Age-Stratified Analysis of the Clinical Efficacy of Subcutaneous Immunotherapy for Allergic Rhinitis in Chinese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. The Inclusion and Exclusion Criteria of Patients
2.3. The Diagnosis of AR
- (1)
- Serum Total IgE and sIgE Measurement: Allergen-specific IgE was detected using the EUROIMMUN immunoblotting system. Serum samples and reagents were loaded into microfluidic cartridges for automated analysis against a panel of 19 common allergens. sIgE levels were graded as follows: Grade 0: <0.35 KU/L; Grade 1: 0.35 to <0.7 KU/L; Grade 2: 0.7 to <3.5 KU/L; Grade 3: 3.5 to <17.5 KU/L; Grade 4: 17.5 to <50 KU/L.
- (2)
- Allergen Skin Prick Test (SPT): SPT was performed using a panel of 18 allergen extracts from ALK (Denmark). Wheal responses were read 15 min after pricking. Results were graded based on the SI value (ratio of the allergen wheal diameter to the histamine wheal diameter). SI = 0 was negative; SI < 0.5 was (+); 0.5 ≤ SI < 1.0 was (++); 1.0 ≤ SI < 2.0 was (+++); SI ≥ 2.0 was (++++).
2.4. Pre- and Post-SCIT Examination and Evaluation
- (1)
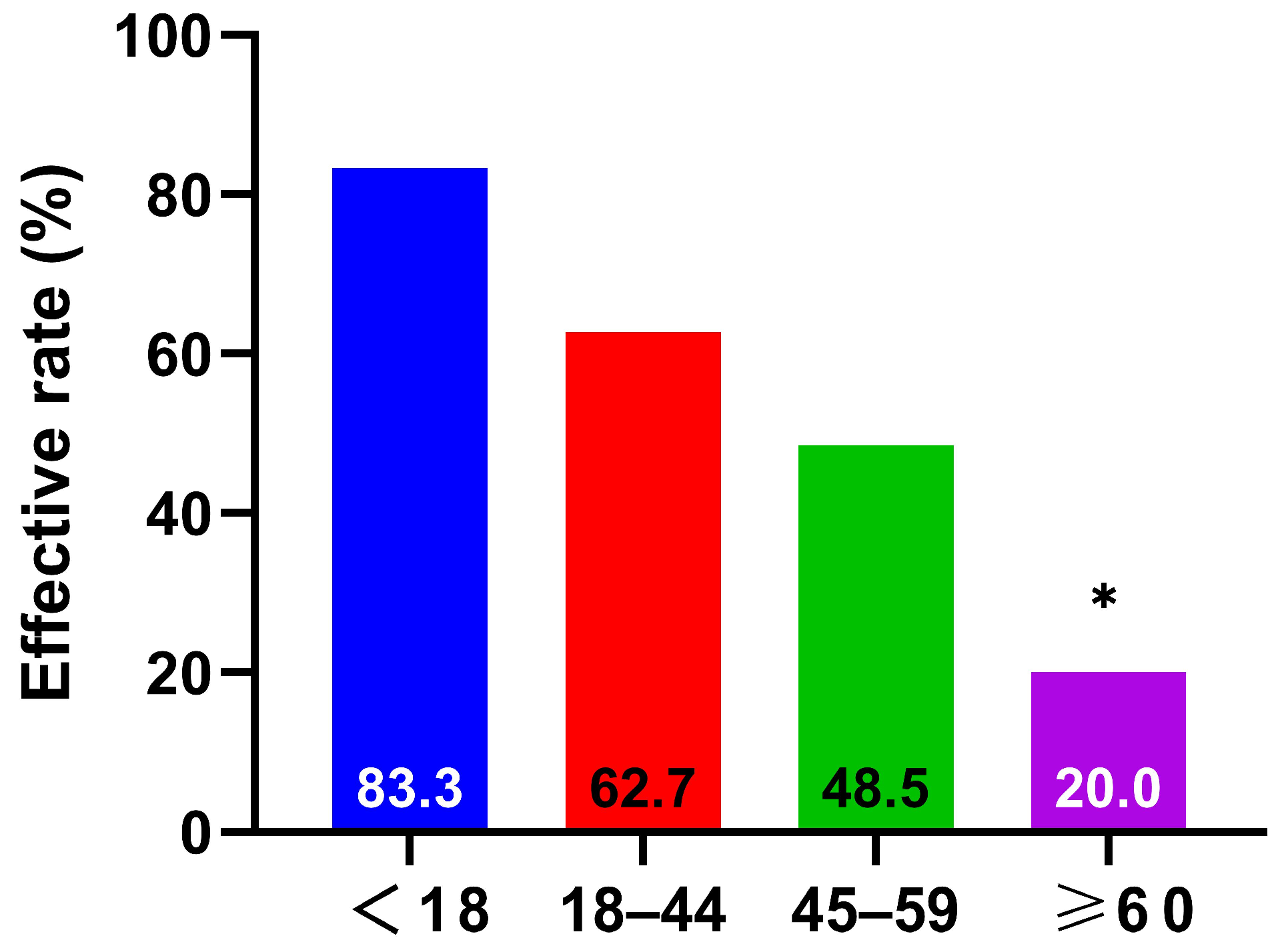
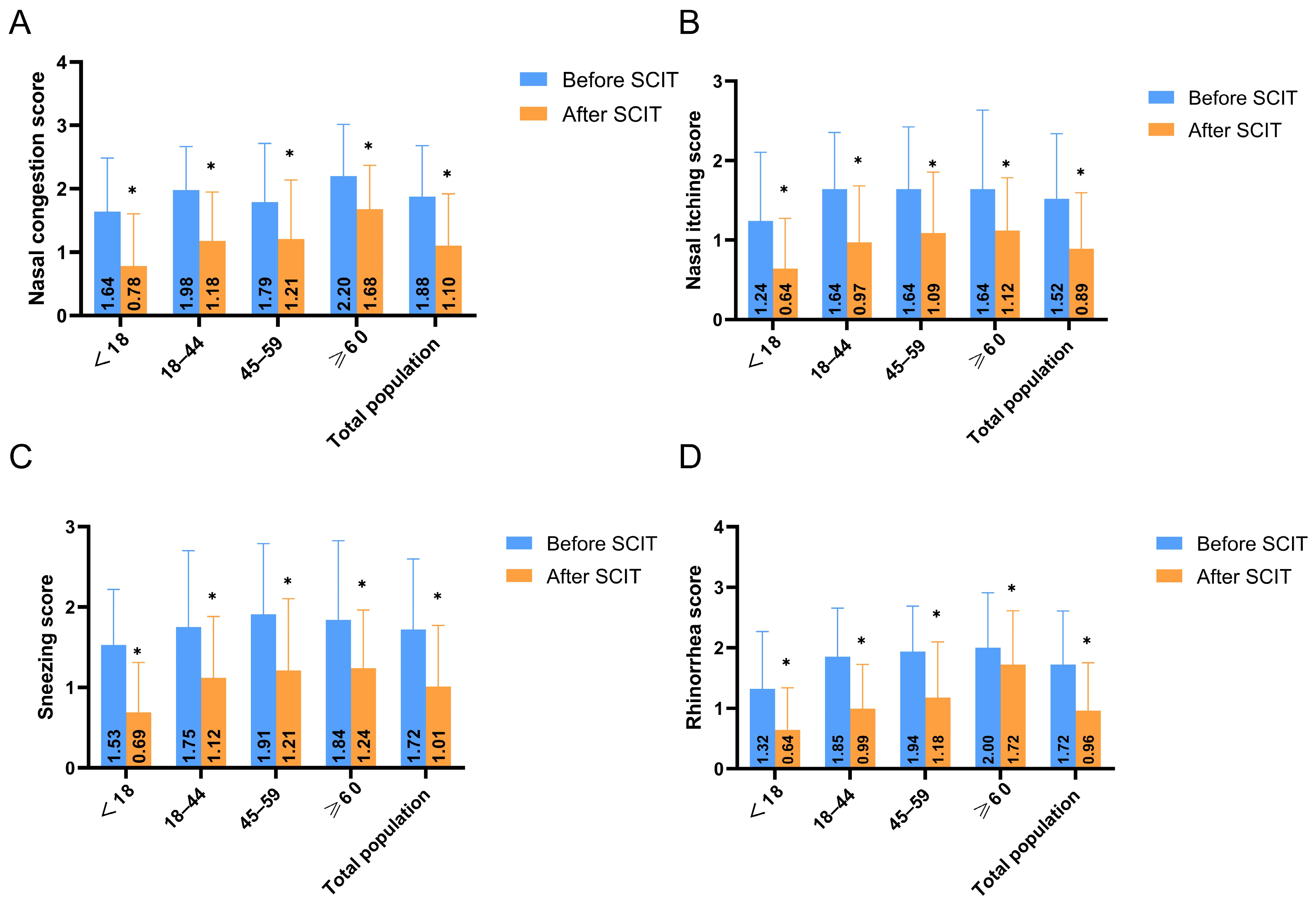
