Mass Spectrometry for Lysine Methylation: Principles, Progress, and Prospects
Abstract
1. Introduction
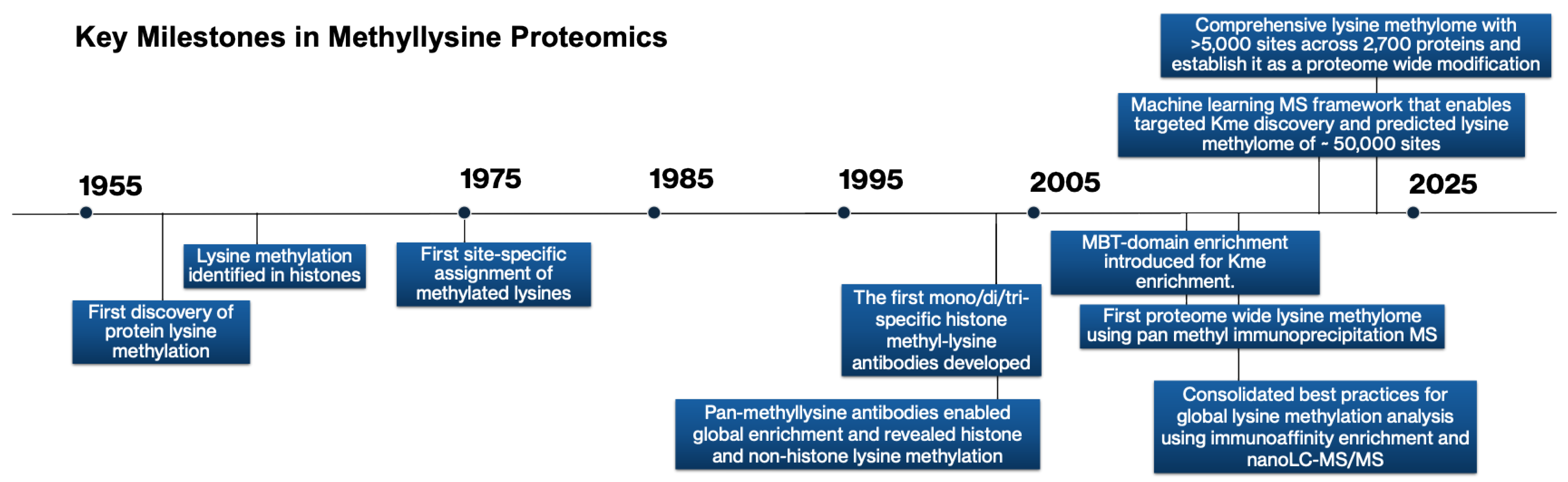
2. Foundations of Early Lysine Methylation Discovery
3. Instrumentation Advances in Lysine Methylation MS Workflows
3.1. Ionization Techniques
3.2. MS/MS Acquisition
3.3. High-Resolution Mass Analyzers
3.4. Fragmentation
3.5. Modern Data Acquisition and Ion Mobility Enhancements
4. Proteomic Workflows for Lysine Methylation: Bottom-Up to Top-Down
4.1. Bottom-Up Methyllysine Proteomics
4.2. Top-Down Methyllysine Proteomics
4.3. Middle-Down Methyllysine Proteomics
4.4. Emerging Hybrid and Next-Gen MS Workflows
5. Proteolytic Constraints in Bottom-Up Analysis of Methylated Peptides
6. Enrichment Strategies for Methylated Peptides
6.1. Biological Recognition-Based Enrichment
6.2. Affinity Chromatography
6.3. Chemical Derivatization
7. Quantitative Labeling Strategies for Lysine Methylation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, K.E.; Gozani, O. An Unexpected Journey: Lysine Methylation across the Proteome. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2014, 1839, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.M.; Moore, K.E.; Green, E.M.; Martín, G.M.; Gozani, O. Proteome-Wide Enrichment of Proteins Modified by Lysine Methylation. Nat. Protoc. 2014, 9, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.E.; Carlson, S.M.; Camp, N.D.; Cheung, P.; James, R.G.; Chua, K.F.; Wolf-Yadlin, A.; Gozani, O. A General Molecular Affinity Strategy for Global Detection and Proteomic Analysis of Lysine Methylation. Mol. Cell 2013, 50, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, K.; Ye, M. Strategies for Large-Scale Analysis of Non-Histone Protein Methylation by LC-MS/MS. Analyst 2017, 142, 3536–3548. [Google Scholar] [CrossRef]
- Bremang, M.; Cuomo, A.; Agresta, A.M.; Stugiewicz, M.; Spadotto, V.; Bonaldi, T. Mass Spectrometry-Based Identification and Characterisation of Lysine and Arginine Methylation in the Human Proteome. Mol. Biosyst. 2013, 9, 2231. [Google Scholar] [CrossRef]
- Afjehi-Sadat, L.; Garcia, B.A. Comprehending Dynamic Protein Methylation with Mass Spectrometry. Curr. Opin. Chem. Biol. 2013, 17, 12–19. [Google Scholar] [CrossRef]
- Berryhill, C.A.; Evans, T.N.; Doud, E.H.; Smith-Kinnaman, W.R.; Hanquier, J.N.; Mosley, A.L.; Cornett, E.M. Quantitative Analysis of Nonhistone Lysine Methylation Sites and Lysine Demethylases in Breast Cancer Cell Lines. J. Proteome Res. 2025, 24, 550–561. [Google Scholar] [CrossRef]
- Berryhill, C.A.; Hanquier, J.N.; Doud, E.H.; Cordeiro-Spinetti, E.; Dickson, B.M.; Rothbart, S.B.; Mosley, A.L.; Cornett, E.M. Global Lysine Methylome Profiling Using Systematically Characterized Affinity Reagents. Sci. Rep. 2023, 13, 377. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, Erasing and Reading Histone Lysine Methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef]
- Han, D.; Huang, M.; Wang, T.; Li, Z.; Chen, Y.; Liu, C.; Lei, Z.; Chu, X. Lysine Methylation of Transcription Factors in Cancer. Cell Death Dis. 2019, 10, 290. [Google Scholar] [CrossRef]
- Bhat, K.P.; Ümit Kaniskan, H.; Jin, J.; Gozani, O. Epigenetics and beyond: Targeting Writers of Protein Lysine Methylation to Treat Disease. Nat. Rev. Drug Discov. 2021, 20, 265–286. [Google Scholar] [CrossRef]
- Rowe, E.M.; Xing, V.; Biggar, K.K. Lysine Methylation: Implications in Neurodegenerative Disease. Brain Res. 2019, 1707, 164–171. [Google Scholar] [CrossRef]
- Husmann, D.; Gozani, O. Histone Lysine Methyltransferases in Biology and Disease. Nat. Struct. Mol. Biol. 2019, 26, 880–889. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subbanna, S. Histone Methylation Regulation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2021, 22, 4654. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, J.; Lei, Z.; Zhu, H.; Rao, D.; Wang, T.; Fu, C.; Zhang, Z.; Xia, L.; Huang, W. Lysine Methylation Modifications in Tumor Immunomodulation and Immunotherapy: Regulatory Mechanisms and Perspectives. Biomark. Res. 2024, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dent, S.Y.R. Histone Modifying Enzymes and Cancer: Going beyond Histones. J. Cell Biochem. 2005, 96, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Connolly, J.; Biggar, K.K. Beyond Histones—The Expanding Roles of Protein Lysine Methylation. FEBS J. 2017, 284, 2732–2744. [Google Scholar] [CrossRef]
- Plch, J.; Hrabeta, J.; Eckschlager, T. KDM5 Demethylases and Their Role in Cancer Cell Chemoresistance. Int. J. Cancer 2019, 144, 221–231. [Google Scholar] [CrossRef]
- Carlson, S.M.; Gozani, O. Nonhistone Lysine Methylation in the Regulation of Cancer Pathways. Cold Spring Harb. Perspect. Med. 2016, 6, a026435. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, J.Y.; Ha, S.J.; Yu, S.; Shin, J.K.; Kim, H.C. Proteome-Wide Identification of Arginine Methylation in Colorectal Cancer Tissues from Patients. Proteome Sci. 2020, 18, 6. [Google Scholar] [CrossRef]
- Serre, N.B.C.; Alban, C.; Bourguignon, J.; Ravanel, S. An Outlook on Lysine Methylation of Non-Histone Proteins in Plants. J. Exp. Bot. 2018, 69, 4569–4581. [Google Scholar] [CrossRef]
- Hart-Smith, G.; Yagoub, D.; Tay, A.P.; Pickford, R.; Wilkins, M.R. Large Scale Mass Spectrometry-Based Identifications of Enzyme-Mediated Protein Methylation Are Subject to High False Discovery Rates. Mol. Cell Proteomics 2016, 15, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, M.; Li, L.; Kaneko, T.; Voss, C.; Zhang, L.; Xia, J.; Li, S.S.C. Affinity Purification of Methyllysine Proteome by Site-Specific Covalent Conjugation. Anal. Chem. 2018, 90, 13876–13881. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Why Always Lysine? The Ongoing Tale of One of the Most Modified Amino Acids. Adv. Biol. Regul. 2016, 60, 144–150. [Google Scholar] [CrossRef]
- Cornett, E.M.; Ferry, L.; Defossez, P.-A.; Rothbart, S.B. Lysine Methylation Regulators Moonlighting Outside the Epigenome. Mol. Cell 2019, 75, 1092–1101. [Google Scholar] [CrossRef]
