiri, S. Incidence of and factors associated with hyponatremia in traumatic cervical spinal cord injury patients. Spinal Cord Ser. Cases 2022, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Rroji, M.; Figurek, A.; Viggiano, D.; Capasso, G.; Spasovski, G. Phosphate in the Context of Cognitive Impairment and Other Neurological Disorders Occurrence in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 7362. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, B.K.; Jeung, K.W.; Youn, C.S.; Lee, D.H.; Lee, S.M.; Heo, T.; Min, Y.I. Prognostic value of serum phosphate level in adult patients resuscitated from cardiac arrest. Resuscitation 2018, 128, 56–62. [Google Scholar] [CrossRef]
- Kuo, G.; Lee, C.-C.; Yang, S.-Y.; Hsiao, Y.C.; Chuang, S.S.; Chang, S.W.; Tu, K.H.; Fan, P.C.; Tian, Y.C.; Chen, Y.C.; et al. Hyperphosphatemia is associated with high mortality in severe burns. PLoS ONE 2018, 13, e0190978. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, X.; Xu, J.; He, M. The U-shaped association between initial serum phosphate level and mortality of traumatic brain injury patients. Front. Neurol. 2025, 16, 1474809. [Google Scholar] [CrossRef]
- Rugg, C.; Bachler, M.; Kammerlander, R.; Niederbrunner, D.; Bösch, J.; Schmid, S.; Kreutziger, J.; Ströhle, M. ICU-Admission Hyperphosphataemia Is Related to Shock and Tissue Damage, Indicating Injury Severity and Mortality in Polytrauma Patients. Diagnostics 2021, 11, 1548. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Pomerantz, F.; Flanagan, S.; Stein, A.; Gordon, W.A.; Ragnarsson, K.T. Parathyroid hormone suppression in spinal cord injury patients is associated with the degree of neurologic impairment and not the level of injury. Arch. Phys. Med. Rehabil. 1997, 78, 692–696. [Google Scholar] [CrossRef]
- Demetriades, D.; Charalambides, K.; Chahwan, S.; Hanpeter, D.; Alo, K.; Velmahos, G.; Murray, J.; Asensio, J. Nonskeletal cervical spine injuries: Epidemiology and diagnostic pitfalls. J. Trauma 2000, 48, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Kelly, D.F.; Counelis, G.J.; Blinman, T.; McArthur, D.L.; Cryer, H.G. Cervical spine trauma associated with moderate and severe head injury: Incidence, risk factors, and injury characteristics. J. Neurosurg. Spine 2002, 96, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Laurer, H.; Maier, B.; El Saman, A.; Lehnert, M.; Wyen, H.; Marzi, I. Distribution of spinal and associated injuries in multiple trauma patients. Eur. J. Trauma Emerg. Surg. 2007, 33, 476–481. [Google Scholar] [CrossRef]
- Ghobrial, G.M.; Amenta, P.S.; Maltenfort, M.; Williams, K.A., Jr.; Harrop, J.S.; Sharan, A.; Jallo, J.; Heller, J.; Ratliff, J.; Prasad, S. Longitudinal incidence and concurrence rates for traumatic brain injury and spine injury—A twenty year analysis. Clin. Neurol. Neurosurg. 2014, 123, 174–180. [Google Scholar] [CrossRef] [PubMed]
- El-Menyar, A.; Mekkodathil, A.; Verma, V.; Wahlen, B.M.; Peralta, R.; Taha, I.; Hakim, S.; Al-Thani, H. Gender Discrepancy in Patients with Traumatic Brain Injury: A Retrospective Study from a Level 1 Trauma Center. Biomed Res. Int. 2022, 2022, 3147340. [Google Scholar] [CrossRef]