- Symptom and Clinical Efficacy Assessment: Following established literature [29,30], four key nasal symptoms (sneezing, rhinorrhea, nasal itching, and nasal congestion) were evaluated using a 4-point scale: 0 = no symptoms; 1 = mild (symptoms present but easily tolerated); 2 = moderate (symptoms bothersome but tolerable); 3 = severe (symptoms intolerable, interfering with daily activities and/or sleep). The symptom improvement rate was calculated using the formula: [(Pre-treatment score − Post-treatment score)/Pre-treatment score] × 100%. Efficacy was categorized as: Marked improvement (>66% improvement), Moderate improvement (26–65% improvement), or Ineffective (<25% improvement) [31,32]. The overall response rate was calculated as the percentage of patients achieving marked or moderate improvement (i.e., >25% improvement).
- (2)
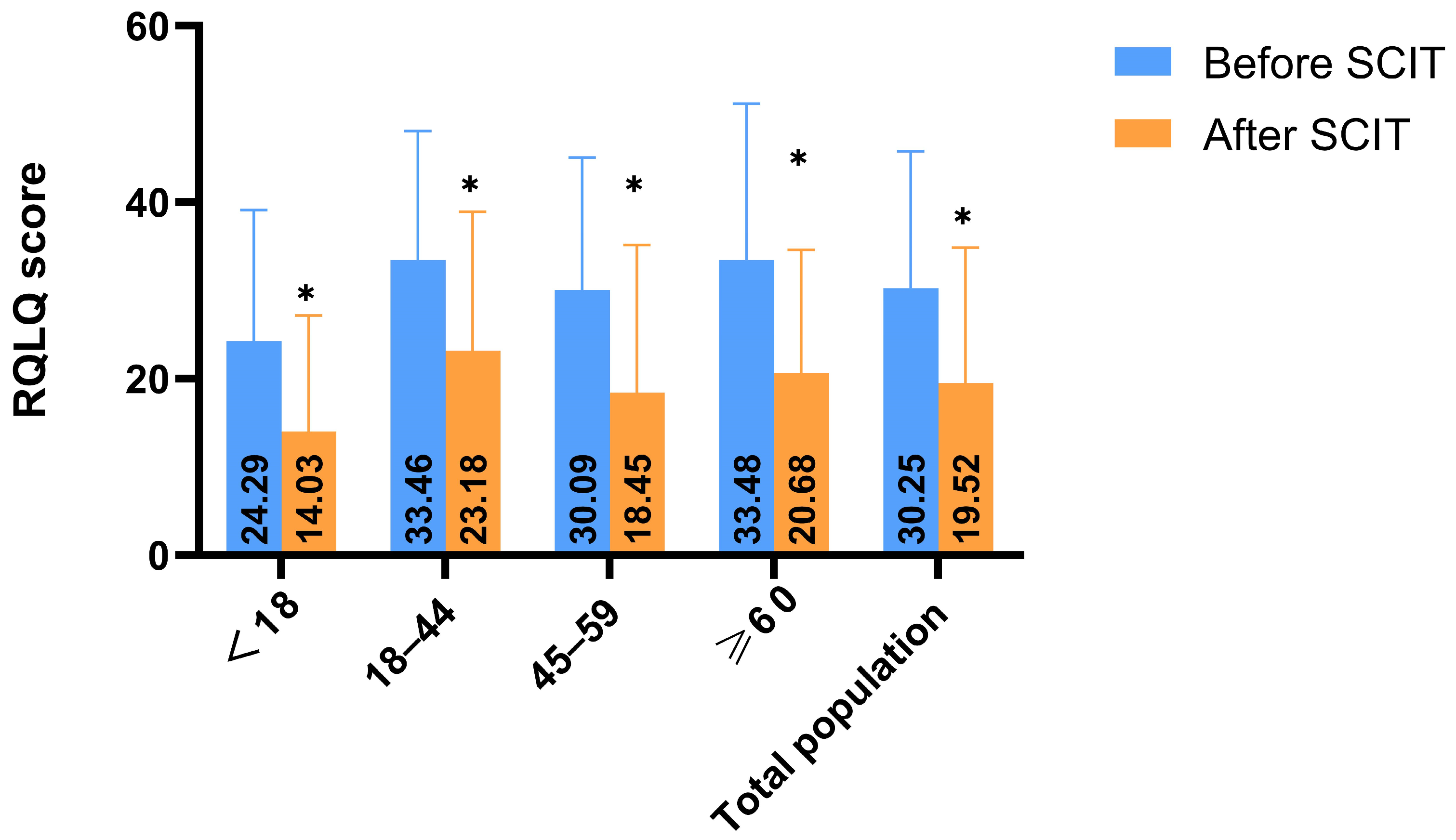
- Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ): Patients completed the RQLQ to assess the impact of AR on daily activities, social functions, sleep quality, nasal and eye symptoms, and emotional well-being. Items were rated on a 0–6 scale (the RQLQ uses a 7-point scale, with 0 = no problem to 6 = extremely problematic), with higher scores indicating greater impairment [33,34].