- Lanouette, S.; Mongeon, V.; Figeys, D.; Couture, J. The Functional Diversity of Protein Lysine Methylation. Mol. Syst. Biol. 2014, 10, 724. [Google Scholar] [CrossRef]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Snijders, A.P.L.; Hung, M.-L.; Wilson, S.A.; Dickman, M.J. Analysis of Arginine and Lysine Methylation Utilizing Peptide Separations at Neutral PH and Electron Transfer Dissociation Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2010, 21, 88–96. [Google Scholar] [CrossRef]
- Levy, D.; Liu, C.L.; Yang, Z.; Newman, A.M.; Alizadeh, A.A.; Utz, P.J.; Gozani, O. A Proteomic Approach for the Identification of Novel Lysine Methyltransferase Substrates. Epigenet. Chromatin 2011, 4, 19. [Google Scholar] [CrossRef]
- Erce, M.A.; Pang, C.N.I.; Hart-Smith, G.; Wilkins, M.R. The Methylproteome and the Intracellular Methylation Network. Proteomics 2012, 12, 564–586. [Google Scholar] [CrossRef]
- Petrossian, T.C.; Clarke, S.G. Uncovering the Human Methyltransferasome. Mol. Cell. Proteom. 2011, 10, M110.000976. [Google Scholar] [CrossRef]
- Nishikori, S.; Hattori, T.; Fuchs, S.M.; Yasui, N.; Wojcik, J.; Koide, A.; Strahl, B.D.; Koide, S. Broad Ranges of Affinity and Specificity of Anti-Histone Antibodies Revealed by a Quantitative Peptide Immunoprecipitation Assay. J. Mol. Biol. 2012, 424, 391–399. [Google Scholar] [CrossRef]
- Rothbart, S.B.; Dickson, B.M.; Raab, J.R.; Grzybowski, A.T.; Krajewski, K.; Guo, A.H.; Shanle, E.K.; Josefowicz, S.Z.; Fuchs, S.M.; Allis, C.D.; et al. An Interactive Database for the Assessment of Histone Antibody Specificity. Mol. Cell 2015, 59, 502–511. [Google Scholar] [CrossRef]
- Cornett, E.M.; Dickson, B.M.; Rothbart, S.B. Analysis of Histone Antibody Specificity with Peptide Microarrays. J. Vis. Exp. 2017, 126, 5512. [Google Scholar] [CrossRef]
- Jiang, Y.; Rex, D.A.B.; Schuster, D.; Neely, B.A.; Rosano, G.L.; Volkmar, N.; Momenzadeh, A.; Peters-Clarke, T.M.; Egbert, S.B.; Kreimer, S.; et al. Comprehensive Overview of Bottom-Up Proteomics Using Mass Spectrometry. ACS Meas. Sci. Au 2024, 4, 338–417. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.H.F.M.; Kubicek, S.; Mechtler, K.; O’Sullivan, R.J.; Derijck, A.A.H.A.; Perez-Burgos, L.; Kohlmaier, A.; Opravil, S.; Tachibana, M.; Shinkai, Y.; et al. Partitioning and Plasticity of Repressive Histone Methylation States in Mammalian Chromatin. Mol. Cell 2003, 12, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Challen, B.; Cramer, R. Advances in Ionisation Techniques for Mass Spectrometry-based Omics Research. Proteomics 2022, 22, 2100394. [Google Scholar] [CrossRef]
- Peters-Clarke, T.M.; Coon, J.J.; Riley, N.M. Instrumentation at the Leading Edge of Proteomics. Anal. Chem. 2024, 96, 7976–8010. [Google Scholar] [CrossRef]
- Ryan, D.J.; Spraggins, J.M.; Caprioli, R.M. Protein Identification Strategies in MALDI Imaging Mass Spectrometry: A Brief Review. Curr. Opin. Chem. Biol. 2019, 48, 64–72. [Google Scholar] [CrossRef]
- Larsen, M.R.; Trelle, M.B.; Thingholm, T.E.; Jensen, O.N. Analysis of Posttranslational Modifications of Proteins by Tandem Mass Spectrometry. Biotechniques 2006, 40, 790–798. [Google Scholar] [CrossRef]
- Han, X.; Aslanian, A.; Yates, J.R. Mass Spectrometry for Proteomics. Curr. Opin. Chem. Biol. 2008, 12, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.; Schurgers, L.; Jankowski, V. Identification and Characterization of Post-Translational Modifications: Clinical Implications. Mol. Aspects Med. 2022, 86, 101066. [Google Scholar] [CrossRef] [PubMed]
- Karpievitch, Y.V.; Polpitiya, A.D.; Anderson, G.A.; Smith, R.D.; Dabney, A.R. Liquid Chromatography Mass Spectrometry-Based Proteomics: Biological and Technological Aspects. Ann. Appl. Stat. 2010, 4, 1797–1823. [Google Scholar] [CrossRef]
- Virág, D.; Dalmadi-Kiss, B.; Vékey, K.; Drahos, L.; Klebovich, I.; Antal, I.; Ludányi, K. Current Trends in the Analysis of Post-Translational Modifications. Chromatographia 2020, 83, 1–10. [Google Scholar] [CrossRef]
- Banerjee, S.; Mazumdar, S. Electrospray Ionization Mass Spectrometry: A Technique to Access the Information beyond the Molecular Weight of the Analyte. Int. J. Anal. Chem. 2012, 2012, 1–40. [Google Scholar] [CrossRef]
- Darie-Ion, L.; Whitham, D.; Jayathirtha, M.; Rai, Y.; Neagu, A.-N.; Darie, C.C.; Petre, B.A. Applications of MALDI-MS/MS-Based Proteomics in Biomedical Research. Molecules 2022, 27, 6196. [Google Scholar] [CrossRef]
- Lubec, G.; Afjehi-Sadat, L. Limitations and Pitfalls in Protein Identification by Mass Spectrometry. Chem. Rev. 2007, 107, 3568–3584. [Google Scholar] [CrossRef]
- Ahrens, C.H.; Wade, J.T.; Champion, M.M.; Langer, J.D. A Practical Guide to Small Protein Discovery and Characterization Using Mass Spectrometry. J. Bacteriol. 2022, 204, e0035321. [Google Scholar] [CrossRef]
- Sousa, P.; Silva, L.; Luís, C.; Câmara, J.S.; Perestrelo, R. MALDI-TOF MS: A Promising Analytical Approach to Cancer Diagnostics and Monitoring. Separations 2023, 10, 453. [Google Scholar] [CrossRef]
- Dixit, S.M.; Polasky, D.A.; Ruotolo, B.T. Collision Induced Unfolding of Isolated Proteins in the Gas Phase: Past, Present, and Future. Curr. Opin. Chem. Biol. 2018, 42, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yau, P.M.; Chandrasekhar, B.; New, R.; Kondrat, R.; Imai, B.S.; Bradbury, M.E. Differentiation between Peptides Containing Acetylated or Tri-methylated Lysines by Mass Spectrometry: An Application for Determining Lysine 9 Acetylation and Methylation of Histone H3. Proteomics 2004, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shuken, S.R. An Introduction to Mass Spectrometry-Based Proteomics. J. Proteome Res. 2023, 22, 2151–2171. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef]
- Neagu, A.-N.; Jayathirtha, M.; Baxter, E.; Donnelly, M.; Petre, B.A.; Darie, C.C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27, 2411. [Google Scholar] [CrossRef]
- Wells, M.J.; McLuckey, S.A. Collision-Induced Dissociation (CID) of Peptides and Proteins. Methods Enzmol. 2005, 402, 148–185. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Zheng, Y.-F.; Wang, W.-C.; Toh, J.-T.; Hsu, Y.-M.; Chien, H.-J.; Chang, C.-J.; Lai, C.-C. Direct Identification of Intact Proteins Using a Low-Resolution Mass Spectrometer with CID. J. Am. Soc. Mass. Spectrom. 2024, 35, 1507–1515. [Google Scholar] [CrossRef]
