Abstract
Background/Objective: The effectiveness of blue-light phototherapy (PT) is mainly dependent on the total dose of light (time under PT and amount of skin exposed) received by infants. The primary aim of this study was the development of a novel, flexible, and stretchable device to provide continuous PT treatment, avoiding temporary interruptions that are often observed in practice, such as during breastfeeding, for example. This study evaluated the biocompatibility of a novel, low-cost blanket equipped with light-emitting diode (LED) lamps designed to maintain therapeutic efficacy while facilitating uninterrupted skin-to-skin contact. Methods: Fourteen New Zealand White rabbits, weighing approximately 2.9 kg and aged 4 months, were randomly assigned to an experimental group (TG, n = 7) or a control group (CG, n = 7). The TG received phototherapy directly on the skin (irradiance: 19.3 [13.0–22.0] µW/cm−2/nm−1) during two 12 h sessions over consecutive days, while the CG remained under identical conditions with the device turned off. Biochemical, hematological, dermatological, and histological parameters, as well as rectal and skin temperatures, were assessed. Results: The results showed no differences in clinical appearance or histological analysis of skin tissue between the groups. Blood analysis indicated a reduction in absolute monocyte counts in the TG compared to the CG (p = 0.049), though levels remained within normal ranges. Skin temperature was consistently higher in the TG, except during the initial measurement. Rectal temperatures were similar on the first day but lower in the TG on the second day (mean 40.3 ± 0.21 °C vs. 40.7 ± 0.32 °C; p = 0.039). Conclusions: Temperature levels remained within physiological limits for both groups throughout the study. The device demonstrated biocompatibility and caused no adverse dermatological, hematological, or biochemical effects.
1. Introduction
Phototherapy is an effective treatment for neonatal jaundice or hyperbilirubinemia. This clinical condition affects 60 to 80% of babies and is responsible for numerous hospitalizations [1]. Phototherapy is the most commonly used alternative for neonatal jaundice when there is no risk of kernicterus (severe encephalopathy) and has advantages over other treatments because of its minimal invasiveness [2]. Bilirubin is produced by red blood cell (RBC) metabolism and is normally eliminated from the body through hepatic conjugation with glucuronic acid and excreted in the bile as bilirubin glucuronides. During the normal metabolism process, RBCs from the degradation of the heme group are converted into biliverdin (green color) and subsequently transformed into the bilirubin molecule (yellow color). Bilirubin circulates in the blood bound to serum albumin, is called indirect or unconjugated bilirubin, and is metabolized in the liver [3,4].
In the liver, the enzyme uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) transforms bilirubin into monoglucuronides and diglucuronide. These water-soluble compounds are excreted in the bile by the transport protein MRP2 [4]. However, in neonates, the hepatic activity of the UGT1A1 enzyme is insufficient, causing bilirubin accumulation and consequently jaundice, also due to the shorter life span of red blood cells at this stage.
Phototherapy consists of exposing the infant to light irradiation, thus preventing bilirubin accumulation and neurotoxicity. Light decomposes the bilirubin molecules and converts them into a water-soluble metabolite that can be eliminated [5].
During phototherapy, light photons act on the bilirubin molecules in nanoseconds, converting them into water-soluble photoproducts of structural isomerization— forming lumirubin, which has an excretion half-life of approximately 1.9 h—and configurational isomerization—bilirubin isomer 4Z, 15E, with an excretion half-life about 13 h. This isomer can be excreted directly, without hepatic metabolism, thus eliminating the metabolite. The existing phototherapeutic systems include sunlight sources, optic fiber blankets, fluorescent lights, halogen spotlights, and light-emitting diode (LED) lamps; the last three systems usually operate from a tripod to photoisomerize bilirubin [6]. Due to environmental pollution, toxicity, and operational lifetime drawbacks, light sources using fluorescent lamps have been replaced by LEDs. When comparing treatments with LED and non-LED devices, LED light therapy has a reduced failure rate, according to data from a systematic review and meta-analysis [6].
Phototherapy conventionally used for neonatal jaundice with remote irradiated lighting affects mothers, as it requires separation from their baby; other drawbacks include continuous crying of the newborn because of the discomfort caused by the lack of clothing and by the eye protection devices required during the treatment, which are stressful for both mother and child [7]. The side effects of phototherapy treatment include hyperthermia and dehydration, mainly caused by lamps that emit heat, especially in tropical countries.
Over the past 70 years, new alternatives have been developed for the management of hyperbilirubinemia, such as blood clearance devices [5], drugs, and devices based on photobiology. In this connection, devices for use close to the human skin, compared to those with light irradiated from a distance, represent advances in terms of humanization of care, increasing parental satisfaction by facilitating bonding, breastfeeding, and the kangaroo method [8].
Technological advances in the manufacture of electronic components and the development of new LED devices and organic light-emitting diodes (OLEDs) for different purposes and applications have enabled the introduction of new forms of light production and delivery devices, such as the use of lamps that do not heat human skin when used at a short distance, enabling more economical, effective, and safer solutions than the use of fluorescent and halogen lamps, since in this case, there is no emission of ultraviolet radiation and there is less heat production [2].
Studies investigating the effects of phototherapy, such as the presence of hydroelectrolytic (dehydration and diarrhea), hematological (change in monocytes), dermatological (skin eruptions), and hyperthermal, have been published [9,10]. Other side effects of phototherapy include chills, eye trauma, increased insensible excretion of water, bronze baby syndrome, eye damage, DNA damage, and nasal obstruction caused by the eye’s protection devices [9,11]. The absorption of water, sodium, and potassium may be impaired in newborns receiving phototherapy, but this effect is transient and resolves after ceasing the treatment. Hematological changes such as leukocyte imbalance, for example, may also occur. Studies have shown an increase in circulating leukocytes after phototherapy [12,13]. Another study corroborates these findings by reporting an increase in the total number of polymorphonuclear cells, such as lymphocytes and monocytes, and arguing that these findings were temporary and without clinical relevance [14]. Dermatological alterations caused by exposure to light as the main triggering factor [12] are also well described.
A review study reports asymptomatic and transient mild or moderate thrombocytopenia (platelet count below 150,000/cm3) that can be observed in 79% of newborns after 48 h of phototherapy [15]. Effects of phototherapy on magnesium serum levels (mild reduction in double phototherapy) [16] and on calcium levels (hypocalcemia—serum calcium < 8 mg/dL) have been described in 12.5% of full-term neonates [17].