- (3)
- Immunological Parameter Measurement: Peripheral blood was collected before treatment and after 6 months of therapy to assess the treatment’s effect on the immune system. The proportions of Tregs (CD4+CD25+CD127low/−) and Bregs (CD19+CD24hiCD38hi) within peripheral blood mononuclear cells (PBMCs) were analyzed by flow cytometry. Briefly, PBMCs were stained with anti-CD4-PE, anti-CD25-FITC, and anti-CD127-PC5 monoclonal antibodies to identify Tregs, and with anti-CD19-PE, anti-CD24-PC5, and anti-CD38-FITC antibodies to identify Bregs (Beckman Coulter, Brea, CA, USA). Isotype-matched IgGs were used as controls for non-specific fluorescence. Data were acquired on a FACSCalibur flow cytometer (Beckman Coulter, USA) and analyzed using Cell Quest 3.3 software (BD, USA).
2.5. SCIT Protocol
2.6. Observation of Adverse Reactions
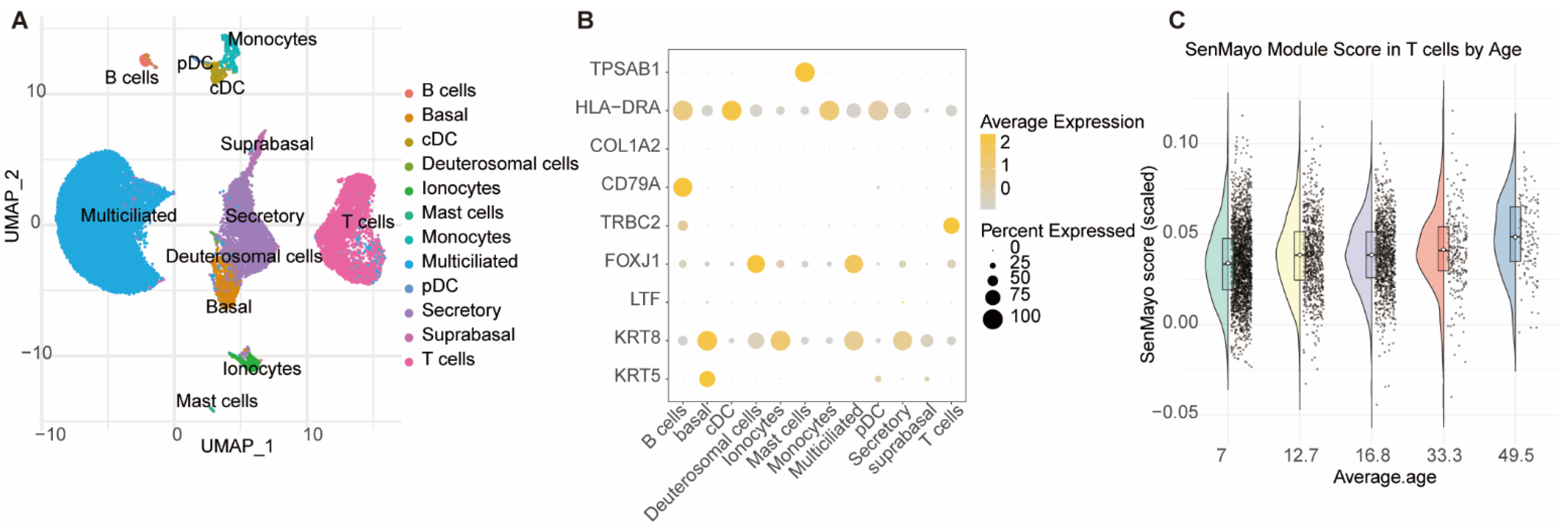
2.7. Single-Cell Transcriptomic Analysis
2.8. Statistical Analysis
3. Results
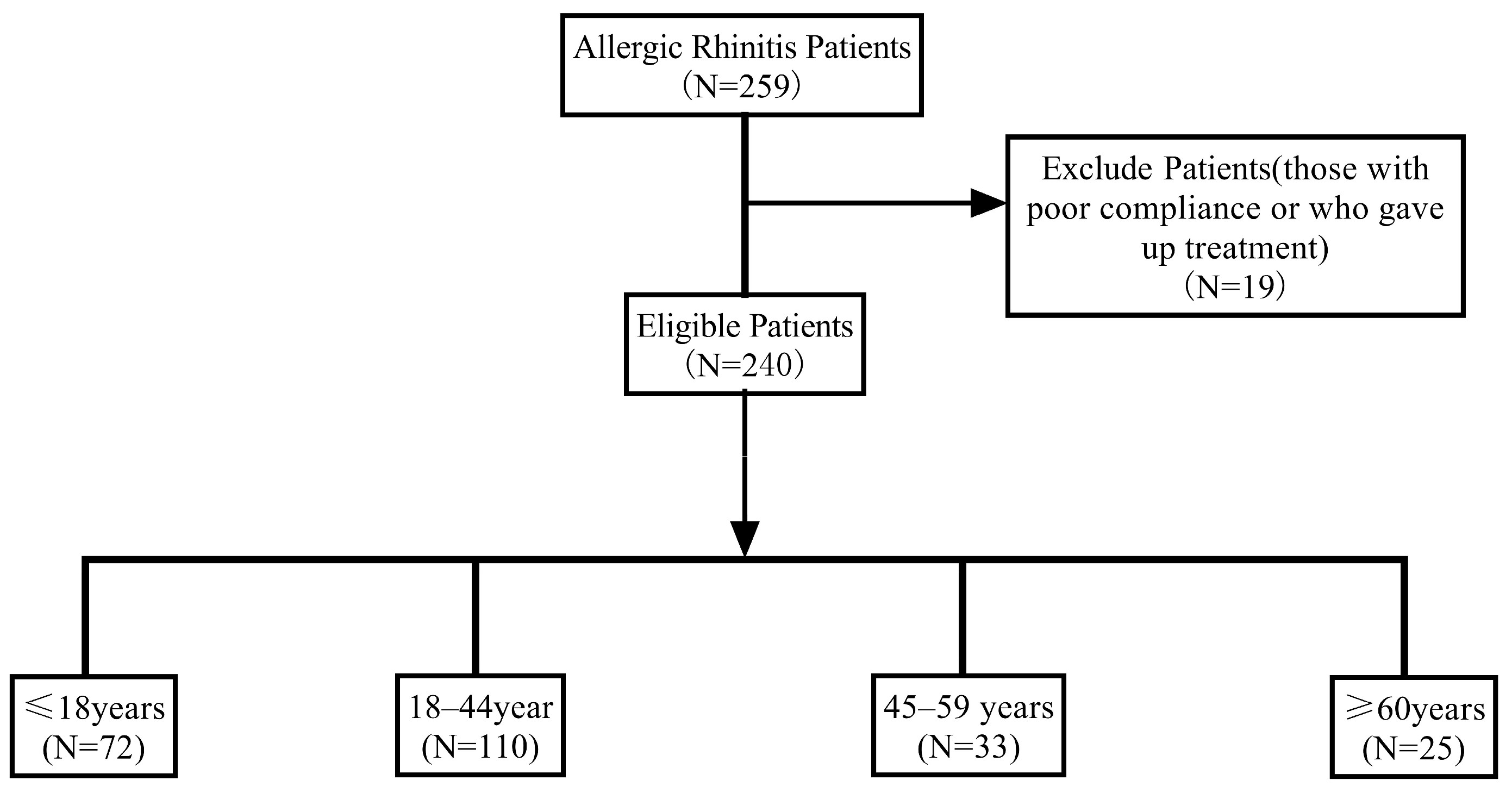
3.1. Study Cohort
3.2. Efficacy Evaluation by Age Stratification
3.2.1. Response Rate by Age Group
3.2.2. Nasal Symptom Scores by Age Group
3.2.3. RQLQ Scores by Age Group
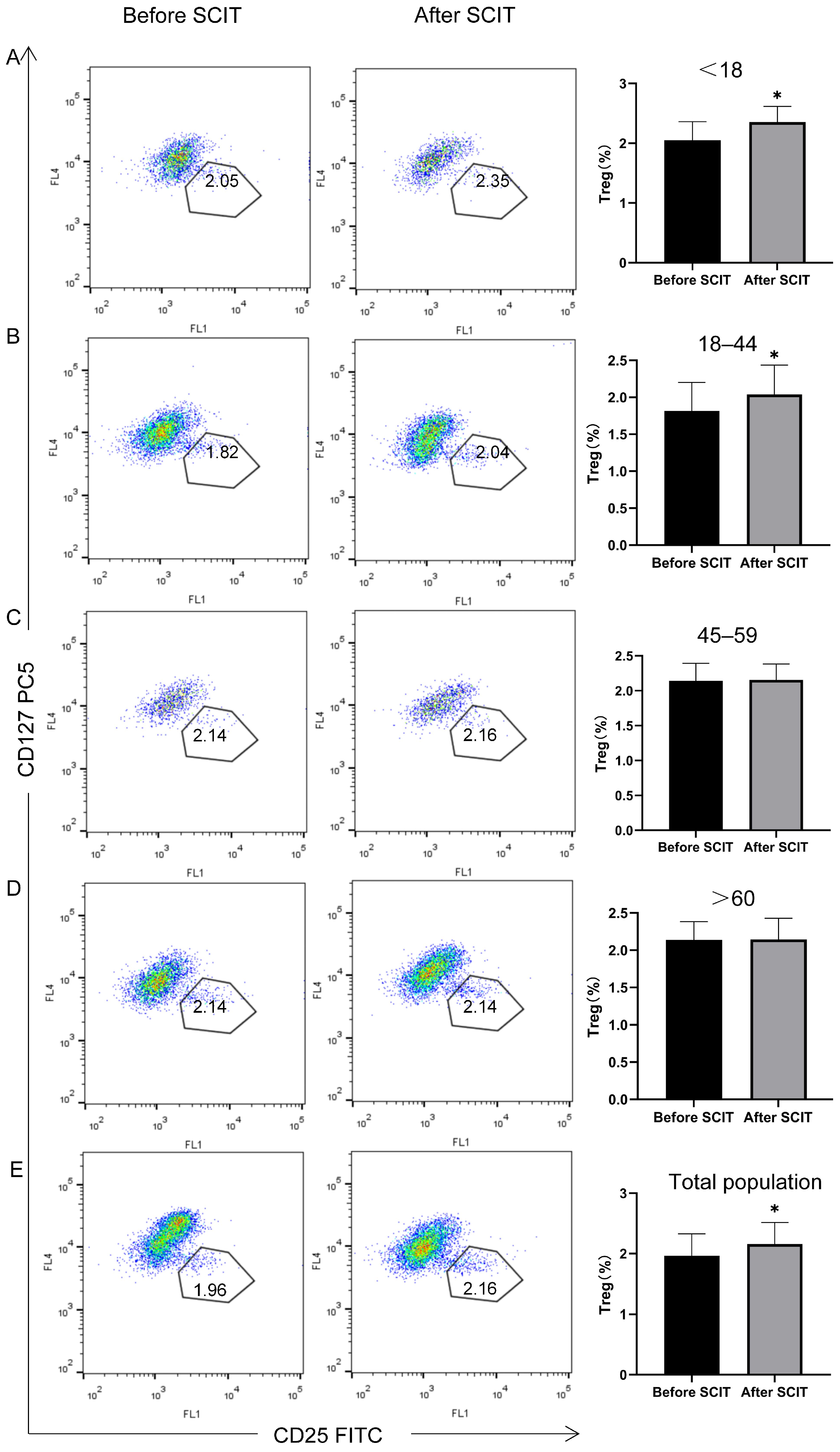
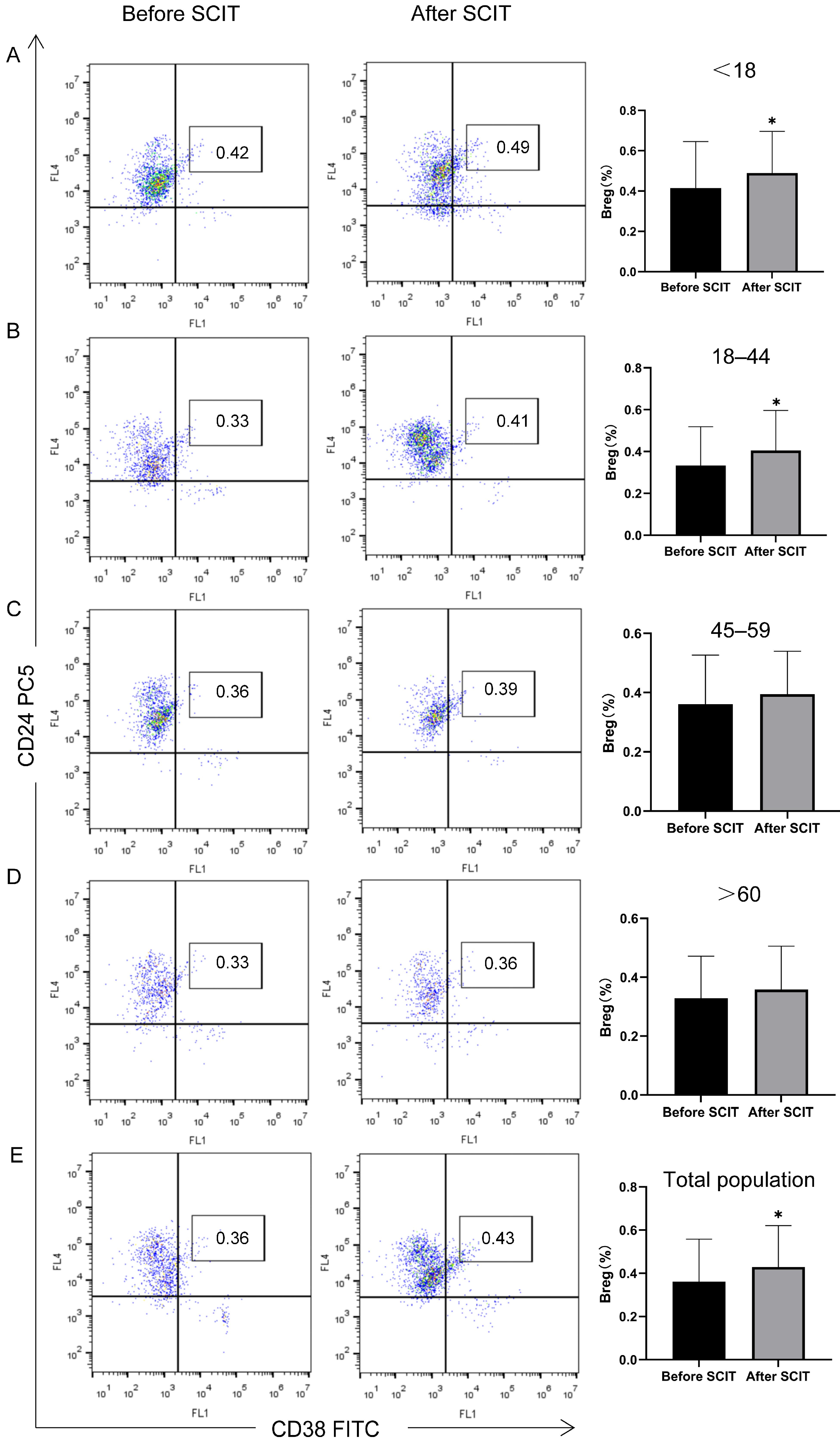
3.2.4. Peripheral Blood Treg and Breg Changes by Age Group
3.3. Adverse Reactions by Age Stratification
3.4. Nasal Mucosal T Cells Exhibit Significant Age-Dependent Senescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCIT | Subcutaneous immunotherapy. |
| AR | Allergic rhinitis. |
| HDM | House dust mite. |
| WHO | World Health Organization. |
| RQLQ | Rhinoconjunctivitis Quality of Life Questionnaire. |
| AIT | Allergen-specific immunotherapy. |
| SLIT | Sublingual immunotherapy. |
| SPT | Skin prick tests. |
| PBMCs | Peripheral blood mononuclear cells. |
| Treg | regulatory T cell(s). |
| Breg | regulatory B cell(s). |
| sIgE | specific immunoglobulin E. |
| CI | confidence interval. |
References
- Zhang, Y.; Zhang, L. Increasing Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol. Res. 2019, 11, 156–169. [Google Scholar] [CrossRef]
- Passali, D.; Cingi, C.; Staffa, P.; Passali, F.; Muluk, N.B.; Bellussi, M.L. The International Study of the Allergic Rhinitis Survey: Outcomes from 4 geographical regions. Asia Pac. Allergy 2018, 8, e7. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol. Res. 2014, 6, 105–113. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, J.; Fu, Q.; He, S.; Li, H.; Liu, Z.; Tan, G.; Tao, Z.; Wang, D.; Wen, W.; et al. Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis. Allergy Asthma Immunol. Res. 2018, 10, 300–353. [Google Scholar] [CrossRef]
- Mortz, C.G.; Andersen, K.E.; Poulsen, L.K.; Kjaer, H.F.; Broesby-Olsen, S.; Bindslev-Jensen, C. Atopic diseases and type I sensitization from adolescence to adulthood in an unselected population (TOACS) with focus on predictors for allergic rhinitis. Allergy 2019, 74, 308–317. [Google Scholar] [CrossRef]
- Drazdauskaitė, G.; Layhadi, J.A.; Shamji, M.H. Mechanisms of Allergen Immunotherapy in Allergic Rhinitis. Curr. Allergy Asthma Rep. 2020, 21, 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, M.; Li, X.; Yan, B.; Liu, J.; Shi, L.; Cao, Z.; Feng, Y.; Liu, W.; et al. Stapokibart for moderate-to-severe seasonal allergic rhinitis: A randomized phase 3 trial. Nat. Med. 2025, 31, 2213–2221. [Google Scholar] [CrossRef]