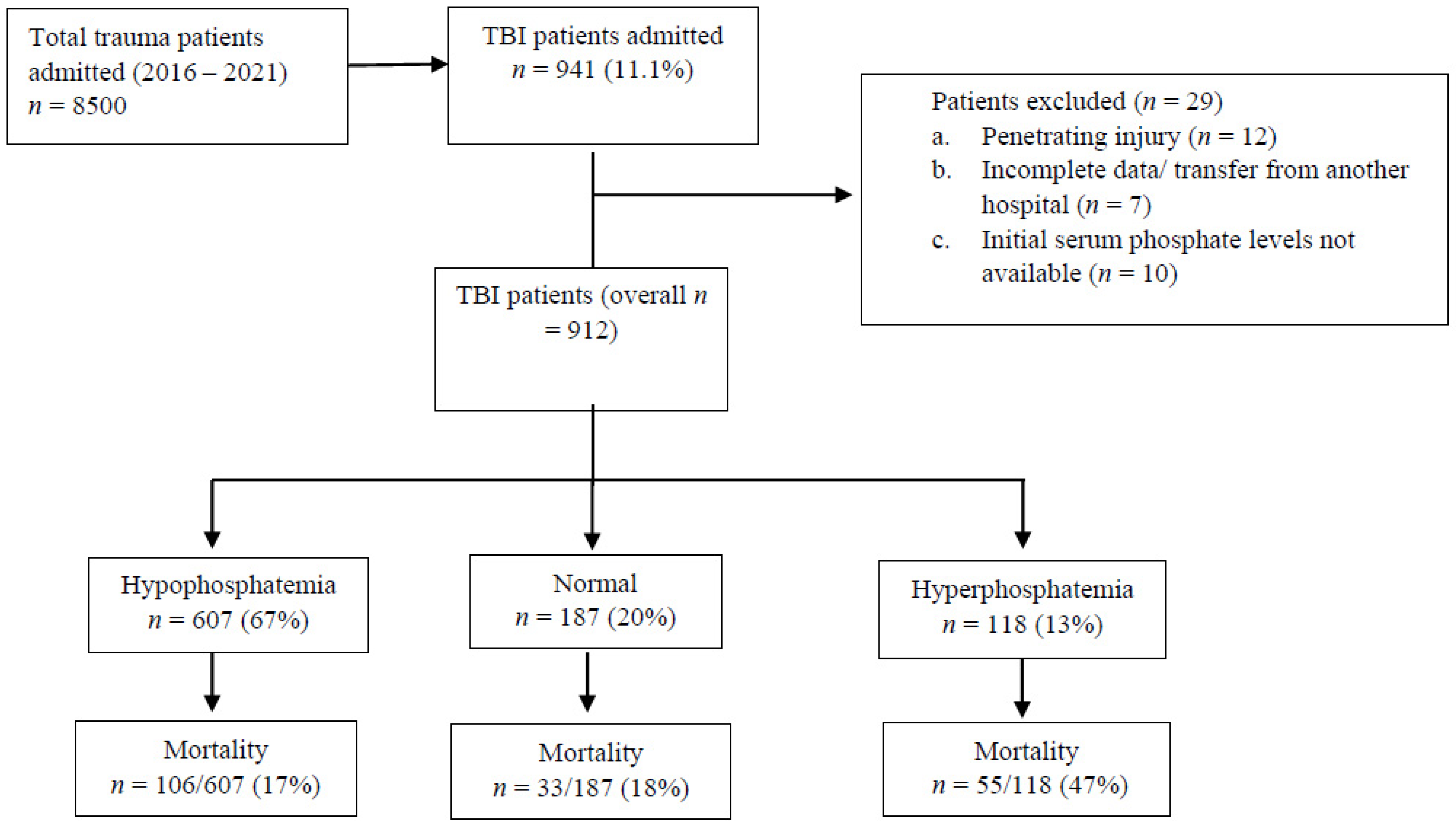
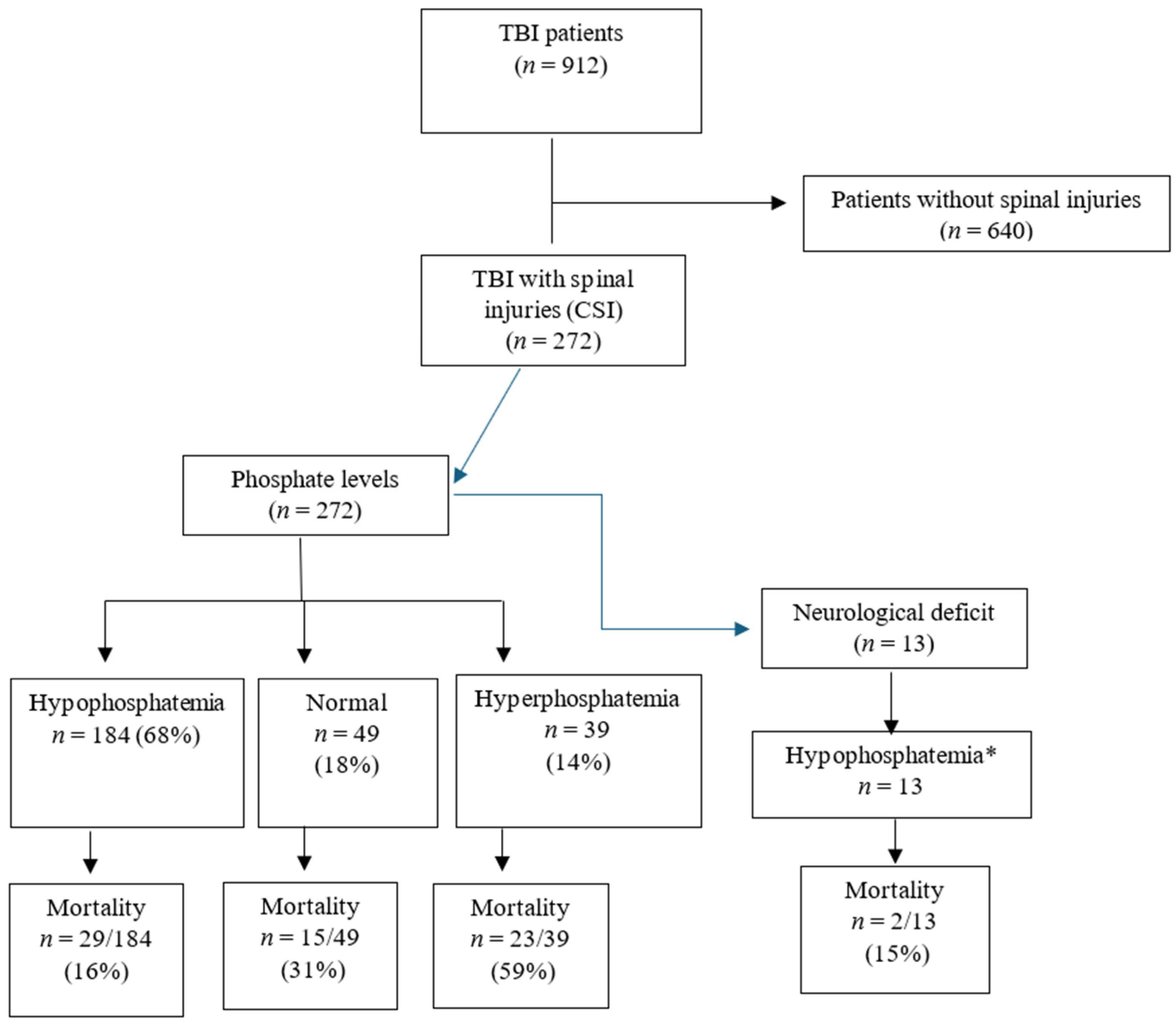
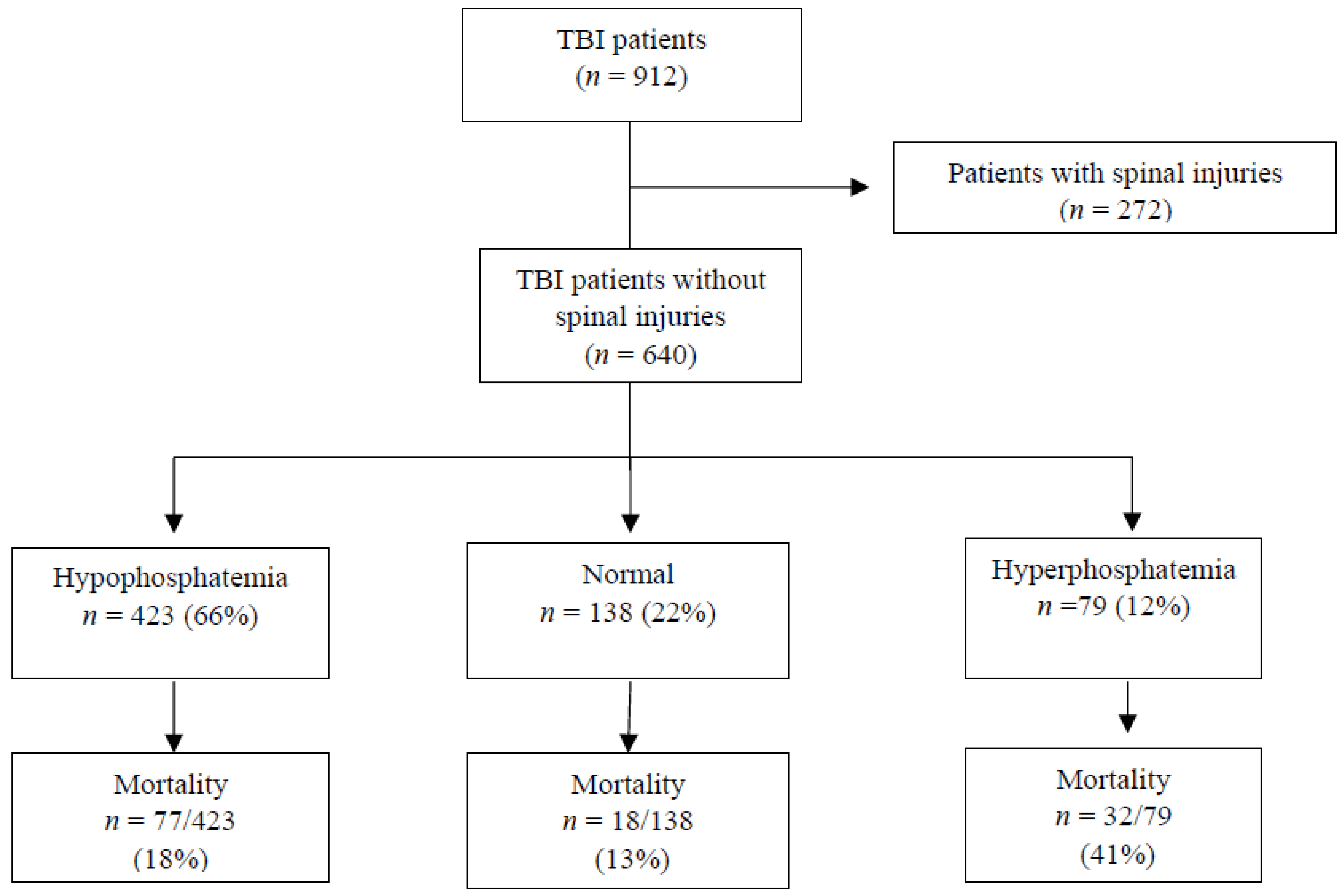
| Variable | Overall TBI (n = 912) | Non-CSI (n = 640) | CSI (n = 272) | p Value |
|---|---|---|---|---|
| Age (Median, IQR) | 30 (2–40) | 30 (22–41) | 31 (23–39) | 0.41 |
| Males | 853 (93.5%) | 598 (93.4%) | 255 (93.8%) | 0.86 |
| TBI type | ||||
| 319 (35.0%) | 222 (34.7%) | 97 (35.7%) | 0.78 |
| 384 (42.1%) | 262 (40.9%) | 122 (44.9%) | 0.27 |
| 204 (22.4%) | 157 (24.5%) | 47 (17.3%) | 0.02 |
| 206 (22.6%) | 154 (24.1%) | 52 (19.1%) | 0.10 |
| Neurological deficit | 13 (1.4%) | - | 13 (4.8%) | - |
| Glasgow Coma Score (Median, IQR) | 3 (3–9) | 3 (3–9) | 3 (3–3) | 0.001 |
| Head AIS (Median, IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.73 |
| Spine AIS (Median, IQR) | 2 (2–2) | - | 2 (2–2) | - |
| Injury Severity Score (Median, IQR) | 27 (17–34) | 26 (17–30) | 29 (22–38) | 0.001 |
| Electrolyte levels (SD) | ||||
| 141.0 (4.5) | 140.7 (4.4) | 141.7 (4.5) | 0.01 |
| 3.8 (0.7) | 3.8 (0.6) | 3.9 (0.7) | 0.28 |
| 1.9 (0.3) | 2.0 (0.3) | 1.9 (0.3) | 0.001 |
| 0.7 (0.1) | 0.7 (0.1) | 0.6 (0.1) | 0.10 |
| 1.0 (0.5) | 1.0 (0.4) | 1.1 (0.5) | 0.11 |
| Hemoglobin level (Median, IQR) | 12.9 (11.2–14.3) | 13.2 (11.4–14.4) | 12.5 (10.8–13.9) | 0.006 |
| Mortality | 194 (21.3%) | 127 (19.8%) | 67 (24.6%) | 0.106 |