The use of gonadal protection during phototherapy treatment was also indicated after a single study reported an increased risk of genital squamous cell carcinoma in men with psoriasis treated with psoralens and exposed to UVA/UVB radiation, which has maintained the indication of protection of patients’ genitalia to the present day [18,19]. Considering the above, with a view to observing such clinical variables during treatment with a new LED device proximal to the skin, an experimental rabbit model was set up for the application of the treatment. In order to improve outcomes for patients with clinical problems, multi-method research approaches by nurse scientists and the use of animal models are required. Thus, the animal models used in translational research serve as analogies for clinical problems seen in humans and are therefore useful for investigative advances [20].
This study evaluated a wearable LED device with a flexible structure for neonatal phototherapy in a randomized experimental trial. The primary outcome was the evaluation of 24 h treatment for dermatologic, histologic, hematologic, biochemical, and temperature changes. The secondary outcome measures were the temperature measurements at 30 cm from the experimental area and the temperature per sensor between the device and the skin. Exploratory measures were used to analyze the effects on gonadal tissue.
2. Materials and Methods
2.1. Wearable Device
The wearable device used in this study measures 15 × 30 cm and has a blue luminous efficiency of 19.3 (13.0–22.0) µw/cm2/nm; it was built in accordance with the requirements of the Agencia Nacional de Vigilância Sanitária (ANVISA, National Agency of Sanitary Surveillance) and the Food and Drug Administration (FDA). The wearable device is based on an optimized, transparent, and laminated PVC multilayer film structure and comprises (i) a surface-mounted blue LED emitter layer for standard to intensive phototherapy, (ii) a glycerol-encapsulated bag for rabbit electrical safety, thermal stability, and comfort, and (iii) aluminum foil as a refractive layer (or backlight assembly) for improved lighting conditions. The bags were effective in controlling the temperature of the device, which prevents thermal injury in skin, while the irradiance increases by approximately 20% when the backlight assembly is used. It is a thin, flexible device that can be molded to the body. The tensile stress–strain profile demonstrated that the wearable device had an elongation of approximately two times its original size.
It is covered with human-skin-biocompatible tissue that allows the passage of light. Light emission acts on bilirubin molecules, converting them into water-soluble products (lumirubin and bilirubin isomer 4Z, 15E) that can be excreted directly without the aid of hepatic metabolism. The absorbable light spectrum occurs in the blue spectrum region, close to 460 nm. It is noteworthy that the 460–490 nm spectrum (blue light) is the most efficient spectrum [21]. Blue light was obtained from high-intensity and waterproof flexible LED strip lights (18 units SMD 5050 LEDs, width = 10 mm, voltage = 12 V, beam angle = 120°, operation temperature from 20 to 50 °C, luminous intensity per LED = 800 mcd, wavelength range from 455 to 470 nm, and lifetime = 30,000 h). This device also comprises a refractive (or backlighting) layer for light-intensity enhancement. The multicomponent structure was heat-sealed at the borders and cooled to bond the films and components together to obtain the wearable phototherapy device.
For temperature maintenance and comfort of the rabbits, water bags (or cooling waterbeds to cushion and support the rabbits’ bodies) were obtained by sealing two PVC films by lamination with heat-sealed borders at different temperatures (TS; 85, 100, and 115 °C) after being filled with water and cooled to bond the films together. In the in vitro assessment, the device’s glycerin bag (a component that prevents thermal injuries to the skin) was effective in controlling the temperature resulting from the use of the device [22].
2.2. In Vivo Study
A pilot study with three rabbits (not included in the final sample) was conducted prior to the main experiment. The sample consisted of 14 New Zealand White (NZW) rabbits that met the inclusion criteria of being acclimated, weighing more than 1.5 kg, and aged more than 90 days. The pilot study excluded rabbits with pre-existing diseases or skin lesions, and rabbits were housed in individual stainless-steel cages prior to the procedure (for acclimatization). Standard feed and water were provided ad libitum throughout the study period. The cages measured 45 × 60 × 40 cm.
The primary outcome assessed the 24 h treatment for dermatological, histological, hematological, biochemical, and temperature changes. Secondary outcomes comprised temperature measurements taken 30 cm away from the experimental area and the temperature measured by a sensor located between the device and the skin. Exploratory measures were utilized to analyze the effects on gonadal tissue.
2.3. Procedure
The rabbits were randomly and individually assigned to one of the numbered cages (Figure 1). Animals were randomized to treatment (TG, n = 7) or control (CG, n = 7) groups using cage number assignment. The rabbits included in the sample were numbered inside the ear from C4 to C17, marked on the inner ear. The ARRIVE 2.0 checklist was used to write this report [23].
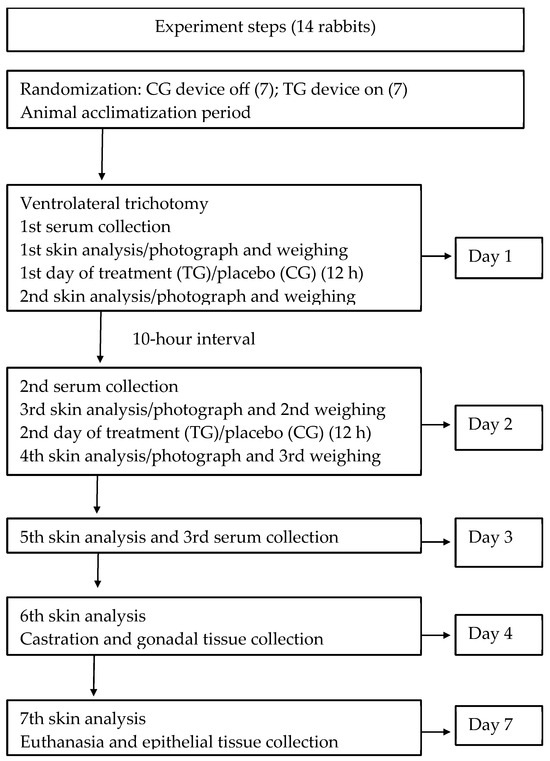
Figure 1.
Overview of experimental study protocol. Schematic illustrating the steps of the biocompatibility study of the new LED phototherapy device in the rabbit model. TG: treatment group (LED on, 12 h/day); CG: control group (device off).
2.4. Treatment
After light sedation to allow skin shaving, the TG animals underwent phototherapy with an LED device with a mean irradiance of 19.3 (13.0–22.0 µw/cm2/nm) in the shaved area located in the right ventrolateral region of the body that was covered by surgical overalls. The treatment was applied for 12 h in a row on Day 1 and for 12 h in a row on Day 2, with a 10 h interval between each session. During the treatment, the animals received water and feed ad libitum. The CG animals received the same procedure adopted for the TG animals, but their device was not connected to the power source, remaining turned off throughout the experiment (sham control).