- Abdullah, B.; Abdul Latiff, A.H.; Manuel, A.M.; Jamli, F.M.; Singh, H.S.D.; Ismail, I.H.; Jahendran, J.; Saniasiaya, J.; Woo, K.C.K.; Khoo, P.C.; et al. Pharmacological Management of Allergic Rhinitis: A Consensus Statement from the Malaysian Society of Allergy and Immunology. J. Asthma Allergy 2022, 15, 983–1003. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Blaiss, M.S.; Derebery, M.J.; Mahr, T.A.; Gordon, B.R.; Sheth, K.K.; Simmons, A.L.; Wingertzahn, M.A.; Boyle, J.M. Burden of allergic rhinitis: Results from the Pediatric Allergies in America survey. J. Allergy Clin. Immunol. 2009, 124, S43–S70. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pinto, B.; Vieira, R.J.; Bognanni, A.; Gil-Mata, S.; Ferreira-da-Silva, R.; Ferreira, A.; Cardoso-Fernandes, A.; Ferreira-Cardoso, H.; Marques-Cruz, M.; Duarte, V.H.; et al. Efficacy and safety of intranasal medications for allergic rhinitis: Network meta-analysis. Allergy 2025, 80, 94–105. [Google Scholar] [CrossRef]
- Bousquet, J.; Pfaar, O.; Togias, A.; Schünemann, H.J.; Ansotegui, I.; Papadopoulos, N.G.; Tsiligianni, I.; Agache, I.; Anto, J.M.; Bachert, C.; et al. 2019 ARIA Care pathways for allergen immunotherapy. Allergy 2019, 74, 2087–2102. [Google Scholar] [CrossRef]
- Atipas, K.; Kanjanawasee, D.; Tantilipikorn, P. Intradermal Allergen Immunotherapy for Allergic Rhinitis: Current Evidence. J. Pers. Med. 2022, 12, 1341. [Google Scholar] [CrossRef]
- Marogna, M.; Tomassetti, D.; Bernasconi, A.; Colombo, F.; Massolo, A.; Di Rienzo Businco, A.; Canonica, G.W.; Passalacqua, G.; Tripodi, S. Preventive effects of sublingual immunotherapy in childhood: An open randomized controlled study. Ann. Allergy Asthma Immunol. 2008, 101, 206–211. [Google Scholar] [CrossRef]
- Inal, A.; Altintas, D.U.; Yilmaz, M.; Karakoc, G.B.; Kendirli, S.G.; Sertdemir, Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J. Investig. Allergol. Clin. Immunol. 2007, 17, 85–91. [Google Scholar]
- Zhang, Y.; Lan, F.; Zhang, L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy 2022, 77, 3309–3319. [Google Scholar] [CrossRef]
- Mortuaire, G.; Michel, J.; Papon, J.F.; Malard, O.; Ebbo, D.; Crampette, L.; Jankowski, R.; Coste, A.; Serrano, E. Specific immunotherapy in allergic rhinitis. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, M.; Yue, W.; Zhou, J.; Li, R.; Lin, J.; Predictive, Y.L. factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int. Arch. Allergy Immunol. 2014, 164, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jung, J.W.; Park, J.W.; Choi, J.H.; Kwon, J.W.; Lee, S.; Kim, J.H.; Lee, S.M.; Ahn, Y.M.; Han, M.Y.; et al. Clinical efficacy of allergen-specific immunotherapy from patient andphysician perspectives. Yonsei Med. J. 2019, 60, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.; Kaambwa, B.; Novielli, N.; Huissoon, A.; Fry-Smith, A.; Meads, C.; Barton, P.; Dretzke, J. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol. Assess. 2013, 17, vi-322. [Google Scholar] [CrossRef]