| Normophosphatemia (n = 187; 20.5%) | Hypophosphatemia (n = 607; 66.6%) | Hyperphosphatemia (n = 118; 12.9%) | p Value | |
|---|---|---|---|---|
| Age * | 28 (21–39) | 31 (24–41) | 26 (15–38) | 0.04 |
| Male | 170 (90.9%) | 579 (95.4%) | 104 (88.1%) | 0.004 |
| Subdural hemorrhage | 60 (32.1%) | 224 (36.9%) | 35 (29.7%) | 0.21 |
| Subarachnoid hemorrhage | 88 (47.1%) | 247 (40.7%) | 49 (41.5%) | 0.30 |
| Extradural hemorrhage | 45 (24.1%) | 142 (23.4%) | 17 (14.4%) | 0.08 |
| Midline shift | 36 (19.3%) | 139 (22.9%) | 31 (26.3%) | 0.34 |
| Neurological deficit | - | 13 (2.1%) | - | - |
| Glasgow Coma Score * | 3 (3–11) | 3 (3–8) | 3 (3–6) | 0.09 |
| Cervical spine injury | 26 (13.9%) | 87 (14.3%) | 22 (18.6%) | 0.45 |
| Thoracic spine injury | 22 (11.8%) | 83 (13.7%) | 15 (12.7%) | 0.79 |
| Lumbar spine injury | 24 (12.8%) | 89 (14.7%) | 18 (15.3%) | 0.78 |
| Injury Severity Score * | 26 (17–34) | 26 (17–33) | 30 (26–38) | 0.001 |
| Ventilatory days * | 4 (1–9) | 4 (1–11) | 3 (1–11) | 0.59 |
| ICU LOS * | 8 (4–14) | 8 (3–16) | 5 (2–17) | 0.07 |
| Mortality | 33 (17.6%) | 106 (17.5%) | 55 (46.6%) | 0.001 |
| Mild TBI (GCS 13–15) (n = 88; 9.7%) | Moderate TBI (GCS 9–12) (n = 144; 15.8%) | Severe TBI (GCS 3–8) (n = 671; 73.6%) | p Value | |
|---|---|---|---|---|
| Age | 33 (26–43) | 31 (23–42) | 29 (22–39) | 0.006 |
| Injury Severity Score | 22 (16–29) | 19 (14–26) | 29 (22–35) | 0.001 |
| Serum phosphate level | 1.03 (0.79–1.22) | 0.97 (0.76–1.21) | 0.92 (0.72–1.21) | 0.001 |
| Hypophosphatemia | 60% | 64% | 68% | 0.02 |