2.5. Temperature Parameters
Skin temperature was monitored using a rectal thermometer and a continuous sensor placed between the shaved skin and the device. The values obtained from both methods were recorded for analysis. Temperature and moisture were properly controlled during the experiment, with the temperature being maintained at 20 °C using wall-split air-conditioning equipment in the room where the rabbits underwent phototherapy.
2.6. Serum Collection
Serum collection was performed at three time points as described in the General Principles of Blood Collection in Animals and in the Protocol for Marginal Ear Vein/Artery Blood Sample Collection [24]. The first collection took place before starting the treatment on Day 1, Time Zero (T0), the second in the morning of Day 2, after 12 h of treatment, Time 1 (T1), and the third in the morning after Day 3, Time 2 (T2), after 24 h of treatment. To perform the laboratory tests, a single puncture of the marginal vein was performed, and the material was collected in a 7.5% Ethylene-Diamine-Tetraacetic Acid (K3-EDTA) anticoagulant tube for blood count and a dry tube for biochemical analysis. To perform the blood count of red blood cells, white blood cells, and platelets and to determine hemoglobin, as well as the mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean platelet volume (MPV), and red cell distribution width (RDW), the material was placed in an automated veterinary cell counter (impedance). The packed cell volume (PCV) was determined by the Strumia microcapillary method (11,400 rpm for 5 min). The differential leukocyte count, along with the morphological evaluation of red blood cells, white blood cells, and platelets, as well as the estimation of platelets per 1000× field, was performed on blood smears stained with commercial hematological dye (Instant-Prov, Newprov, Pinhais, PR, Brazil), following the recommendations defined by Jain [25]. The platelet count in a hemocytometer was performed according to the methodology described by Brecher et al. [26]. For this purpose, 2 mL of a 1% ammonium oxalate solution was homogenized with 20 µL of blood for five minutes in a vortex. Then, both sides of the hemocytometer were filled, followed by incubation in a moist chamber for 20 min. The platelets contained in the 5 diagonal quadrants of the central grid of the chamber on both sides were counted, and the concentration of platelets/µL was obtained after multiplying by a factor of 2525. For the biochemical analyses, the serum tubes were centrifuged after collection and taken to the laboratory for analysis. The serum biochemistry assessment was performed on automated biochemical equipment (Roche® Cobas Mira Plus, Mannheim, Germany) using kits (Bioclin®, Belo Horizonte, MG, Brazil and Labtest®, Lagoa Santa, MG, Brazil) according to the manufacturer’s recommendations. Biochemical analyses included alanine aminotransferase (ALT), alkaline phosphatase (AP), gamma-glutamyltransferase (GGT), urea, creatinine, total protein, albumin, and total, indirect, and direct bilirubin [26].
2.7. Macroscopic Clinical Evaluation
After shaving the rabbits’ hair, images and a macroscopic evaluation of the skin were obtained before and after each treatment session, at 24, 48, 72, and 96 h. The camera used for the photography was a compact digital Nikon 4300 with automatic mode, macro function activated, and a resolution of 800 × 600 pixels. The classification of skin appearance was conducted in pairs by the investigator and a dermatologist with 24 years of experience in the function, following the adapted Draize Assessment model [27] and photographic record.
2.8. Surgical Procedure
Testicular tissue exeresis was performed on Day 4. The animals underwent surgery on a surgical table; they were placed in the supine position for orchiectomy (castration) surgery. The animals were anesthetized with 5% isoflurane, and a vaporizing mask was placed for anesthesia and oxygen supply. After shaving the hair in the inner part of the gonad region, the area was disinfected with 70% alcohol, followed by a 10% povidone iodine solution to prevent infection in the surgical area. A standard approach was used in the right anterior region, exposing the gonad through a 3–4 cm vertical incision for bilateral orchiectomy (castration) surgery. The gonadal tissue was extracted, followed by fixation in 10% neutral buffered formalin for analysis. The incision sites were cleaned with saline and sutured layer-by-layer. Antibiotics and analgesic medications were administered.
2.9. Histological Analysis
Rabbits were euthanized on Day 7, with an intravenous overdose of thiopental sodium 150 mg/kg, complying with the American Veterinary Medical Association (AVMA) guidelines [28]. The tissues were then collected (only on Day 7) and dehydrated using a 10% buffered formalin solution for histological evaluation. Samples of skin tissue and gonads were embedded in paraffin and cut into thin sections. Samples were stained with hematoxylin and eosin (H&E) to assess the presence of cellular changes. The slides were mounted, and the images were examined with a conventional 400 × Opticam microscope (Axio Vision Rel 4.8, Carl Zeiss®, Carl Zeiss, Germany, high-powered microscope at ×100, ×200, and ×400 magnification) by a senior pathologist with 30 years of experience. The presence or absence of alterations in the epidermis, dermis, muscular morphological structure, intensity and composition of the inflammatory infiltrate, and other findings were described.
2.10. Statistical Treatment for Data Analysis
The sample size calculation was based on the incidence of rabbits with a body temperature above 40 °C during 12 h of monitoring. The following assumptions were made: (i) the probability of a rabbit having a body temperature above 40 °C is constant, independent between rabbits, and equal to 0.15; (ii) using a binomial distribution with parameters n and p = 0.15, it was estimated that 22 rabbits were required to ensure a 2.8% probability of observing zero rabbits exceeding 40 °C. The experiment’s power was set at 80%, requiring 11 rabbits per group. After the pilot with 3 animals, 22 animals were obtained; however, eight animals died during the habitat transfer and the start of the acclimatization period and were not replaced, leaving 7 rabbits per group. Data collection was stopped for this number of rabbits due to constant repetition of the same responses in the measurements.
Data are expressed as the mean and standard deviation (SD). Confidence intervals of 95% were calculated, and hypothesis tests were performed, setting zero for the mean difference of the values of any hematological and biochemical response variable. Analysis was performed using SPSS version 21.0 (IBM, Armonk, NY, USA). Temperature data were collected longitudinally before and after an intervention, thus allowing observation of the data behavior according to time. Comparisons between groups regarding numerical variables were performed using two-way ANOVA and the Mann–Whitney U test. The results were considered statistically significant at p < 0.05. The dataset is available in the Mendeley Data (Digital Commons Data) repository [29].
3. Results
3.1. Biochemical and Hematological Parameters
Regarding the analysis of the biochemical laboratory parameters, the values collected at times T0, T1, and T2 were within the normal range for rabbits, according to the data presented in Table 1.