- Ren, L.; Wang, C.; Xi, L.; Gao, Y.; Zhang, Y.; Zhang, L. Long-term efficacy of HDM-SCIT in pediatric and adult patients with allergic rhinitis. Allergy Asthma Clin. Immunol. 2023, 19, 20. [Google Scholar] [CrossRef]
- Bozek, A.; Ignasiak, B.; Filipowska, B.; Jarzab, J. House dust mite sublingual immunotherapy: A double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin. Exp. Allergy 2013, 43, 242–248. [Google Scholar] [CrossRef]
- Bożek, A.; Kołodziejczyk, K.; Kozłowska, R.; Canonica, G.W. Evidence of the efficacy and safety of house dust mite subcutaneous immunotherapy in elderly allergic rhinitis patients: A randomized, double-blind placebo-controlled trial. Clin. Transl. Allergy 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Bozek, A.; Kolodziejczyk, K.; Krajewska-Wojtys, A.; Jarzab, J. Pre-seasonal, subcutaneous immunotherapy: A double-blinded, placebo-controlled study in elderly patients with an allergy to grass. Ann. Allergy Asthma Immunol. 2016, 116, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.F.; Manti, S.; Papale, M.; Amato, M.; Licari, A.; Marseglia, G.L.; Leonardi, S. Nasal Nitric Oxide and Nasal Cytology as Predictive Markers of Short-Term Sublingual Allergen-Specific Immunotherapy Efficacy in Children with Allergic Rhinitis. Am J. Rhinol. Allergy 2022, 36, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J.; Gao, Y.; Cao, F.; Xiong, W.; Wang, C.; Zhang, Y.; Zhang, L. Clinical response to subcutaneous immunotherapy at 3 years in allergic rhinitis patients is predicted by short-term treatment effectiveness. Clin. Transl. Allergy 2023, 13, e12223. [Google Scholar] [CrossRef]
- Scadding, G.K.; Kariyawasam, H.H.; Scadding, G.; Mirakian, R.; Buckley, R.J.; Dixon, T.; Durham, S.R.; Farooque, S.; Jones, N.; Leech, S.; et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin. Exp. Allergy 2017, 47, 856–889. [Google Scholar] [CrossRef]
- Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Chinese guideline for diagnosis and treatment of allergic rhinitis (2022, revision). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2022, 57, 106–129. [Google Scholar] [CrossRef]
- Pfaar, O.; Demoly, P.; Gerth van Wijk, R.; Bonini, S.; Bousquet, J.; Canonica, G.W.; Durham, S.R.; Jacobsen, L.; Malling, H.J.; Mösges, R.; et al. Recommendations for the standardization of clinicaloutcomes used in allergen immunotherapy trials forallergic rhinoconjunctivitis: An EAACI Position Paper. Allergy 2014, 69, 854–867. [Google Scholar] [CrossRef]
- Demoly, P.; Corren, J.; Creticos, P.; De Blay, F.; Gevaert, P.; Hellings, P.; Kowal, K.; Le Gall, M.; Nenasheva, N.; Passalacqua, G.; et al. A 300 IR sublingual tablet is an effective, safe treatment for house dust mite-induced allergic rhinitis: An international, double-blind, placebo-controlled, randomized phase III clinical trial. J. Allergy Clin. Immunol. 2021, 147, 1020–1030.e10. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Baena-Cagnani, C.E.; Bousquet, J.; Bousquet, P.J.; Lockey, R.F.; Malling, H.J.; Passalacqua, G.; Potter, P.; Valovirta, E. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a world allergy organization (WAO) taskforce. Allergy 2007, 62, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yin, J.; Wang, X.; Ma, T.; Lan, T.; Song, Q.; Guo, Y. Relationship between serum inhibitory activity for IgE and efficacy of Artemisia pollen subcutaneous immunotherapy for allergic rhinitis: A preliminary self-controlled study. Allergy Asthma Clin. Immunol. 2020, 16, 18. [Google Scholar] [CrossRef]