- Hseiky, A.; Crespo, M.; Kieffer-Jaquinod, S.; Fenaille, F.; Pflieger, D. Small Mass but Strong Information: Diagnostic Ions Provide Crucial Clues to Correctly Identify Histone Lysine Modifications. Proteomes 2021, 9, 18. [Google Scholar] [CrossRef]
- Ma, X. Recent Advances in Mass Spectrometry-Based Structural Elucidation Techniques. Molecules 2022, 27, 6466. [Google Scholar] [CrossRef]
- Abdelhameed, A.S.; Kadi, A.A.; Abdel-Aziz, H.A.; Angawi, R.F.; Attwa, M.W.; Al-Rashood, K.A. Multistage Fragmentation of Ion Trap Mass Spectrometry System and Pseudo-MS3 of Triple Quadrupole Mass Spectrometry Characterize Certain (E)-3-(Dimethylamino)-1-Arylprop-2-En-1-Ones: A Comparative Study. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Makarov, A.; Denisov, E.; Kholomeev, A.; Balschun, W.; Lange, O.; Strupat, K.; Horning, S. Performance Evaluation of a Hybrid Linear Ion Trap/Orbitrap Mass Spectrometer. Anal. Chem. 2006, 78, 2113–2120. [Google Scholar] [CrossRef]
- May, J.C.; McLean, J.A. Ion Mobility-Mass Spectrometry: Time-Dispersive Instrumentation. Anal. Chem. 2015, 87, 1422–1436. [Google Scholar] [CrossRef]
- Yang, L.; Gu, Y.; Gong, H.; Liu, Z.; Xiong, X.; Fang, X. Development and Research of the Quadrupole Mass Spectrometry Simulation Model with the Entire Ion Optics System. Sci. Rep. 2025, 15, 7510. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Lee, K.; Jang, K.-S.; Kim, Y.-G.; Park, S.-H.; Lee, C.-S.; Kim, B.-G. Low Mass Cutoff Evasion with Qz Value Optimization in Ion Trap. Anal. Biochem. 2009, 387, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.; Chen, A.; Schubmehl, M.; Kolonko, K.J.; Barnes, G.L. Exploring the Effects of Methylation on the CID of Protonated Lysine: A Combined Experimental and Computational Approach. J. Am. Soc. Mass. Spectrom. 2021, 32, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Farhi, J.; Emenike, B.; Lee, R.S.; Sad, K.; Fawwal, D.V.; Beusch, C.M.; Jones, R.B.; Verma, A.K.; Jones, C.Y.; Foroozani, M.; et al. Dynamic In Vivo Mapping of the Methylproteome Using a Chemoenzymatic Approach. J. Am. Chem. Soc. 2025, 147, 7214–7230. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.L.; Kolonko, K.J.; Lucas, K.; Chen, A.; Schubmehl, M. Comparing the Collision-Induced Dissociation of Trimethyl Lysine+ and Acetyl Lysine-H+. Chem. Phys. Lett. 2023, 833, 140907. [Google Scholar] [CrossRef]
- Olsen, J.V.; Macek, B.; Lange, O.; Makarov, A.; Horning, S.; Mann, M. Higher-Energy C-Trap Dissociation for Peptide Modification Analysis. Nat. Methods 2007, 4, 709–712. [Google Scholar] [CrossRef]
- Silzel, J.W.; Julian, R.R. RDD-HCD Provides Variable Fragmentation Routes Dictated by Radical Stability. J. Am. Soc. Mass. Spectrom. 2023, 34, 452–458. [Google Scholar] [CrossRef]
- Révész, Á.; Hevér, H.; Steckel, A.; Schlosser, G.; Szabó, D.; Vékey, K.; Drahos, L. Collision Energies: Optimization Strategies for Bottom-up Proteomics. Mass. Spectrom. Rev. 2023, 42, 1261–1299. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, J.K.; Pinto, A.F.M.; Yates, J.R. Energy Dependence of HCD on Peptide Fragmentation: Stepped Collisional Energy Finds the Sweet Spot. J. Am. Soc. Mass. Spectrom. 2013, 24, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Riley, N.M.; Coon, J.J. The Role of Electron Transfer Dissociation in Modern Proteomics. Anal. Chem. 2018, 90, 40–64. [Google Scholar] [CrossRef] [PubMed]
- Lermyte, F.; Valkenborg, D.; Loo, J.A.; Sobott, F. Radical Solutions: Principles and Application of Electron-Based Dissociation in Mass Spectrometry-Based Analysis of Protein Structure. Mass. Spectrom. Rev. 2018, 37, 750–771. [Google Scholar] [CrossRef]
- Jeanne Dit Fouque, K.; Miller, S.A.; Pham, K.; Bhanu, N.V.; Cintron-Diaz, Y.L.; Leyva, D.; Kaplan, D.; Voinov, V.G.; Ridgeway, M.E.; Park, M.A.; et al. Top-“Double-Down” Mass Spectrometry of Histone H4 Proteoforms: Tandem Ultraviolet-Photon and Mobility/Mass-Selected Electron Capture Dissociations. Anal. Chem. 2022, 94, 15377–15385. [Google Scholar] [CrossRef]
- Jeanne Dit Fouque, K.; Kaplan, D.; Voinov, V.G.; Holck, F.H.V.; Jensen, O.N.; Fernandez-Lima, F. Proteoform Differentiation Using Tandem Trapped Ion Mobility, Electron Capture Dissociation, and ToF Mass Spectrometry. Anal. Chem. 2021, 93, 9575–9582. [Google Scholar] [CrossRef]
- Voinov, V.G.; Deinzer, M.L.; Beckman, J.S.; Barofsky, D.F. Electron Capture, Collision-Induced, and Electron Capture-Collision Induced Dissociation in Q-TOF. J. Am. Soc. Mass. Spectrom. 2011, 22, 607–611. [Google Scholar] [CrossRef]
- Voinov, V.G.; Hoffman, P.D.; Bennett, S.E.; Beckman, J.S.; Barofsky, D.F. Electron Capture Dissociation of Sodium-Adducted Peptides on a Modified Quadrupole/Time-of-Flight Mass Spectrometer. J. Am. Soc. Mass. Spectrom. 2015, 26, 2096–2104. [Google Scholar] [CrossRef]
- Kim, M.; Pandey, A. Electron Transfer Dissociation Mass Spectrometry in Proteomics. Proteomics 2012, 12, 530–542. [Google Scholar] [CrossRef]
- Mikesh, L.M.; Ueberheide, B.; Chi, A.; Coon, J.J.; Syka, J.E.P.; Shabanowitz, J.; Hunt, D.F. The Utility of ETD Mass Spectrometry in Proteomic Analysis. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2006, 1764, 1811–1822. [Google Scholar] [CrossRef]
- Syka, J.E.P.; Coon, J.J.; Schroeder, M.J.; Shabanowitz, J.; Hunt, D.F. Peptide and Protein Sequence Analysis by Electron Transfer Dissociation Mass Spectrometry. Proc. Natl. Acad. Sci. USA 2004, 101, 9528–9533. [Google Scholar] [CrossRef] [PubMed]
- Greer, S.M.; Sidoli, S.; Coradin, M.; Schack Jespersen, M.; Schwämmle, V.; Jensen, O.N.; Garcia, B.A.; Brodbelt, J.S. Extensive Characterization of Heavily Modified Histone Tails by 193 Nm Ultraviolet Photodissociation Mass Spectrometry via a Middle–Down Strategy. Anal. Chem. 2018, 90, 10425–10433. [Google Scholar] [CrossRef] [PubMed]