Table 1.
Biochemical parameters of New Zealand rabbits submitted to phototherapy treatment using an LED device.
When comparing with the Anova model to evaluate at the three time points (T0, T1, and T2), there was a difference in urea (T0 > T1, T2) and creatinine (T1 < T2), as shown in Table 2.

Table 2.
Multiple comparisons of biochemical parameters of New Zealand rabbits submitted to phototherapy treatment using an LED device.
The descriptive analysis, regarding the hematological parameters (Table 3), indicated that the relative values of the parameters between the groups were similar. There was no statistically significant difference in all collections performed at time points T0 and T1; however, at T2, at the end of the treatment, only one statistical difference (p = 0.049) in the absolute value of monocytes was observed.

Table 3.
Hematological parameters of New Zealand rabbits submitted to phototherapy treatment using an LED device.
In the T2 evaluation, the ± CG presented higher average absolute values of monocytes (564.1 ± 310.0137) compared to the TG (278.4 ± 152.5766). However, these data do not show statistical significance in the relative values of monocytes.
When comparing the time points before and after treatment for the TG animals, the mean monocyte count decreased from 336.6 cells/mm3 before phototherapy to 278.4 cells/mm3 after phototherapy.
3.2. Dermatological and Histological Parameters of Skin and Gonads
Dermatological evaluations revealed no treatment-associated lesions in either the control (CG, n = 7) or experimental (TG, n = 7) groups. Only one change in the skin was identified, described in Appendix B (Table A2); however, after assessment it was described as just a melanin deposit. The anatomopathological analyses also confirm the findings at the cellular level in both the control (CG, n = 6) and experimental (TG, n = 7) groups, with representative micrographs presented in Figure 2. All animals showed a good general condition, the absence of signs of alterations that could be attributed to the treatment used, and typical hair growth for the species.
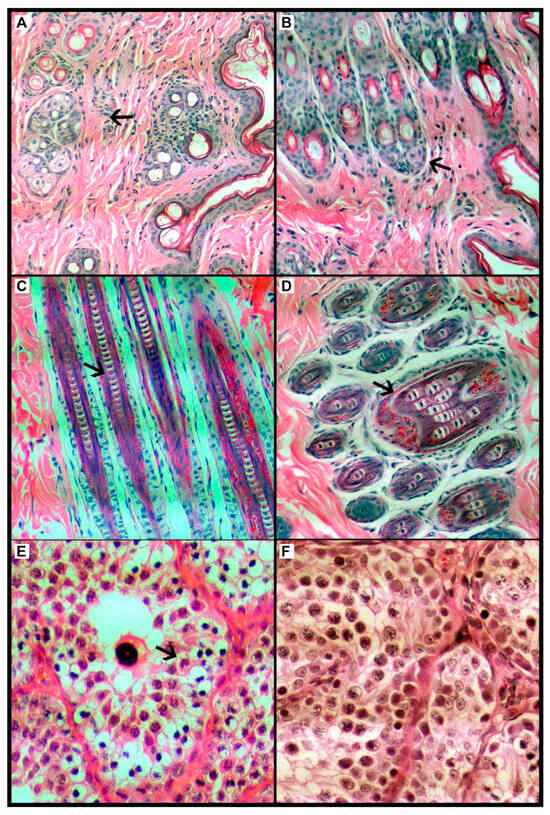
Figure 2.
Optical photomicrograph of the Oryctolagus cuniculus rabbit species dermis and epidermis; comparison of control group (left) and treatment group (right). (A) Control group (CG), histological tissue within normal limits, considering the keratin layer, then the epidermis, and finally the cutaneous appendages such as sebaceous and sweat glands and hair follicles intermingled with the supporting connective tissue. (B). Treatment group (TG), histological tissue within normal standards, considering the keratin layer, then the epidermal layer and finally the skin appendages such as sebaceous and sweat glands and hair follicles intermingled with the supporting connective tissue. (C) CG, the main hair structures can be seen in this sagittal segment, such as the cortex, medulla and cuticle. All are within normal ranges. (D). TG, this sagittal segment shows the main hair structures, such as the cortex, medulla, and cuticle. All are within normal ranges. HE stain. Magnification 200×. (E) CG, histological aspect of rabbit’s testicle, with focus on one of the seminiferous tubules (arrow). The basal layer consisting of delicate connective tissue, right after the germ cells in their different degrees of maturity. Thus, it was considered within normality standards. (F) In the TG, the rabbit testicle is focused on one of the seminiferous tubules (arrow). Basal layer consisting of delicate connective tissue, right after the germ cells in their different degrees of maturity. Thus, it is considered within normality standards. HE stain. Magnification 400×. The arrow indicates the cell structure in (A,B), the follicle in (C,D), and the seminiferous tubule in (E,F).
As for the evaluation of the gonadal tissue, all samples showed seminiferous tubules and a basal layer made up of delicate connective tissue, followed by germ cells in their different degrees of maturity. Thus, the tissue samples were considered within normality standards.
3.3. Thermal Parameters Results
At a distance of 30 cm, the mean temperature ranged from 18.9 ± 0.31 °C to 19.6 ± 1.02 °C. As for the moisture parameters at a 30 cm distance and in the room, measured by a thermo-hygrometer during the 24 h treatment, they varied so that the lowest moisture level recorded 30 cm from the experiment reached 55.4 ± 5.2554 and the mean highest moisture level reached 58.9 ± 6.9864. The lowest moisture level recorded in the experiment room was 55.1 ± 9.8899, and the highest moisture level recorded was 59.7 ± 9.2864.
With the exception of the first assessment (p = 0.109), the temperature measured by the sensor showed a statistically significant difference between the two groups, higher in the TG (see Table A1 in Appendix A).
On the first day, the rectal temperature did not differ significantly; however, on Day 2, in the T4 evaluation, the final time point corresponding to the final hours of treatment (Figure 3), it was observed that the temperature was lower in the TG, with a mean of 40.3 (±0.2116) compared to the CG with a mean of 40.7 (±0.3259) (p = 0.039).
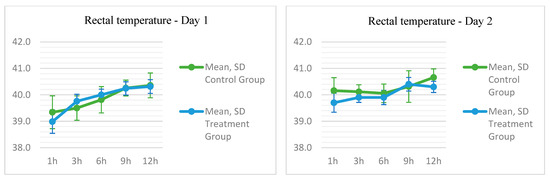
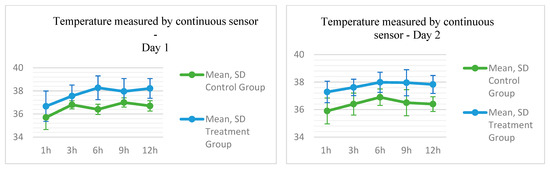
Figure 3.