- Juniper, E.F.; Thompson, A.K.; Ferrie, P.J.; Roberts, J.N. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J. Allergy Clin. Immunol. 1999, 104, 364–369. [Google Scholar] [CrossRef]
- Blaiss, M.S.; Gronskyte Juhl, R.; Siew, L.Q.C.; Hammerby, E.; Devillier, P. Determining the minimal important differences in the RQLQ score with grass and tree allergy immunotherapy versus placebo in adults with moderate-to-severe allergy. Allergy 2022, 77, 1843–1851. [Google Scholar] [CrossRef]
- Winkley, K.; Banerjee, D.; Bradley, T.; Koseva, B.; Cheung, W.A.; Selvarangan, R.; Pastinen, T.; Grundberg, E. Immune cell residency in the nasal mucosa may partially explain respiratory disease severity across the age range. Sci. Rep. 2021, 11, 15927. [Google Scholar] [CrossRef]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guidelines: Anaphylaxis (2021 update). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Fan, W.; Wang, K.; Yu, Q.; Si, L.; van der Smagt, P.; Tang, J.; Chen, N. Sequential model for predicting patient adherence in subcutaneous immunotherapy for allergic rhinitis. Front. Pharmacol. 2024, 15, 1371504. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Devillier, P.; Schmitt, J.; Demoly, P.; Hilberg, O.; DuBuske, L.; Hass, N.; Klok, T.; Beutner, C. Adherence and persistence in allergen immunotherapy (APAIT): A reporting checklist for retrospective studies. Allergy 2023, 78, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Dhivert-Donnadieu, H.; Bousquet, J. Vaccinations aux allergenes chez l′enfant [Vaccination with allergens in children]. Allerg. Immunol. 2000, 32, 397–401. (In French) [Google Scholar] [PubMed]
- Duman Senol, H.; Topyildiz, E.; Ekici, B.; Gulen, F.; Demir, E. Effectiveness and adverse reactions to subcutaneous immunotherapy in children with allergic rhinitis/asthma. Int. J. Pediatr. Otorhinolaryngol. 2022, 162, 111292. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.C.; Wang, N.; Zhou, P.C.; Chen, C.L.; Song, J.; Pan, L.; Liao, B.; Zhang, X.-H.; Yang, Y.-S.; et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2019, 144, 118–128. [Google Scholar] [CrossRef]
- Shamji, M.H.; Sharif, H.; Layhadi, J.A.; Zhu, R.; Kishore, U.; Renz, H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J. Allergy Clin. Immunol. 2022, 149, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Gootjes, C.; Zwaginga, J.J.; Roep, B.O.; Nikolic, T. Defining Human Regulatory T Cells beyond FOXP3: The Need to Combine Phenotype with Function. Cells 2024, 13, 941. [Google Scholar] [CrossRef]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Kouzegaran, S.; Zamani, M.A.; Faridhosseini, R.; Rafatpanah, H.; Rezaee, A.; Yousefzadeh, H.; Movahed, R.; Azad, F.J.; Tehrani, H. Immunotherapy in Allergic Rhinitis: It’s Effect on the Immune System and Clinical Symptoms. Open Access Maced. J. Med. Sci. 2018, 6, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Yang, L.T.; Qiu, S.Q.; Yang, G.; Luo, X.Q.; Miao, B.P.; Geng, X.-R.; Liu, Z.-Q.; Liu, J.; Wen, Z.; et al. Combination of specific allergen and probiotics induces specific regulatory B cells and enhances specific immunotherapy effect on allergic rhinitis. Oncotarget 2016, 7, 54360–54369. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Hulse, K.E.; Tan, B.K.; Schleimer, R.P. B-lymphocyte lineage cells and the respiratory system. J. Allergy Clin. Immunol. 2013, 131, 933–957. [Google Scholar] [CrossRef]