| Hyperphosphatemia | 8% | 12% | 14% | 0.02 |
| Serum glucose-phosphate ratio (SD) | 9.34 (51) | 9.34 (5.1) | 11.17 (7.7) | 0.004 |
| Intubation | 45 (51.1%) | 97 (67.4%) | 666 (99.3%) | 0.001 |
| Ventilatory days | 1 (0–5) | 1 (0–5) | 6 (2–12) | 0.001 |
| TICU days | 4 (2–11) | 5 (3–11) | 10 (4–17) | 0.001 |
| Mortality | 6 (6.8%) | 8 (5.6%) | 179 (26.7%) | 0.001 |
| Variables | Correlation and p-Value | Overall TBI | Non-CSI | CSI (n = 272) |
|---|---|---|---|---|
| Serum Sodium | Pearson Correlation | 0.016 | −0.008 | 0.048 |
| Sig. (2-tailed) | 0.628 | 0.849 | 0.428 | |
| N | 912 | 640 | 272 | |
| Serum Potassium | Pearson Correlation | 0.267 | 0.279 | 0.242 |
| Sig. (2-tailed) | 0.001 | 0.001 | 0.001 | |
| N | 912 | 640 | 272 | |
| Serum Calcium | Pearson Correlation | 0.000 | 0.020 | −0.016 |
| Sig. (2-tailed) | 0.997 | 0.620 | 0.795 | |
| N | 912 | 640 | 272 | |
| Serum Magnesium | Pearson Correlation | 0.373 | 0.290 | 0.544 |
| Sig. (2-tailed) | 0.001 | 0.001 | 0.001 | |
| N | 912 | 640 | 272 | |
| Cervical Spine AIS | Pearson Correlation | −0.103 | - | −0.219 |
| Sig. (2-tailed) | 0.234 | - | 0.090 | |
| N | 134 | - | 61 | |
| Thoracic Spine AIS | Pearson Correlation | −0.118 | - | −0.155 |
| Sig. (2-tailed) | 0.194 | - | 0.263 | |
| N | 122 | - | 54 | |
| Lumbar Spine AIS | Pearson Correlation | −0.097 | - | −0.150 |
| Sig. (2-tailed) | 0.273 | - | 0.293 | |
| N | 130 | - | 51 | |
| Injury severity score (ISS) | Pearson Correlation | 0.202 | 0.159 | 0.270 |