- Riley, N.M.; Westphall, M.S.; Coon, J.J. Activated Ion-Electron Transfer Dissociation Enables Comprehensive Top-Down Protein Fragmentation. J. Proteome Res. 2017, 16, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, S.J.; Chrisman, P.A.; McLuckey, S.A. Electron-Transfer Ion/Ion Reactions of Doubly Protonated Peptides: Effect of Elevated Bath Gas Temperature. Anal. Chem. 2005, 77, 5662–5669. [Google Scholar] [CrossRef]
- Ko, B.J.; Brodbelt, J.S. Enhanced Electron Transfer Dissociation of Peptides Modified at C-Terminus with Fixed Charges. J. Am. Soc. Mass. Spectrom. 2012, 23, 1991–2000. [Google Scholar] [CrossRef]
- Li, M.; Zhong, X.; Feng, Y.; Li, L. Novel Isobaric Tagging Reagent Enabled Multiplex Quantitative Glycoproteomics via Electron-Transfer/Higher-Energy Collisional Dissociation (EThcD) Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2022, 33, 1874–1882. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, X.; Feng, Y.; Kent, K.C.; Li, L. Improving Data Quality and Preserving HCD-Generated Reporter Ions with EThcD for Isobaric Tag-Based Quantitative Proteomics and Proteome-Wide PTM Studies. Anal. Chim. Acta 2017, 968, 40–49. [Google Scholar] [CrossRef]
- Liao, R.; Zheng, D.; Nie, A.; Zhou, S.; Deng, H.; Gao, Y.; Yang, P.; Yu, Y.; Tan, L.; Qi, W.; et al. Sensitive and Precise Characterization of Combinatorial Histone Modifications by Selective Derivatization Coupled with RPLC-EThcD-MS/MS. J. Proteome Res. 2017, 16, 780–787. [Google Scholar] [CrossRef]
- Ma, F.; Sun, R.; Tremmel, D.M.; Sackett, S.D.; Odorico, J.; Li, L. Large-Scale Differentiation and Site Specific Discrimination of Hydroxyproline Isomers by Electron Transfer/Higher-Energy Collision Dissociation (EThcD) Mass Spectrometry. Anal. Chem. 2018, 90, 5857–5864. [Google Scholar] [CrossRef]
- Kessler, A.L.; Fort, K.L.; Resemann, H.C.; Krüger, P.; Wang, C.; Koch, H.; Hauschild, J.-P.; Marino, F.; Heck, A.J.R. Increased EThcD Efficiency on the Hybrid Orbitrap Excedion Pro Mass Analyzer Extends the Depth in Identification and Sequence Coverage of HLA Class I Immunopeptidomes. Mol. Cell. Proteom. 2025, 24, 101049. [Google Scholar] [CrossRef]
- Frese, C.K.; Altelaar, A.F.M.; van den Toorn, H.; Nolting, D.; Griep-Raming, J.; Heck, A.J.R.; Mohammed, S. Toward Full Peptide Sequence Coverage by Dual Fragmentation Combining Electron-Transfer and Higher-Energy Collision Dissociation Tandem Mass Spectrometry. Anal. Chem. 2012, 84, 9668–9673. [Google Scholar] [CrossRef]
- Brodbelt, J.S.; Morrison, L.J.; Santos, I. Ultraviolet Photodissociation Mass Spectrometry for Analysis of Biological Molecules. Chem. Rev. 2020, 120, 3328–3380. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.R. The Mechanism Behind Top-Down UVPD Experiments: Making Sense of Apparent Contradictions. J. Am. Soc. Mass. Spectrom. 2017, 28, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Sipe, S.N.; Brodbelt, J.S. Impact of Charge State on 193 Nm Ultraviolet Photodissociation of Protein Complexes. Phys. Chem. Chem. Phys. 2019, 21, 9265–9276. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.D.; Greer, S.M.; Brodbelt, J.S. Integrating Carbamylation and Ultraviolet Photodissociation Mass Spectrometry for Middle-Down Proteomics. Anal. Chem. 2017, 89, 11772–11778. [Google Scholar] [CrossRef]
- Cotham, V.C.; Horton, A.P.; Lee, J.; Georgiou, G.; Brodbelt, J.S. Middle-Down 193-Nm Ultraviolet Photodissociation for Unambiguous Antibody Identification and Its Implications for Immunoproteomic Analysis. Anal. Chem. 2017, 89, 6498–6504. [Google Scholar] [CrossRef]
- Hellinger, J.; Brodbelt, J.S. Impact of Charge State on Characterization of Large Middle-Down Sized Peptides by Tandem Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2024, 35, 1647–1656. [Google Scholar] [CrossRef]
- Fornelli, L.; Srzentić, K.; Toby, T.K.; Doubleday, P.F.; Huguet, R.; Mullen, C.; Melani, R.D.; dos Santos Seckler, H.; DeHart, C.J.; Weisbrod, C.R.; et al. Thorough Performance Evaluation of 213 Nm Ultraviolet Photodissociation for Top-down Proteomics. Mol. Cell. Proteom. 2020, 19, 405–420. [Google Scholar] [CrossRef]
- Picotti, P.; Aebersold, R. Selected Reaction Monitoring–Based Proteomics: Workflows, Potential, Pitfalls and Future Directions. Nat. Methods 2012, 9, 555–566. [Google Scholar] [CrossRef]
- Hartel, N.G.; Chew, B.; Qin, J.; Xu, J.; Graham, N.A. Deep Protein Methylation Profiling by Combined Chemical and Immunoaffinity Approaches Reveals Novel PRMT1 Targets. Mol. Cell. Proteom. 2019, 18, 2149–2164. [Google Scholar] [CrossRef]
- Müller, F.; Kolbowski, L.; Bernhardt, O.M.; Reiter, L.; Rappsilber, J. Data-Independent Acquisition Improves Quantitative Cross-Linking Mass Spectrometry. Mol. Cell. Proteom. 2019, 18, 786–795. [Google Scholar] [CrossRef]
- Doerr, A. DIA Mass Spectrometry. Nat. Methods 2015, 12, 35. [Google Scholar] [CrossRef]
- Lou, R.; Shui, W. Acquisition and Analysis of DIA-Based Proteomic Data: A Comprehensive Survey in 2023. Mol. Cell. Proteom. 2024, 23, 100712. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-Independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell. Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, L. Data-independent Acquisition Proteomics Methods for Analyzing Post-translational Modifications. Proteomics 2023, 23, 2200046. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, K.; Fahrner, M.; Brombacher, E.; Seredynska, A.; Maldacker, M.; Kreutz, C.; Schmidt, A.; Schilling, O. Data-Independent Acquisition: A Milestone and Prospect in Clinical Mass Spectrometry-Based Proteomics. Mol. Cell. Proteom. 2024, 23, 100800. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Reiter, L.; Denu, J.M.; Dowell, J.A. Quantification of SAHA-Dependent Changes in Histone Modifications Using Data-Independent Acquisition Mass Spectrometry. J. Proteome Res. 2015, 14, 3252–3262. [Google Scholar] [CrossRef]
- Sidoli, S.; Lin, S.; Xiong, L.; Bhanu, N.V.; Karch, K.R.; Johansen, E.; Hunter, C.; Mollah, S.; Garcia, B.A. Sequential Window Acquisition of All Theoretical Mass Spectra (SWATH) Analysis for Characterization and Quantification of Histone Post-Translational Modifications. Mol. Cell. Proteom. 2015, 14, 2420–2428. [Google Scholar] [CrossRef]