Temperature parameters measured by a rectal thermometer and sensor in New Zealand rabbits submitted to phototherapy treatment using an LED device.
4. Discussion
The blanket employed in this study has been shown to be safe in animal models, thus enabling its translation to clinical studies, as it did not yield significant dermatological, histological, laboratory, biochemical, or hematological alterations.
Previous studies in infants reported that remote LED phototherapy increased eosinophils and basophils, decreased leukocytes and neutrophils, but did not alter monocyte or lymphocyte counts [30]. Our investigation differed in that it used a rabbit animal model as well as a proximal light device; the experiment did not cause hematological alterations in eosinophils and basophils but only in serum monocyte count.
In this connection, these findings suggest that the reduced number of monocytes has minor clinical relevance, since a minimum variation in the hematological parameters is expected and the values are within the reference range for the species. Also, in the groups, the standard deviation is high (high dispersion), and the mean is far apart. In the absolute monocyte count, the difference between the groups was statistically significant. Although the absolute monocyte count showed a statistically significant difference (p = 0.049), the relative monocyte values remained similar between groups, suggesting minimal clinical relevance.
Monocytes, once released into circulation, have a half-life of 1 to 2 days due to CCR2/chemokine receptor expression; they respond to this monocyte chemotactic protein and are drawn to sites of tissue injury to participate in inflammatory and phagocytic functions. Experiments indicate that they are predominant in wound infiltrates up to 6 h after muscle injury or tissue trauma. These inflammatory monocytes finally differentiate into classically activated M1 macrophages and, subsequently, into different types of dendritic cells [31]. The histological analyses of the tissues collected, in which cellular inflammatory infiltrates containing macrophages were not found, corroborate our laboratory data. Therefore, these findings suggest that the difference found may not be related to migration to the tissues but to the decrease or maintenance of cell expression (production) or some other factor.
In the literature, one study highlights the analysis of biochemical parameters that present alterations pre- and post-phototherapy in jaundiced neonates [32,33]. The main changes, as a result of the light therapy, are associated with the decline in serum levels of total cholesterol (p < 0.05), triglycerides (<0.005), very-low-density lipoprotein (VLDL) (<0.005), uric acid, creatinine, total serum proteins, albumin, and the serum electrolytes sodium, potassium, chloride, and calcium (p < 0.001 in each case) [32]. However, in our study, no difference in the biochemical parameter values was found in the TG before and after treatment.
When reviewing the biochemical parameters at T0, T1, and T2, we found a difference in urea (T0 > T1, T2) and creatinine (T1 < T2) when performing repeated measures Anova comparisons (p < 0.05).
Regarding dermatological and histological clinical parameters, a new macroscopic finding on the skin was analyzed by histopathological examination; however, it was found to be only a deposit of melanin. Melanocytes make up 1–2% of the epidermal cells, while keratinocytes, which produce keratin, constitute over 95% of the epidermal cells. Melanin absorbs the ultraviolet (UV) photons as well as the free radicals induced by exposure to UV radiation before these free radicals interact with other cellular components. Melanosomes distributed throughout the epidermis provide a highly protective screen that absorbs and scatters harmful UV radiation [31]. It is believed that in this study, the accumulation of skin pigment has arisen as a result of the hair removal and due to exposure to LED light proximal to the skin for 24 h during the treatment and subsequently to the natural lighting of the bioterium facility until euthanasia occurred.
Regarding gonadal pathology, the effect on gonad tissues was an exploratory outcome also investigated in our study. Blinded histopathological analyses performed on rabbit gonadal tissue revealed that the gonadal structures were preserved.
The literature reports only one study that found an increased risk of genital squamous cell carcinoma in humans associated with exposure to PUVA and UVB radiation [18]. As a consequence, gonadal protection during phototherapy has been required. Other experimental studies with newborn rats present results that indicate interference of phototherapy in spermatogenesis, such as a decrease in spermatogonia in the tubules and a decrease in Sertoli cells in the sperm [34,35]. However, these findings were not similar in humans [15].
Regarding the measured temperatures, it was expected that there would be significant differences between the TG with the device turned on and the CG with the device turned off. However, temperature monitoring proximal to the skin revealed that the values remained within normal parameters. Regarding the systemic temperature variation, the groups were similar because, despite the levels being within normal limits in both groups, the difference in the p-value of p = 0.039 was smaller than the measurement bias of the device, which was ±0.4.
In vitro clinical trials with bilirubin solution and blue light were previously conducted by the authors and described in other studies [36,37]. A recent systematic review and meta-analysis of randomized controlled clinical trials indicated that intermittent blue-light therapy achieves a higher overall efficacy rate and significantly shorter treatment times, with a decrease in the time to clinical resolution of jaundice [38].
It is concluded that the device is biocompatible with the skin and is not the cause of any injury based on an animal model without hyperbilirubinemia. In clinical practice in neonatal patients, the device could perform a function similar to the fur that shields rabbits from cold and heat by acting as a thermal insulator for neonates. It could minimize changes in ambient temperature that may affect infants’ thermoregulation. This is particularly relevant because it is common for newborns, especially premature and low-birth-weight infants, to have difficulty producing enough heat to compensate for heat loss [39].
Reports in the literature indicate the development of recent innovations, with tests still performed in vitro to evaluate wearable OLED devices [40]; however, the LED device tested in our study is a new form of light delivery that has an advantage in that it is low cost.
5. Conclusions
The narrowband blue LED wearable device did not cause clinically relevant dermatological, histological, biochemical, or hematological alterations in this non-hyperbilirubinemic rabbit model. These findings suggest that the transfer of understanding and its use in neonatal phototherapy facilities can be performed on an experimental basis. The primary finding was that the blanket had minimal impact on skin temperature. Nevertheless, continuous monitoring of temperature is recommended to ensure treatment safety.
A limitation of this study is that the animals were not submitted to the procedure that causes hyperbilirubinemia. Other laboratory parameters to be assessed in babies that were not controlled in this study are the changes in magnesium, calcium, and vitamin D before and after treatment.