- Fan, K.; Jin, L.; Yu, S. Roles of regulatory B cells in the pathogenesis of allergic rhinitis. Allergol. Immunopathol. 2022, 50, 7–15. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Lombardo, N.; Terracciano, R.; Navalesi, P.; Savino, R.; Pelaia, G. Biological mechanisms underlying the clinical effects of allergen-specific immunotherapy in asthmatic children. Expert. Opin. Biol. Ther. 2018, 18, 197–204. [Google Scholar] [CrossRef]
- Wang, M.; Gu, Z.; Yang, J.; Zhao, H.; Cao, Z. Changes among TGFbeta1(+) Breg cells and helper T cell subsets in a murine model of allergic rhinitis with prolonged OVA challenge. Int. Immunopharmacol. 2019, 69, 347–357. [Google Scholar] [CrossRef]
- Palomares, O.; Akdis, M.; Martin-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Terada, T.; Tsujimoto, N.; Morie, Y.; Ishida, T.; Takahashi, H.; Hamaguchi, J.; Tabuchi, Y.; Doi, K.; Noro, K.; et al. Regulatory T and B cells in peripheral blood of subcutaneous immunotherapy-treated Japanese cedar pollinosis patients. Immunotherapy 2019, 11, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, N.; Liu, Z. Advances in predictive and monitoring biomarkers for clinical efficacy of subcutaneous immunotherapy in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 55, 527–531. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Durham, I.J.A.S.R. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy 2018, 73, 765–798. [Google Scholar] [CrossRef]
- Pablo, R.D.R.; Vidal, C.; Just, J.; Tabar, A.I.; Sanchez-Machin, I.; Eberle, P.; Borja, J.; Bubel, P.; Pfaar, O.; Demoly, P.; et al. The European Survey on Ad- verse Systemic Reactions in Allergen Immunotherapy (EASSI): A paediatric assessment. Pediatr. Allergy Immunol. 2017, 28, 60–70. [Google Scholar] [CrossRef]
- Nacaroglu, H.T.; Erdem, S.B.; Sumer, O.; Karaman, S.; Karkıner, C.S.U.; Asilsoy, S.; Gunay, I.; Can, D. Local and systemic reactions to subcutaneous allergen immunotherapy. Ann. Allergy Asthma Immunol. 2016, 116, 349–353. [Google Scholar] [CrossRef]
- Epstein, T.G.; Liss, G.M.; Murphy-Berendts, K.; Bernstein, D.I. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008–2012: An update on fatal and nonfatal systemic allergic reactions. J. Allergy Clin. Immunol. Pract. 2014, 2, 161–167. [Google Scholar] [CrossRef]
- Lim, C.E.; Sison, C.P.; Ponda, P. Comparison of pediatric and adult systemic reactions to subcutaneous immunotherapy. J. Allergy Clin. Immunol. Pract. 2017, 5, 1241–1247.e2. [Google Scholar] [CrossRef]
- Coutinho, C.; Lourenço, T.; Fernandes, M.; Neto, M.; Lopes, A.; Santos, A.S.; Barbosa, M.P. Subcutaneous immunotherapy with aeroallergens: Safety profile assessment. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 77–83. [Google Scholar] [CrossRef]