- Robinson, A.E.; Binek, A.; Venkatraman, V.; Searle, B.C.; Holewinski, R.J.; Rosenberger, G.; Parker, S.J.; Basisty, N.; Xie, X.; Lund, P.J.; et al. Lysine and Arginine Protein Post-Translational Modifications by Enhanced DIA Libraries: Quantification in Murine Liver Disease. J. Proteome Res. 2020, 19, 4163–4178. [Google Scholar] [CrossRef]
- Shi, T.; Su, D.; Liu, T.; Tang, K.; Camp, D.G.; Qian, W.; Smith, R.D. Advancing the Sensitivity of Selected Reaction Monitoring-based Targeted Quantitative Proteomics. Proteomics 2012, 12, 1074–1092. [Google Scholar] [CrossRef]
- Gillette, M.A.; Carr, S.A. Quantitative Analysis of Peptides and Proteins in Biomedicine by Targeted Mass Spectrometry. Nat. Methods 2013, 10, 28–34. [Google Scholar] [CrossRef]
- Peterson, A.C.; Russell, J.D.; Bailey, D.J.; Westphall, M.S.; Coon, J.J. Parallel Reaction Monitoring for High Resolution and High Mass Accuracy Quantitative, Targeted Proteomics. Mol. Cell. Proteom. 2012, 11, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Charih, F.; Liu, H.; Ruiz-Blanco, Y.B.; Stalker, L.; Chopra, A.; Connolly, J.; Adhikary, H.; Frensemier, K.; Hoekstra, M.; et al. Proteome-Wide Prediction of Lysine Methylation Leads to Identification of H2BK43 Methylation and Outlines the Potential Methyllysine Proteome. Cell Rep. 2020, 32, 107896. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; Ridgeway, N.H.; Biggar, K.K. Characterization of KDM5 Lysine Demethylase Family Substrate Preference and Identification of Novel Substrates. J. Biochem. 2023, 173, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Brunner, A.-D.; Koch, S.; Koch, H.; Lubeck, M.; Krause, M.; Goedecke, N.; Decker, J.; Kosinski, T.; Park, M.A.; et al. Online Parallel Accumulation–Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol. Cell. Proteom. 2018, 17, 2534–2545. [Google Scholar] [CrossRef]
- Fuller, C.N.; Valadares Tose, L.; Vitorino, F.N.L.; Bhanu, N.V.; Panczyk, E.M.; Park, M.A.; Garcia, B.A.; Fernandez-Lima, F. Bottom-up Histone Post-Translational Modification Analysis Using Liquid Chromatography, Trapped Ion Mobility Spectrometry, and Tandem Mass Spectrometry. J. Proteome Res. 2024, 23, 3867–3876. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Martínez-Val, A.; Steigerwald, S.; Rüther, P.; Fort, K.L.; Arrey, T.N.; Harder, A.; Makarov, A.; Olsen, J.V. A Compact Quadrupole-Orbitrap Mass Spectrometer with FAIMS Interface Improves Proteome Coverage in Short LC Gradients. Mol. Cell. Proteom. 2020, 19, 716–729. [Google Scholar] [CrossRef]
- Hebert, A.S.; Prasad, S.; Belford, M.W.; Bailey, D.J.; McAlister, G.C.; Abbatiello, S.E.; Huguet, R.; Wouters, E.R.; Dunyach, J.-J.; Brademan, D.R.; et al. Comprehensive Single-Shot Proteomics with FAIMS on a Hybrid Orbitrap Mass Spectrometer. Anal. Chem. 2018, 90, 9529–9537. [Google Scholar] [CrossRef]
- Deng, W.; Sha, J.; Xue, F.; Jami-Alahmadi, Y.; Plath, K.; Wohlschlegel, J. High-Field Asymmetric Waveform Ion Mobility Spectrometry Interface Enhances Parallel Reaction Monitoring on an Orbitrap Mass Spectrometer. Anal. Chem. 2022, 94, 15939–15947. [Google Scholar] [CrossRef]
- Shvartsburg, A.A.; Zheng, Y.; Smith, R.D.; Kelleher, N.L. Ion Mobility Separation of Variant Histone Tails Extending to the “Middle-down” Range. Anal. Chem. 2012, 84, 4271–4276. [Google Scholar] [CrossRef]
- Shvartsburg, A.A.; Zheng, Y.; Smith, R.D.; Kelleher, N.L. Separation of Variant Methylated Histone Tails by Differential Ion Mobility. Anal. Chem. 2012, 84, 6317–6320. [Google Scholar] [CrossRef]
- Swearingen, K.E.; Moritz, R.L. High-Field Asymmetric Waveform Ion Mobility Spectrometry for Mass Spectrometry-Based Proteomics. Expert Rev. Proteom. 2012, 9, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Cesare, J.; Rekowski, M.; Clark, Z.; Thornton, J.; Washburn, M. Analysis of FAIMS for the Study of Affinity-Purified Protein Complexes Using the Orbitrap Ascend Tribrid Mass Spectrometer. Mol. Omics 2025, 21, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Sidoli, S.; Bhanu, N.V.; Karch, K.R.; Wang, X.; Garcia, B.A. Complete Workflow for Analysis of Histone Post-Translational Modifications Using Bottom-up Mass Spectrometry: From Histone Extraction to Data Analysis. J. Vis. Exp. 2016, 111, 54112. [Google Scholar] [CrossRef]
- Duong, V.-A.; Lee, H. Bottom-Up Proteomics: Advancements in Sample Preparation. Int. J. Mol. Sci. 2023, 24, 5350. [Google Scholar] [CrossRef]
- Dupree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of This Field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef]
- Brandi, J.; Noberini, R.; Bonaldi, T.; Cecconi, D. Advances in Enrichment Methods for Mass Spectrometry-Based Proteomics Analysis of Post-Translational Modifications. J. Chromatogr. A 2022, 1678, 463352. [Google Scholar] [CrossRef]
- Dau, T.; Bartolomucci, G.; Rappsilber, J. Proteomics Using Protease Alternatives to Trypsin Benefits from Sequential Digestion with Trypsin. Anal. Chem. 2020, 92, 9523–9527. [Google Scholar] [CrossRef]
- Chick, J.M.; Kolippakkam, D.; Nusinow, D.P.; Zhai, B.; Rad, R.; Huttlin, E.L.; Gygi, S.P. A Mass-Tolerant Database Search Identifies a Large Proportion of Unassigned Spectra in Shotgun Proteomics as Modified Peptides. Nat. Biotechnol. 2015, 33, 743–749. [Google Scholar] [CrossRef]
- Wu, S.; Lourette, N.M.; Tolić, N.; Zhao, R.; Robinson, E.W.; Tolmachev, A.V.; Smith, R.D.; Paša-Tolić, L. An Integrated Top-Down and Bottom-Up Strategy for Broadly Characterizing Protein Isoforms and Modifications. J. Proteome Res. 2009, 8, 1347–1357. [Google Scholar] [CrossRef]
- Chen, W.; Ding, Z.; Zang, Y.; Liu, X. Characterization of Proteoform Post-Translational Modifications by Top-Down and Bottom-Up Mass Spectrometry in Conjunction with Annotations. J. Proteome Res. 2023, 22, 3178–3189. [Google Scholar] [CrossRef]