These findings will directly inform hospital-based clinical trials, where the device’s unique design enables therapeutic efficacy while preserving mother–infant bonding during phototherapy. Successful knowledge transfer hinges on (1) optimizing exposure cycles to accommodate breastfeeding intervals, (2) establishing safety thresholds for 72 h continuous use, and (3) validating performance in neonates with hyperbilirubinemia. By overcoming these translational challenges, this technology has the potential to transform neonatal care, replacing conventional isolation-based systems with a biocompatible solution that reduces bilirubin while preserving mother–infant bonding.
Author Contributions
T.F.N.: conceptualization (lead), methodology, investigation, writing—review and editing, data curation, original draft (lead). S.C.M.B.: formal analysis; methodology, review and editing. J.C.L.: formal analysis; methodology, review and editing. R.F.B.: formal analysis, methodology, review and editing, funding acquisition. L.d.A.D.J.: investigation, data curation. G.S.L.: investigation. L.P.F.A.: formal analysis, review and editing. N.S.R.: formal analysis, review and editing. S.E.V.: investigation, data curation. H.L.: conceptualization (supporting), formal analysis, review and editing (equal). C.N.d.N.: investigation, data curation, formal analysis. R.J.: formal analysis, conceptualization (lead), methodology, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), grant numbers 88882.433178/2019-01, 88887.481612/2020-00, and AUXPE No. 3703/2025.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Universidade Estadual Paulista (UNESP)-Faculdade de Medicina Veterinária de Botucatu (approval number: 1376/2021; approval date: 27 July 2021). The experiments with animals were performed in 2021, complying with the protocol approved by the Animal Ethics Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available in the Mendeley Data Repository [29].
Acknowledgments
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) for financial support, as well as UNESP—Unipex—Botucatu, UNESP—Upeclin—Botucatu, the Instituto Nacional de Eletrônica Orgânica (INEO)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ALT | Alanine Aminotransferase |
| ANOVA | Analysis of Variance |
| ANVISA | Agencia Nacional de Vigilância Sanitária |
| AP | Alkaline Phosphatase |
| AVMA | American Veterinary Medical Association |
| CG | Control Group |
| EDTA K3 | Ethylene-Diamine-Tetraacetic Acid |
| FDA | Food and Drug Administration |
| GGT | Gamma-Glutamyltransferase |
| H&E | Hematoxylin and Eosin |
| L | Liters |
| LED | Light-Emitting Diode |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| MPV | Mean Platelet Volume |
| MRP2 | Transport Protein |
| NZW | New Zealand White |
| OLEDs | Organic Light-Emitting Diodes |
| PCV | Packed Cell Volume |
| PR | Paraná |
| PUVA | Psoralen Ultraviolet A Radiation |
| RBC | Red Blood Cell |
| RDW | Red Cell Distribution Width |
| SD | Standard Deviation |
| SP | São Paulo |
| T | Time |
| TG | Experimental Group |
| UGT1A1 | Glucuronosyltransferase |
| UI | International Unit |
| UNESP | Universidade Estadual Paulista |
| UV | Ultraviolet |
| UVB | Ultraviolet B |
| VLDL | Very-Low-Density Lipoprotein |
| cm2 | Square Centimeter(s) |
| cm3 | Cubic Centimeter(s) |
| dL | Deciliter |
| g/dL | Grams per Deciliter |
| h | Hours |
| kg | Kilograms |
| mL | Milliliter |
| mg | Milligrams |
| n | Sample Size |
| nm | Nanometer |
| p | p-Value |
| rpm | Revolutions per Minute |
| °C | Degree Celsius |
| µL | Microliters |
| µW | Microwatt |
Appendix A

Table A1.
Temperature parameters recorded using both a skin sensor and rectal thermometer in New Zealand rabbits exposed to phototherapy using an LED device.
Table A1.
Temperature parameters recorded using both a skin sensor and rectal thermometer in New Zealand rabbits exposed to phototherapy using an LED device.
| Control (n 7) | Treatment (n 7) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Min. | Max. | Mean | Median | SD | Min. | Max. | p | |
| Temperature per sensor between device and skin in °C | |||||||||||
| Day 1 | |||||||||||
| Skin sensor T. D1T0 | 35.7 | 35.9 | 1.0404 | 33.5 | 36.8 | 36.7 | 36.4 | 1.3149 | 34.6 | 38.3 | 0.109 |
| Skin sensor T. D1T1 | 36.8 | 36.8 | 0.3498 | 36.2 | 37.2 | 37.6 | 37.8 | 0.9502 | 35.7 | 38.6 | 0.046 |
| Skin sensor T. D1T2 | 36.4 | 36.5 | 0.4488 | 35.8 | 37.1 | 38.3 | 38.3 | 1.0291 | 36.5 | 39.9 | 0.006 |
| Skin sensor T. D1T3 | 37.0 | 36.9 | 0.4018 | 36.5 | 37.7 | 38.0 | 38.5 | 1.1103 | 35.8 | 38.9 | 0.047 |
| Skin sensor T. D1T4 | 36.7 | 36.8 | 0.4488 | 35.8 | 37.1 | 38.2 | 38.8 | 0.8552 | 36.8 | 38.9 | 0.008 |
| Day 2 | |||||||||||
| Skin sensor T. D2T0 | 35.9 | 35.9 | 0.9414 | 34.3 | 37.0 | 37.3 | 37.3 | 0.7798 | 36.1 | 38.3 | 0.011 |
| Skin sensor T. D2T1 | 36.4 | 36.5 | 0.8000 | 35.5 | 37.4 | 37.6 | 37.5 | 0.5984 | 36.9 | 38.5 | 0.015 |
| Skin sensor T. D2T2 | 36.9 | 36.6 | 0.5984 | 36.3 | 37.8 | 38.0 | 38.3 | 0.7335 | 36.9 | 38.6 | 0.012 |
| Skin sensor T. D2T3 | 36.5 | 36.6 | 0.9517 | 35.4 | 38.3 | 38.0 | 38.5 | 0.9484 | 36.5 | 38.7 | 0.029 |
| Skin sensor T. D2T4 | 36.4 | 36.5 | 0.5407 | 35.4 | 37.0 | 37.8 | 38.0 | 0.6525 | 36.8 | 38.7 | 0.004 |
| Rectal Temperature | |||||||||||
| Day 1 | |||||||||||
| Rectal T. D1T0 | 39.3 | 39.4 | 0.6214 | 38.4 | 40.4 | 39.0 | 38.9 | 0.4413 | 38.6 | 39.9 | 0.200 |
| Rectal T. D1T1 | 39.5 | 39.5 | 0.4619 | 38.7 | 40.1 | 39.8 | 39.8 | 0.2637 | 39.2 | 40.0 | 0.244 |