- Sani, S.; Gupta, R.; Fonacier, L.; Aquino, M. Risk stratification of systemic reactions to subcutaneous immunotherapy: A retrospective study. Allergy Asthma Proc. 2019, 40, 338–342. [Google Scholar] [CrossRef]
- Di Bona, D.; Magistà, S.; Masciopinto, L.; Lovecchio, A.; Loiodice, R.; Bilancia, M.; Albanesi, M.; Caiaffa, M.F.; Nettis, E.; Macchia, L.; et al. Safety and treatment compliance of subcutaneous immunotherapy: A 30-year retrospective study. Respir. Med. 2020, 161, 105843. [Google Scholar] [CrossRef] [PubMed]

| ≤18 Years (n = 72) | 18–44 Year (n = 110) | 45–59 Years (n = 33) | ≥60 Years (n = 25) | Total (n = 240) | |
|---|---|---|---|---|---|
| Age | 11.25 ± 3.368 | 31.47 ± 7.342 | 50.73 ± 2.820 | 63.44 ± 2.200 | 31.38 ± 17.629 |
| Sex | |||||
| Male | 45 (62.5%) | 61 (55.5%) | 18 (54.5%) | 16 (64.0%) | 140 (58.3%) |
| Female | 27 (37.5%) | 49 (44.5%) | 15 (45.5%) | 9 (36.0%) | 100 (41.7%) |
| Medical history (non-study disease) | |||||
| Yes | 9 (12.5%) | 73 (66.4%) | 23 (69.7%) | 23 (92.0%%) | 128 (53.3%) |
| No | 63 (87.5) | 37 (33.6%) | 10 (30.3%) | 2 (8.0%) | 112 (46.7%) |
| TNSS | 5.72 ± 2321 | 7.22 ± 2.272 | 7.27 ± 2.730 | 7.68 ± 3.038 | 6.83 ± 2.534 |
| RQLQ | 24.29 ± 14.815 | 33.46 ± 14.638 | 30.09 ± 14.9999 | 33.48 ± 17.676 | 30.25 ± 15.523 |
| ≤18 Years (n = 72) | 18–44 Year (n = 110) | 45–59 Years (n = 33) | ≥60 Years (n = 25) | Total (n = 240) | |
|---|---|---|---|---|---|
| Treg (%) | |||||
| Before SCIT | 2.05 ± 0.31 | 1.82 ± 0.39 | 2.14 ± 0.25 | 2.14 ± 0.25 | 1.96 ± 0.36 |
| After SCIT | 2.35 ± 0.27 * | 2.04 ± 0.40 * | 2.16 ± 0.23 | 2.14 ± 0.28 | 2.16 ± 0.36 * |
| Breg (%) | |||||
| Before SCIT | 0.42 ± 0.23 | 0.33 ± 0.18 | 0.36 ± 0.17 | 0.33 ± 0.14 | 0.36 ± 0.20 |
| After SCIT | 0.49 ± 0.21 * | 0.41 ± 0.19 * | 0.39 ± 0.15 | 0.36 ± 0.15 | 0.43 ± 0.19 * |
| ≤18 Years (n = 72) | 18–44 Years (n = 110) | 45–59 Years (n = 33) | ≥60 Years (n = 25) | Total (n = 240) | p | |
|---|---|---|---|---|---|---|
| Systemic reactions | 2 (5.13%) | 3 (4.76%) | 0 (0%) | 0 (0%) | 5 (4.17%) | 0.818 |
| Local reactions | 5 (12.82%) | 3 (4.76%) | 1 (9.09%) | 0 (0%) | 9 (7.5%) | 0.340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Fan, K.; Zhou, S.; Wang, Y.; Tan, S.; Long, B.; Yu, S. Age-Stratified Analysis of the Clinical Efficacy of Subcutaneous Immunotherapy for Allergic Rhinitis in Chinese Patients. Biomedicines 2025, 13, 2831. https://doi.org/10.3390/biomedicines13112831
Jin L, Fan K, Zhou S, Wang Y, Tan S, Long B, Yu S. Age-Stratified Analysis of the Clinical Efficacy of Subcutaneous Immunotherapy for Allergic Rhinitis in Chinese Patients. Biomedicines. 2025; 13(11):2831. https://doi.org/10.3390/biomedicines13112831
Chicago/Turabian StyleJin, Ling, Kai Fan, Shican Zhou, Yang Wang, Shiwang Tan, Bojin Long, and Shaoqing Yu. 2025. "Age-Stratified Analysis of the Clinical Efficacy of Subcutaneous Immunotherapy for Allergic Rhinitis in Chinese Patients" Biomedicines 13, no. 11: 2831. https://doi.org/10.3390/biomedicines13112831
APA StyleJin, L., Fan, K., Zhou, S., Wang, Y., Tan, S., Long, B., & Yu, S. (2025). Age-Stratified Analysis of the Clinical Efficacy of Subcutaneous Immunotherapy for Allergic Rhinitis in Chinese Patients. Biomedicines, 13(11), 2831. https://doi.org/10.3390/biomedicines13112831