- Chen, B.; Brown, K.A.; Lin, Z.; Ge, Y. Top-Down Proteomics: Ready for Prime Time? Anal. Chem. 2018, 90, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Scotcher, J.; Wu, S.; Chu, R.K.; Tolić, N.; Ntai, I.; Thomas, P.M.; Fellers, R.T.; Early, B.P.; Zheng, Y.; et al. The First Pilot Project of the Consortium for Top-down Proteomics: A Status Report. Proteomics 2014, 14, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.S.; Loo, J.A.; Tsybin, Y.O.; Liu, X.; Wu, S.; Chamot-Rooke, J.; Agar, J.N.; Paša-Tolić, L.; Smith, L.M.; Ge, Y. Top-down Proteomics. Nat. Rev. Methods Primers 2024, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Cristobal, A.; Marino, F.; Post, H.; van den Toorn, H.W.P.; Mohammed, S.; Heck, A.J.R. Toward an Optimized Workflow for Middle-Down Proteomics. Anal. Chem. 2017, 89, 3318–3325. [Google Scholar] [CrossRef]
- Sidoli, S.; Garcia, B.A. Middle-down Proteomics: A Still Unexploited Resource for Chromatin Biology. Expert Rev. Proteom. 2017, 14, 617–626. [Google Scholar] [CrossRef]
- Wu, C.; Tran, J.C.; Zamdborg, L.; Durbin, K.R.; Li, M.; Ahlf, D.R.; Early, B.P.; Thomas, P.M.; Sweedler, J.V.; Kelleher, N.L. A Protease for “middle-down” Proteomics. Nat. Methods 2012, 9, 822–824. [Google Scholar] [CrossRef]
- Kundinger, S.R.; Bishof, I.; Dammer, E.B.; Duong, D.M.; Seyfried, N.T. Middle-Down Proteomics Reveals Dense Sites of Methylation and Phosphorylation in Arginine-Rich RNA-Binding Proteins. J. Proteome Res. 2020, 19, 1574–1591. [Google Scholar] [CrossRef]
- Moradian, A.; Kalli, A.; Sweredoski, M.J.; Hess, S. The Top-down, Middle-down, and Bottom-up Mass Spectrometry Approaches for Characterization of Histone Variants and Their Post-translational Modifications. Proteomics 2014, 14, 489–497. [Google Scholar] [CrossRef]
- Coradin, M.; Mendoza, M.R.; Sidoli, S.; Alpert, A.J.; Lu, C.; Garcia, B.A. Bullet Points to Evaluate the Performance of the Middle-down Proteomics Workflow for Histone Modification Analysis. Methods 2020, 184, 86–92. [Google Scholar] [CrossRef]
- Agarwal, A.; Diedrich, J.K.; Julian, R.R. Direct Elucidation of Disulfide Bond Partners Using Ultraviolet Photodissociation Mass Spectrometry. Anal. Chem. 2011, 83, 6455–6458. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.; Talbert, L.E.; Akkawi, N.; Julian, R.R. Simplified Identification of Disulfide, Trisulfide, and Thioether Pairs with 213 Nm UVPD. Analyst 2018, 143, 5176–5184. [Google Scholar] [CrossRef]
- Cleland, T.P.; DeHart, C.J.; Fellers, R.T.; VanNispen, A.J.; Greer, J.B.; LeDuc, R.D.; Parker, W.R.; Thomas, P.M.; Kelleher, N.L.; Brodbelt, J.S. High-Throughput Analysis of Intact Human Proteins Using UVPD and HCD on an Orbitrap Mass Spectrometer. J. Proteome Res. 2017, 16, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Jeanne Dit Fouque, K.; Ridgeway, M.E.; Park, M.A.; Fernandez-Lima, F. Trapped Ion Mobility Spectrometry, Ultraviolet Photodissociation, and Time-of-Flight Mass Spectrometry for Gas-Phase Peptide Isobars/Isomers/Conformers Discrimination. J. Am. Soc. Mass. Spectrom. 2022, 33, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Jeanne Dit Fouque, K.; Hard, E.R.; Balana, A.T.; Kaplan, D.; Voinov, V.G.; Ridgeway, M.E.; Park, M.A.; Anderson, G.A.; Pratt, M.R.; et al. Top/Middle-Down Characterization of α-Synuclein Glycoforms. Anal. Chem. 2023, 95, 18039–18045. [Google Scholar] [CrossRef]
- Nagy, K.; Sándor, P.; Vékey, K.; Drahos, L.; Révész, Á. The Enzyme Effect: Broadening the Horizon of MS Optimization to Nontryptic Digestion in Proteomics. J. Am. Soc. Mass. Spectrom. 2025, 36, 299–308. [Google Scholar] [CrossRef]
- Vandermarliere, E.; Mueller, M.; Martens, L. Getting Intimate with Trypsin, the Leading Protease in Proteomics. Mass. Spectrom. Rev. 2013, 32, 453–465. [Google Scholar] [CrossRef]
- Danko, K.; Lukasheva, E.; Zhukov, V.A.; Zgoda, V.; Frolov, A. Detergent-Assisted Protein Digestion-On the Way to Avoid the Key Bottleneck of Shotgun Bottom-Up Proteomics. Int. J. Mol. Sci. 2022, 23, 13903. [Google Scholar] [CrossRef]
- Lund, P.J.; Lehman, S.M.; Garcia, B.A. Quantitative Analysis of Global Protein Lysine Methylation by Mass Spectrometry. Methods Enzymol. 2019, 626, 475–498. [Google Scholar] [CrossRef]
- Miller, R.M.; Smith, L.M. Overview and Considerations in Bottom-up Proteomics. Analyst 2023, 148, 475–486. [Google Scholar] [CrossRef]
- Tsiatsiani, L.; Heck, A.J.R. Proteomics beyond Trypsin. FEBS J. 2015, 282, 2612–2626. [Google Scholar] [CrossRef]
- Garcia, B.A.; Mollah, S.; Ueberheide, B.M.; Busby, S.A.; Muratore, T.L.; Shabanowitz, J.; Hunt, D.F. Chemical Derivatization of Histones for Facilitated Analysis by Mass Spectrometry. Nat. Protoc. 2007, 2, 933–938. [Google Scholar] [CrossRef]
- Karch, K.R.; Sidoli, S.; Garcia, B.A. Identification and Quantification of Histone PTMs Using High-Resolution Mass Spectrometry. Methods Enzymol. 2016, 574, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Plazas-Mayorca, M.D.; Zee, B.M.; Young, N.L.; Fingerman, I.M.; LeRoy, G.; Briggs, S.D.; Garcia, B.A. One-Pot Shotgun Quantitative Mass Spectrometry Characterization of Histones. J. Proteome Res. 2009, 8, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Searfoss, R.M.; Karki, R.; Lin, Z.; Robison, F.; Garcia, B.A. An Optimized and High-Throughput Method for Histone Propionylation and Data-Independent Acquisition Analysis for the Identification and Quantification of Histone Post-Translational Modifications. J. Am. Soc. Mass. Spectrom. 2023, 34, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rojas, M.; Fuller, C.N.; Valadares Tose, L.; Willetts, M.; Park, M.A.; Bhanu, N.V.; Garcia, B.A.; Fernandez-Lima, F. Histone Modification Screening Using Liquid Chromatography, Trapped Ion Mobility Spectrometry, and Time-Of-Flight Mass Spectrometry. J. Vis. Exp. 2024, 203, 65589. [Google Scholar] [CrossRef]
- Giansanti, P.; Tsiatsiani, L.; Low, T.Y.; Heck, A.J.R. Six Alternative Proteases for Mass Spectrometry–Based Proteomics beyond Trypsin. Nat. Protoc. 2016, 11, 993–1006. [Google Scholar] [CrossRef]
- Yan, L.; Zheng, M.; Fan, M.; Yao, R.; Zou, K.; Feng, S.; Wu, M. A Chemoselective Enrichment Strategy for In-Depth Coverage of the Methyllysine Proteome. Angew. Chem. Int. Ed. 2024, 63, e202408564. [Google Scholar] [CrossRef]
- Emenike, B.; Czabala, P.; Farhi, J.; Swaminathan, J.; Anslyn, E.V.; Spangle, J.; Raj, M. Tertiary Amine Coupling by Oxidation for Selective Labeling of Dimethyl Lysine Post-Translational Modifications. J. Am. Chem. Soc. 2024, 146, 10621–10631. [Google Scholar] [CrossRef]