| Rectal T. D1T2 | 39.8 | 39.9 | 0.4981 | 39.1 | 40.4 | 40.0 | 40.0 | 0.2160 | 39.7 | 40.3 | 0.520 |
| Rectal T. D1T3 | 40.3 | 40.3 | 0.2992 | 39.9 | 40.7 | 40.2 | 40.1 | 0.2507 | 40.0 | 40.6 | 0.846 |
| Rectal T. D1T4 | 40.4 | 40.1 | 0.4685 | 39.9 | 41.1 | 40.3 | 40.3 | 0.2610 | 40.0 | 40.8 | 0.796 |
| Day 2 | |||||||||||
| Rectal T. D2T0 | 40.2 | 40.0 | 0.4928 | 39.6 | 40.9 | 39.7 | 39.7 | 0.3592 | 39.1 | 40.1 | 0.096 |
| Rectal T. D2T1 | 40.1 | 40.1 | 0.2673 | 39.6 | 40.5 | 40.0 | 39.9 | 0.1864 | 39.7 | 40.2 | 0.294 |
| Rectal T. D2T2 | 40.1 | 40.1 | 0.3505 | 39.6 | 40.6 | 39.9 | 39.9 | 0.2690 | 39.6 | 40.4 | 0.480 |
| Rectal T. D2T3 | 40.3 | 40.2 | 0.5984 | 39.7 | 41.5 | 40.4 | 40.4 | 0.2573 | 40.0 | 40.6 | 0.440 |
| Rectal T. D2T4 | 40.7 | 40.7 | 0.3259 | 40.1 | 41.1 | 40.3 | 40.3 | 0.2116 | 40.1 | 40.7 | 0.039 |
Note. The abbreviation T. refers to Temperature in degrees Celsius (°C). The letter D corresponds to the Day (D1, Day 1 and D2, Day 2) and T corresponds to the Time of the data collection interval, where T0 is the starting time, T1 is the next three hours, T2 is the 6th hour, T3 is the 9th hour, and T4 is the end time, the 12th hour of each day.
Appendix B

Table A2.
Evaluation of erythema, edema, vesiculobullous lesions, exulcerations, and ulcerations based on Draize’s method (1959) (adapted).
Table A2.
Evaluation of erythema, edema, vesiculobullous lesions, exulcerations, and ulcerations based on Draize’s method (1959) (adapted).
| Groups | Control (n 7) | Treatment (n 7) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin Assessment | R2 | R4 | R6 | R8 | R10 | R12 | R14 | R4 | R6 | R5 | R7 | R9 | R11 | R13 | |
| T 0 Day 1 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 1 Day 1 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 2 Day 2 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 3 Day 2 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 4 Day 3 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 5 Day 5 | Erythema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 6 Day 7 | Erythema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Legend: T = clinical skin analysis. R = Rabbit. Skin assessment aspect classification. Erythema formation: no erythema (0); mild erythema (barely noticeable) (1); well-defined erythema (2); moderate-to-severe erythema (3); severe erythema (violet red) (4). Edema formation: no edema (0); mild edema (barely noticeable) (1); well-defined edema (borders smaller than 1 mm) (2); severe edema (borders smaller than 1mm) (3). Vesico-blister formation: no formation (0); vesicle formation (less than 0.5 cm) (1); blister formation (greater than 0.5 cm) (2); formation of several blisters (larger than 0.5 cm) (3). Formation of exulcerations or ulcerations: no formation (0); mild exulceration formation (1); formation of multiple exulcerations (2); formation of ulceration (3). * One rabbit died during anesthetic sedation (information drawn from the necropsy report N16).
References
- Lin, Q.; Zhu, D.; Chen, C.; Feng, Y.; Shen, F.; Wu, Z. Risk factors for neonatal hyperbilirubinemia: A systematic review and meta-analysis. Transl. Pediatr. 2022, 11, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Seung Lee, J.; Kim, J.; Ye, Y.; Kim, T. Materials and device design for advanced phototherapy systems. Adv. Drug Deliv. Rev. 2022, 186, 114339. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Longo, D.L.; Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Loscalzo, J. Medicina Interna de Harrison, 20th ed.; AMGH: Porto Alegre, Brazil, 2020. [Google Scholar]
- Martinez, J.B.; Dantas, M.; Voltarelli, J.C. Semiologia Geral e Especializada; Guanabara Koogan: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Wang, Y.; Wei, R.; Zhao, W.; Zhao, C. Bilirubin Removal by Polymeric Adsorbents for Hyperbilirubinemia Therapy. Macromol. Biosci. 2023, 23, 2200567. [Google Scholar] [CrossRef] [PubMed]
- Novoa, R.H.; Huaman, K.; Caballero, P. Light-Emitting Diode (LED) Phototherapy Versus Non-LED Phototherapy Devices for Hyperbilirubinemia in Neonates: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2022, 40, 1618–1628. [Google Scholar] [CrossRef]
- Nascimento, T.F.; de Avila, M.A.G.; Bocchi, S.C.M. From suffering to resignation: Grounded Theory approach to maternal experience with newborn in phototherapy. Rev. Bras. Saúde Matern. Infant. 2018, 18, 143–151. [Google Scholar] [CrossRef]
- Føreland, A.M.; Rosenberg, L.; Johannessen, B. Nurses’ experiences using conventional overhead phototherapy versus fibreoptic blankets for the treatment of neonatal hyperbilirubinemia. J. Neonatal Nurs. 2016, 22, 108–114. [Google Scholar] [CrossRef]
- Shahriarpanah, S.; Haji Ebrahim Tehrani, F.; Davati, A.; Ansari, I. Effect of Phototherapy on Serum Level of Calcium, Magnesium and Vitamin D in Infants with Hyperbilirubinemia. Iran. J. Pathol. 2018, 13, 357–362. [Google Scholar]
- Shawky, A.H.; Refaee, A. Risk factors for short-term side effects of phototherapy in neonatal jaundice. Arch. Dis. Child. 2021, 106, A124–A125. [Google Scholar] [CrossRef]
- Xiong, T.; Qu, Y.; Cambier, S.; Mu, D. The side effects of phototherapy for neonatal jaundice: What do we know? What should we do? Eur. J. Pediatr. 2011, 170, 1247–1255. [Google Scholar] [CrossRef]
- Jahanshahifard, S.; Ahmadpour-Kacho, M.; Pasha, Y. Effects of phototherapy on Cytokines′ levels and white blood cells in term neonate with hyperbilirubinemia. J. Clin. Neonatol. 2012, 1, 139. [Google Scholar] [CrossRef]