| Sig. (2-tailed) | 0.001 | 0.001 | 0.001 | |
| N | 912 | 640 | 272 | |
| Initial serum lactate level | Pearson Correlation | 0.302 | 0.246 | 0.420 |
| Sig. (2-tailed) | 0.001 | 0.001 | 0.001 | |
| N | 836 | 640 | 257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Menyar, A.; Mekkodathil, A.; Khan, N.A.; Asim, M.; Joseph, B.; Al-Thani, H. Significance of Initial Serum Phosphate Imbalance in Traumatic Brain Injury with and Without Concomitant Spinal Injuries: Retrospective Analysis. Biomedicines 2025, 13, 2858. https://doi.org/10.3390/biomedicines13122858
El-Menyar A, Mekkodathil A, Khan NA, Asim M, Joseph B, Al-Thani H. Significance of Initial Serum Phosphate Imbalance in Traumatic Brain Injury with and Without Concomitant Spinal Injuries: Retrospective Analysis. Biomedicines. 2025; 13(12):2858. https://doi.org/10.3390/biomedicines13122858
Chicago/Turabian StyleEl-Menyar, Ayman, Ahammed Mekkodathil, Naushad A. Khan, Mohammad Asim, Bellal Joseph, and Hassan Al-Thani. 2025. "Significance of Initial Serum Phosphate Imbalance in Traumatic Brain Injury with and Without Concomitant Spinal Injuries: Retrospective Analysis" Biomedicines 13, no. 12: 2858. https://doi.org/10.3390/biomedicines13122858
APA StyleEl-Menyar, A., Mekkodathil, A., Khan, N. A., Asim, M., Joseph, B., & Al-Thani, H. (2025). Significance of Initial Serum Phosphate Imbalance in Traumatic Brain Injury with and Without Concomitant Spinal Injuries: Retrospective Analysis. Biomedicines, 13(12), 2858. https://doi.org/10.3390/biomedicines13122858