- Wang, K.; Dong, M.; Mao, J.; Wang, Y.; Jin, Y.; Ye, M.; Zou, H. Antibody-Free Approach for the Global Analysis of Protein Methylation. Anal. Chem. 2016, 88, 11319–11327. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, R. Decoding the Protein Methylome: Identification, Validation, and Functional Insights. Bioorg. Med. Chem. 2025, 118, 118056. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Galka, M.; Mori, E.; Liu, X.; Lin, Y.-F.; Wei, R.; Pittock, P.; Voss, C.; Dhami, G.; Li, X.; et al. A Method for Systematic Mapping of Protein Lysine Methylation Identifies Functions for HP1β in DNA Damage Response. Mol. Cell 2013, 50, 723–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, R.; Zacharias, L.; Wooding, K.M.; Peng, W.; Mechref, Y. Glycoprotein Enrichment Analytical Techniques. Methods Enzymol. 2017, 585, 397–429. [Google Scholar] [CrossRef]
- Gaurav, N.; Kutateladze, T.G. Non-Histone Binding Functions of PHD Fingers. Trends Biochem. Sci. 2023, 48, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, T.; Geoghegan, V.L.; Thomas, B.; Ridlova, G.; Trudgian, D.C.; Acuto, O. A Method for Large-Scale Identification of Protein Arginine Methylation. Mol. Cell. Proteom. 2012, 11, 1489–1499. [Google Scholar] [CrossRef]
- Ning, Z.; Star, A.T.; Mierzwa, A.; Lanouette, S.; Mayne, J.; Couture, J.-F.; Figeys, D. A Charge-Suppressing Strategy for Probing Protein Methylation. Chem. Commun. 2016, 52, 5474–5477. [Google Scholar] [CrossRef]
- Guo, H.; Wang, R.; Zheng, W.; Chen, Y.; Blum, G.; Deng, H.; Luo, M. Profiling Substrates of Protein Arginine N-Methyltransferase 3 with S-Adenosyl-l-Methionine Analogues. ACS Chem. Biol. 2014, 9, 476–484. [Google Scholar] [CrossRef]
- Olsen, J.B.; Cao, X.-J.; Han, B.; Chen, L.H.; Horvath, A.; Richardson, T.I.; Campbell, R.M.; Garcia, B.A.; Nguyen, H. Quantitative Profiling of the Activity of Protein Lysine Methyltransferase SMYD2 Using SILAC-Based Proteomics. Mol. Cell. Proteom. 2016, 15, 892–905. [Google Scholar] [CrossRef]
- Ong, S.-E.; Mittler, G.; Mann, M. Identifying and Quantifying in Vivo Methylation Sites by Heavy Methyl SILAC. Nat. Methods 2004, 1, 119–126. [Google Scholar] [CrossRef]
- Ong, S.; Mann, M. Identifying and Quantifying Sites of Protein Methylation by Heavy Methyl SILAC. Curr. Protoc. Protein Sci. 2006, 46, 119–126. [Google Scholar] [CrossRef]
- Ong, S.-E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Geoghegan, V.; Guo, A.; Trudgian, D.; Thomas, B.; Acuto, O. Comprehensive Identification of Arginine Methylation in Primary T Cells Reveals Regulatory Roles in Cell Signalling. Nat. Commun. 2015, 6, 6758. [Google Scholar] [CrossRef]
- Clarke, S.G. The Ribosome: A Hot Spot for the Identification of New Types of Protein Methyltransferases. J. Biol. Chem. 2018, 293, 10438–10446. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Schäfer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Hamon, C. Tandem Mass Tags: A Novel Quantification Strategy for Comparative Analysis of Complex Protein Mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed Protein Quantitation in Saccharomyces Cerevisiae Using Amine-Reactive Isobaric Tagging Reagents. Mol. Cell. Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.; Satpathy, S.; Kanashova, T.; Avanessian, S.C.; Kane, M.H.; Clauser, K.R.; Mertins, P.; Carr, S.A.; Kuster, B. TMT Labeling for the Masses: A Robust and Cost-Efficient, In-Solution Labeling Approach. Mol. Cell. Proteom. 2019, 18, 1468–1478. [Google Scholar] [CrossRef]
- McAlister, G.C.; Nusinow, D.P.; Jedrychowski, M.P.; Wühr, M.; Huttlin, E.L.; Erickson, B.K.; Rad, R.; Haas, W.; Gygi, S.P. MultiNotch MS3 Enables Accurate, Sensitive, and Multiplexed Detection of Differential Expression across Cancer Cell Line Proteomes. Anal. Chem. 2014, 86, 7150–7158. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, X.; Kelleher, N.L. Epiproteomics: Quantitative Analysis of Histone Marks and Codes by Mass Spectrometry. Curr. Opin. Chem. Biol. 2016, 33, 142–150. [Google Scholar] [CrossRef]
- Wang, D.; Ma, M.; Huang, J.; Gu, T.-J.; Cui, Y.; Li, M.; Wang, Z.; Zetterberg, H.; Li, L. Boost-DiLeu: Enhanced Isobaric N,N-Dimethyl Leucine Tagging Strategy for a Comprehensive Quantitative Glycoproteomic Analysis. Anal. Chem. 2022, 94, 11773–11782. [Google Scholar] [CrossRef]
- Peck Justice, S.A.; McCracken, N.A.; Victorino, J.F.; Qi, G.D.; Wijeratne, A.B.; Mosley, A.L. Boosting Detection of Low-Abundance Proteins in Thermal Proteome Profiling Experiments by Addition of an Isobaric Trigger Channel to TMT Multiplexes. Anal. Chem. 2021, 93, 7000–7010. [Google Scholar] [CrossRef]
- Yi, L.; Tsai, C.-F.; Dirice, E.; Swensen, A.C.; Chen, J.; Shi, T.; Gritsenko, M.A.; Chu, R.K.; Piehowski, P.D.; Smith, R.D.; et al. Boosting to Amplify Signal with Isobaric Labeling (BASIL) Strategy for Comprehensive Quantitative Phosphoproteomic Characterization of Small Populations of Cells. Anal. Chem. 2019, 91, 5794–5801. [Google Scholar] [CrossRef]
- Budnik, B.; Levy, E.; Harmange, G.; Slavov, N. SCoPE-MS: Mass Spectrometry of Single Mammalian Cells Quantifies Proteome Heterogeneity during Cell Differentiation. Genome Biol. 2018, 19, 161. [Google Scholar] [CrossRef]
- Specht, H.; Emmott, E.; Petelski, A.A.; Huffman, R.G.; Perlman, D.H.; Serra, M.; Kharchenko, P.; Koller, A.; Slavov, N. Single-Cell Proteomic and Transcriptomic Analysis of Macrophage Heterogeneity Using SCoPE2. Genome Biol. 2021, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Webber, K.G.I.; Madisyn Johnston, S.; Boekweg, H.; Lindgren, C.M.; Liang, Y.; Nydegger, A.; Xie, X.; Tsang, T.; Jayatunge, D.A.D.N.; et al. Data-Dependent Acquisition with Precursor Coisolation Improves Proteome Coverage and Measurement Throughput for Label-Free Single-Cell Proteomics. Angew. Chem. Int. Ed. 2023, 62, e202303415. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Leduc, A.; Wallmann, G.; Huffman, R.G.; Willetts, M.; Khan, S.; Specht, H.; Ralser, M.; Demichev, V.; Slavov, N. Increasing the Throughput of Sensitive Proteomics by PlexDIA. Nat. Biotechnol. 2023, 41, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Truong, T.; Saxton, A.J.; Boekweg, H.; Payne, S.H.; Van Ry, P.M.; Kelly, R.T. HyperSCP: Combining Isotopic and Isobaric Labeling for Higher Throughput Single-Cell Proteomics. Anal. Chem. 2023, 95, 8020–8027. [Google Scholar] [CrossRef]