- Zarkesh, M.; Dalili, S.; Fallah, M.; Heidarzadeh, A.; Rad, A. The effect of neonatal phototherapy on serum level of interlukin-6 and white blood cells′ count. J. Clin. Neonatol. 2016, 5, 189. [Google Scholar] [CrossRef]
- Mrkaić, L.; Kamenov, B.; Najman, S.; Dimitrijević, H.; Mitrović, V.; Maglajlić, S. Neonatal immune system changes caused by phototherapy. Srp. Arh. Celok. Lek. 1994, 122 (Suppl. 1), 36–37. [Google Scholar] [PubMed]
- Yurdakök, M. Phototherapy in the newborn: What’s new? J. Pediatr. Neonatal Individ. Med. 2015, 4, e040255. [Google Scholar] [CrossRef]
- Eghbalian, F.; Shabani, S.; Faradmal, J.; Jenabi, E. Effects of Phototherapy on the Serum Magnesium Level in Neonates with Indirect Hyperbilirubinemia: A Prospective Cohort Study. Int. J. Pediatr. 2022, 2022, 5439630. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, K.; Mani, S.; Vasudevan, N.; Preethi, S.; Krishnamoorthy, N.; Pratibha, R.K.; Sundar, S. Effect of Light-Emitting Diode Phototherapy on Serum Calcium Levels in Neonates with Jaundice. Cureus 2022, 14, e23938. [Google Scholar] [CrossRef]
- Bouceiro Mendes, R.; Alpalhão, M.; Filipe, P. UVB phototherapy in the treatment of vitiligo: State of the art and clinical perspectives. Photodermatol. Photoimmunol. Photomed. 2022, 38, 215–223. [Google Scholar] [CrossRef]
- Stern, R.S. Genital Tumors among Men with Psoriasis Exposed to Psoralens and Ultraviolet A Radiation (PUVA) and Ultraviolet B Radiation. N. Engl. J. Med. 1990, 322, 1093–1097. [Google Scholar] [CrossRef]
- Tkacs, N.C.; Thompson, H.J. From Bedside to Bench and Back Again: Research Issues in Animal Models of Human Disease. Biol. Res. Nurs. 2006, 8, 78–88. [Google Scholar] [CrossRef]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for Neonatal Jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef]
- Lacerda, G.S. Sistema Fototerápico Vestível para Tratamento Contínuo da Icterícia Neonatal. Ph.D. Thesis, Universidade Federal de Ouro Preto, Ouro Preto, Brazil, 2019. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, M.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Jain, N. Schalm’s Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1986; pp. 20–86. [Google Scholar]
- Brecher, G.; Cronkite, E.P. Morphology and Enumeration of Human Blood Platelets. J. Appl. Physiol. 1950, 3, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Draize, J.H. Dermal toxicity. Apprais. Safe Chem. Foods Drugs Cosmet. 1959, 46–59. [Google Scholar]
- Leary, S.L. American Veterinary Medical Association Guidelines for the Euthanasia of Animals—Version 20; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Nascimento, T.F.; Cristina Mangini Bocchi, S.; César Lyra, J.; Fernando Bianchi, R.; Silveira Lacerda, G.; Patrícia Fernandes Abadde, L.; Rocha, N.S.; Vieira, S.E.; Langoni, H.; do Nascimento, C.N.; et al. Evaluation of Thermal; Hemato-Histological and Dermatological Biocompatibility of a LED Device for Neonatal Phototherapy. Mendeley Data 2023. [Google Scholar] [CrossRef]
- Altuntas, N.; Dogan, O.; Kislal, F. Effect of Phototherapy on Neutrophil VCS Parameters and White Blood Cells. J. Coll. Physicians Surg. Pak. 2019, 29, 453–455. [Google Scholar] [CrossRef]
- Souza, C.D.; Eren, M.V. Monocytes and Macrophages and Their Disorders. In Schalm’s Veterinary Hematology; Wiley: Hoboken, NJ, USA, 2022; pp. 386–394. [Google Scholar] [CrossRef]
- Suneja, S.; Rajani, K.; Saxena, R. Effect of phototherapy on biochemical parameters in neonatal hyperbilirubinemia patients: A clinical insight. Indian J. Neonatal Med. Res. 2018, 6, 13–18. [Google Scholar]
- Akpinar Gozetici, M.; Kizilelma Yigit, A.; Beser, O.F. Effect of Phototherapy on Serum Electrolyte Levels. Cerrahpasa Med. J. 2021, 45, 16–20. [Google Scholar] [CrossRef]
- Cetinkursun, S.; Demirbag, S.; Cincik, M.; Baykal, B.; Gunal, A. Effects of phototherapy on newborn rat testicles. Arch. Androl. 2006, 52, 61–70. [Google Scholar] [CrossRef]
- Koç, H.; Altunhan, H.; Dilsiz, A.; Kaymakçi, A.; Duman, S.; Oran, B.; Erkul, I. Testicular Changes in Newborn Rats Exposed to Phototherapy. Pediatr. Dev. Pathol. 1999, 2, 333–336. [Google Scholar] [CrossRef]
- Savedra, R.M.L.; Fonseca, A.M.T.; Silva, M.M.; Bianchi, R.F.; Siqueira, M.F. White LED phototherapy as an improved treatment for neonatal jaundice. Rev. Sci. Instrum. 2021, 92, 064101. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.R.; Tannure, A.M.; Cardoso, L.C.; Siqueira, M.F.; Bianchi, A.C.G.; Bianchi, R.F. Colorimetric dosimeter to promote most efficient use of neonatal phototherapy. Sens. Actuators B Chem. 2016, 240, 1003–1008. [Google Scholar] [CrossRef]
- Wu, R.; Wen, L. Meta-analysis of the efficacy of different blue light therapy methods for neonatal jaundice. J. Matern. -Fetal Neonatal Med. 2024, 38, 1. [Google Scholar] [CrossRef]
- Treesirichod, A.; Eiamkulbutr, S.; Laohathai, P.; Vongbhavit, K.; Panburana, J. The efficacy of infrared filter window film to prevent hyperthermia in neonatal hyperbilirubinemia with conventional phototherapy: A randomized control trial. Pediatr. Neonatol. 2022, 63, 489–495. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, Y.; Kwon, J.H.; Ihm, C.; Kim, S.Y.; Choi, K.C. Wearable Photomedicine for Neonatal Jaundice Treatment Using Blue Organic Light-Emitting Diodes (OLEDs): Toward Textile-Based Wearable Phototherapeutics. Adv. Sci. 2022, 9, 2204622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).