- Orsburn, B.C.; Yuan, Y.; Bumpus, N.N. Insights into Protein Post-Translational Modification Landscapes of Individual Human Cells by Trapped Ion Mobility Time-of-Flight Mass Spectrometry. Nat. Commun. 2022, 13, 7246. [Google Scholar] [CrossRef]
- Huffman, R.G.; Leduc, A.; Wichmann, C.; Di Gioia, M.; Borriello, F.; Specht, H.; Derks, J.; Khan, S.; Khoury, L.; Emmott, E.; et al. Prioritized Mass Spectrometry Increases the Depth, Sensitivity and Data Completeness of Single-Cell Proteomics. Nat. Methods 2023, 20, 714–722. [Google Scholar] [CrossRef]
- Mun, D.-G.; Bhat, F.A.; Joshi, N.; Sandoval, L.; Ding, H.; Jain, A.; Peterson, J.A.; Kang, T.; Pujari, G.P.; Tomlinson, J.L.; et al. Diversity of Post-Translational Modifications and Cell Signaling Revealed by Single Cell and Single Organelle Mass Spectrometry. Commun. Biol. 2024, 7, 884. [Google Scholar] [CrossRef]
- Cutler, R.; Corveleyn, L.; Ctortecka, C.; Cantlon, J.; Jacome Vaca, S.A.; Deforce, D.; Vijg, J.; Dhaenens, M.; Papanastasiou, M.; Carr, S.A.; et al. Mass Spectrometry-Based Profiling of Single-Cell Histone Post-Translational Modifications to Dissect Chromatin Heterogeneity. bioRxiv 2024. [Google Scholar] [CrossRef]
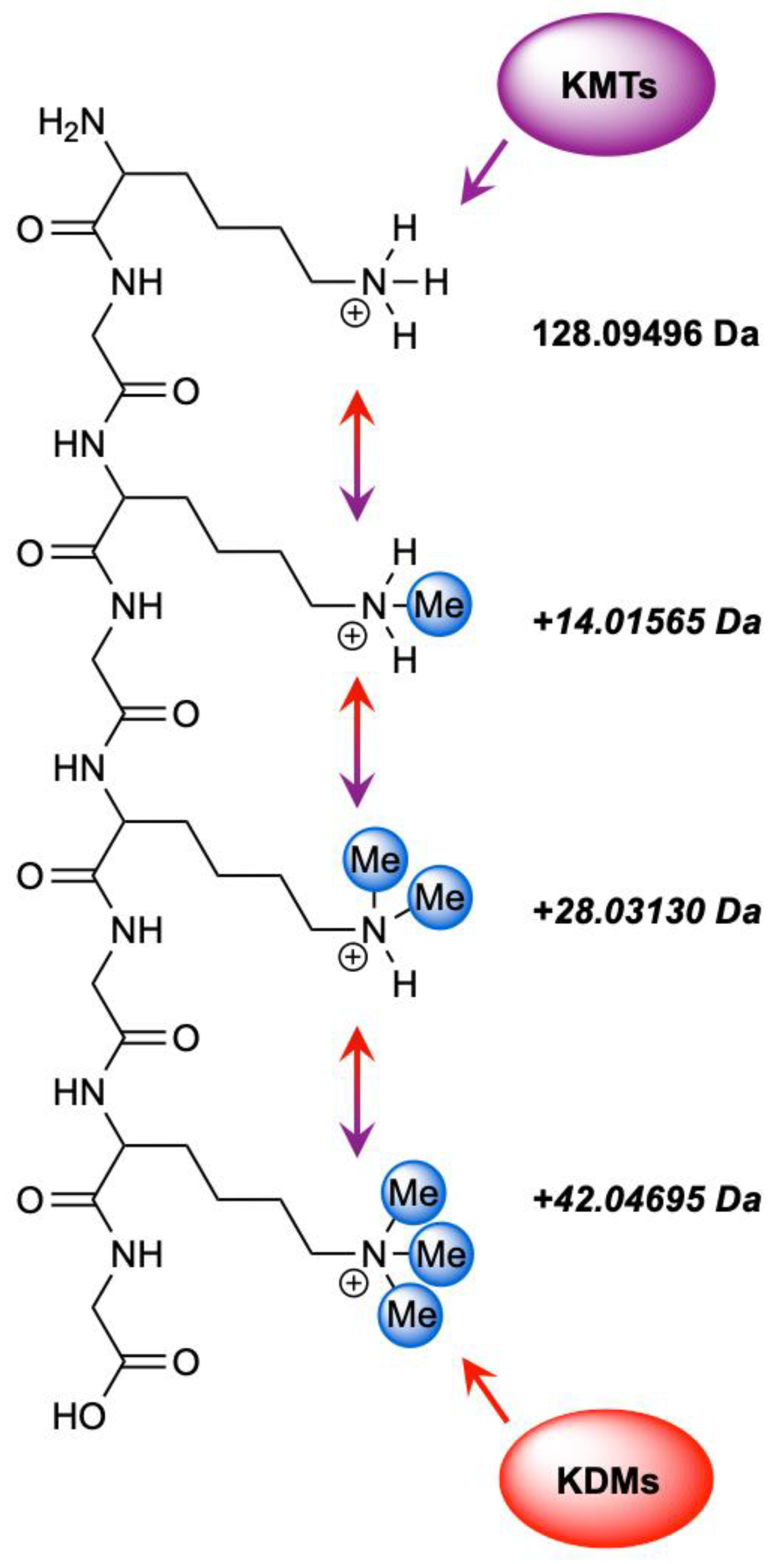
| Modification Type | Alteration | Observed Mass Shift (Da) | Spectral Ambiguity | Deviation from Target PTM Mass (Da) |
|---|---|---|---|---|
| Acetylation | K→KAc | +42.0106 | Kme3 mimic | 0.03635 Da |
| Carbamylation | K→Kcarb | +43.0058 | Kme3 mimic | 0.95885 Da |
| Formylation | K→Kfo | +27.9949 | Kme2 mimic | 0.0364 Da |
| Amino Acid Substitution | Val→Thr | +15.9949 | Kme1 mimic | 1.97925 Da |
| Ala→Ser | +15.9949 | Kme1 mimic | 1.97925 Da | |
| Phe→Tyr | +15.9949 | Kme1 mimic | 1.97925 Da | |
| Lys→Arg | +28.0070 | Kme2 mimic | 0.02430 Da | |
| Cys→Met | +28.0313 | Kme2 mimic | 0→identical | |
| Ala→Val | +28.0313 | Kme2 mimic | 0→identical | |
| Deamidation | Glu→Gln | −0.9840 | Misleading spectrum | 0.9840 Da |
| Asp→Asn | +0.9840 | Misleading spectrum | 0.9840 Da |
| Instrument Class | Mass Analyzer | Mass Range (m/z) | Resolution | ΔMass at 100 m/z (Da) | Mass Accuracy | Applications | Key Limitations |
|---|---|---|---|---|---|---|---|
| HIGH | FT-ICR | 50–10,000 | >5 M | 0.00002 | <1 ppm | High-confidence distinction of Kme states in intact proteins. | High cost; Slow acquisition |
| Orbitrap | 50–6000 | 140–500 K | 0.0002 | <1 ppm | Accurate distinction of Kme states. | Lower resolving power compared with FT-ICR | |
| Q-TOF | 50–40,000 | 20–80 K | 0.001 | <5 ppm | Rapid analysis with moderate resolution for lysine methylation states. | Limited resolution for near-isobaric Kme states | |
| LOW | Ion Trap | 50–2000 | 5–25 K | 0.004 | 5–50 ppm | Routine peptide sequencing and structural characterization. | Inability to resolve isobaric lysine methylation states; Moderate mass accuracy |
| QqQ | 50–4000 | 1–5 K | 0.02 | >100 ppm | High-throughput targeted quantification. | Inability to resolve isobaric Kme states; Poor mass accuracy |
| Workflow | Bottom-Up | Middle-Down | Top-Down |
|---|---|---|---|
| Resolution | Site-level | Domain-level | Proteoform-level |
| Analyte | Peptide (5–30 aa) | Polypeptide (25–90 aa) | Intact protein (>100 aa) |
| Proteolysis | Full (trypsin, Lys-C) | Partial/Limited (Glu-C, Asp-N) | None |
| Fragmentation | CID, HCD, ETD, EThcD, AI-ETD | ETD, AI-ETD, EThcD, UVPD | ECD, ETD, AI-ETD, UVPD |
| Enrichment Strategies | Immunoaffinity (pan- or state-specific antibodies); reader domain affinity; chromatographic separation (SCX, HILIC, IEF); chemical derivatization | Chromatographic or charge-based fractionation (SCX, HILIC); chemical derivatization; occasional antibody or reader-domain pull-downs | Rare; protein-level immunoprecipitation or fractionation (e.g., IEF) |
| Quantification | Discovery: LFQ, SILAC, hM-SILAC, TMT/iTRAQ Targeted: PRM, SRM/MRM | Discovery: LFQ, SILAC, hM-SILAC, TMT/iTRAQ Targeted: PRM, targeted DIA | Discovery: LFQ Targeted: PRM (rare) |
| Applications | Global methylome profiling; site-specific quantification of lysine methylation; comparative analysis across conditions | Mapping of combinatorial histone modifications; domain-level analysis of PTM crosstalk; profiling structured protein regions | Proteoform-resolved methylation analysis; characterization of intact isoforms and variant-specific methylation states |
| Strengths | High analytical depth and throughput; established informatics for FDR control and site localization | Resolves combinatorial PTM states and histone tail variants | Enables direct proteoform mapping and distinction of isobaric or isomeric PTMs |
| Limitations | Loss of proteoform context; missed cleavages; limited ability to resolve combinatorial PTMs | Lower coverage and throughput; complex fragmentation spectra | Incomplete fragmentation, low dynamic range, and high computational demand |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cumming, M.G.; Biggar, K.K. Mass Spectrometry for Lysine Methylation: Principles, Progress, and Prospects. Biomedicines 2025, 13, 2825. https://doi.org/10.3390/biomedicines13112825
Cumming MG, Biggar KK. Mass Spectrometry for Lysine Methylation: Principles, Progress, and Prospects. Biomedicines. 2025; 13(11):2825. https://doi.org/10.3390/biomedicines13112825
Chicago/Turabian StyleCumming, Mackenzie G., and Kyle K. Biggar. 2025. "Mass Spectrometry for Lysine Methylation: Principles, Progress, and Prospects" Biomedicines 13, no. 11: 2825. https://doi.org/10.3390/biomedicines13112825
APA StyleCumming, M. G., & Biggar, K. K. (2025). Mass Spectrometry for Lysine Methylation: Principles, Progress, and Prospects. Biomedicines, 13(11), 2825. https://doi.org/10.3390/biomedicines13112825





