Relative Effects of Brachytherapy and Beam Radiation for DCIS on Subsequent Invasive Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Cohort Creation
2.3. Matching
3. Results
3.1. Unmatched Cohort
3.2. Matched Brachytherapy vs. Omission of Radiation
3.3. Matched Brachytherapy vs. Beam Radiation
3.4. Site of Ipsilateral Breast Invasive Subsequent Event Relative to Initial DCIS Location
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBI | Partial breast irradiation |
| WBI | Whole breast irradiation |
| BCS | Breast-conserving surgery |
References
- Fisher, E.R.; Costantino, J.; Fisher, B.; Palekar, A.S.; Paik, S.M.; Suarez, C.M.; Wolmark, N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Five-year observations concerning lobular carcinoma in situ. Cancer 1996, 78, 1403–1416. [Google Scholar] [CrossRef]
- Wärnberg, F.; Garmo, H.; Emdin, S.; Hedberg, V.; Adwall, L.; Sandelin, K.; Ringberg, A.; Karlsson, P.; Arnesson, L.-G.; Anderson, H.; et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J. Clin. Oncol. 2014, 32, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; Litière, S.; Werutsky, G.; Julien, J.-P.; Fentiman, I.S.; Agresti, R.; Rouanet, P.; de Lara, C.T.; Bartelink, H.; Duez, N.; et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J. Clin. Oncol. 2013, 31, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Pinder, S.E.; Ellis, I.O.; Forsyth, S.; Bundred, N.J.; Forbes, J.F.; Bishop, H.; Fentiman, I.S.; George, W.D. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011, 12, 21–29. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.; Winter, K.A.; Woodward, W.; Kuerer, H.M.; Sneige, N.; Rakovitch, E.; Smith, B.L.; Germain, I.; Hartford, A.C.; O’ROurke, M.A.; et al. Randomized Phase III Trial Evaluating Radiation Following Surgical Excision for Good-Risk Ductal Carcinoma In Situ: Long-Term Report From NRG Oncology/RTOG 9804. J. Clin. Oncol. 2021, 39, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Marrazzo, L.; Saieva, C.; Desideri, I.; Scotti, V.; Simontacchi, G.; Bonomo, P.; Greto, D.; Mangoni, M.; Scoccianti, S.; et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 2020, 38, 4175–4183. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Julian, J.A.; Berrang, T.S.; Kim, D.-H.; Germain, I.; Nichol, A.M.; Akra, M.; Lavertu, S.; Germain, F.; Fyles, A.; et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet 2019, 394, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Strnad, V.; Polgár, C.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Łyczek, J.; Guinot, J.L.; Miguelez, C.G.; et al. Accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy compared with whole-breast irradiation with boost for early breast cancer: 10-year results of a GEC-ESTRO randomised, phase 3, non-inferiority trial. Lancet Oncol. 2023, 24, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; A Ganz, P.; Parda, D.S.; Scheier, M.F.; et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Anderson, S.; Redmond, C.; Fisher, B. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: Pathological findings from NSABP protocol B-06. Semin. Surg. Oncol. 1992, 8, 161–166. [Google Scholar] [PubMed]
- Fisher, B.; Dignam, J.; Wolmark, N.; Mamounas, E.; Costantino, J.; Poller, W.; Fisher, E.R.; Wickerham, D.L.; Deutsch, M.; Margolese, R.; et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J. Clin. Oncol. 1998, 16, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, I.L.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Anderson, S.J.; Julian, T.B.; Land, S.R.; Margolese, R.G.; Swain, S.M.; Costantino, J.P.; et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J. Natl. Cancer Inst. 2011, 103, 478–488. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, T.J.; Blair, S.L.; Hosseini, A.; Harismendy, O.; Wallace, A.M. HER2-Overexpressing Ductal Carcinoma In Situ Associated with Increased Risk of Ipsilateral Invasive Recurrence, Receptor Discordance with Recurrence. Cancer Prev. Res. Phila. Pa. 2020, 13, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.E.; Barnes, N.L.P.; Cramer, A.; Johnson, R.; Cheema, K.; Morris, J.; Howe, M.; Bundred, N.J. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2013. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Hansen, B.; Klopfer, S. Optimal Full Matching and Related Designs Via Network Flows. J. Comput. Graph. Stat. 2012, 15, 609–627. [Google Scholar] [CrossRef]
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract. Radiat. Oncol. 2017, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
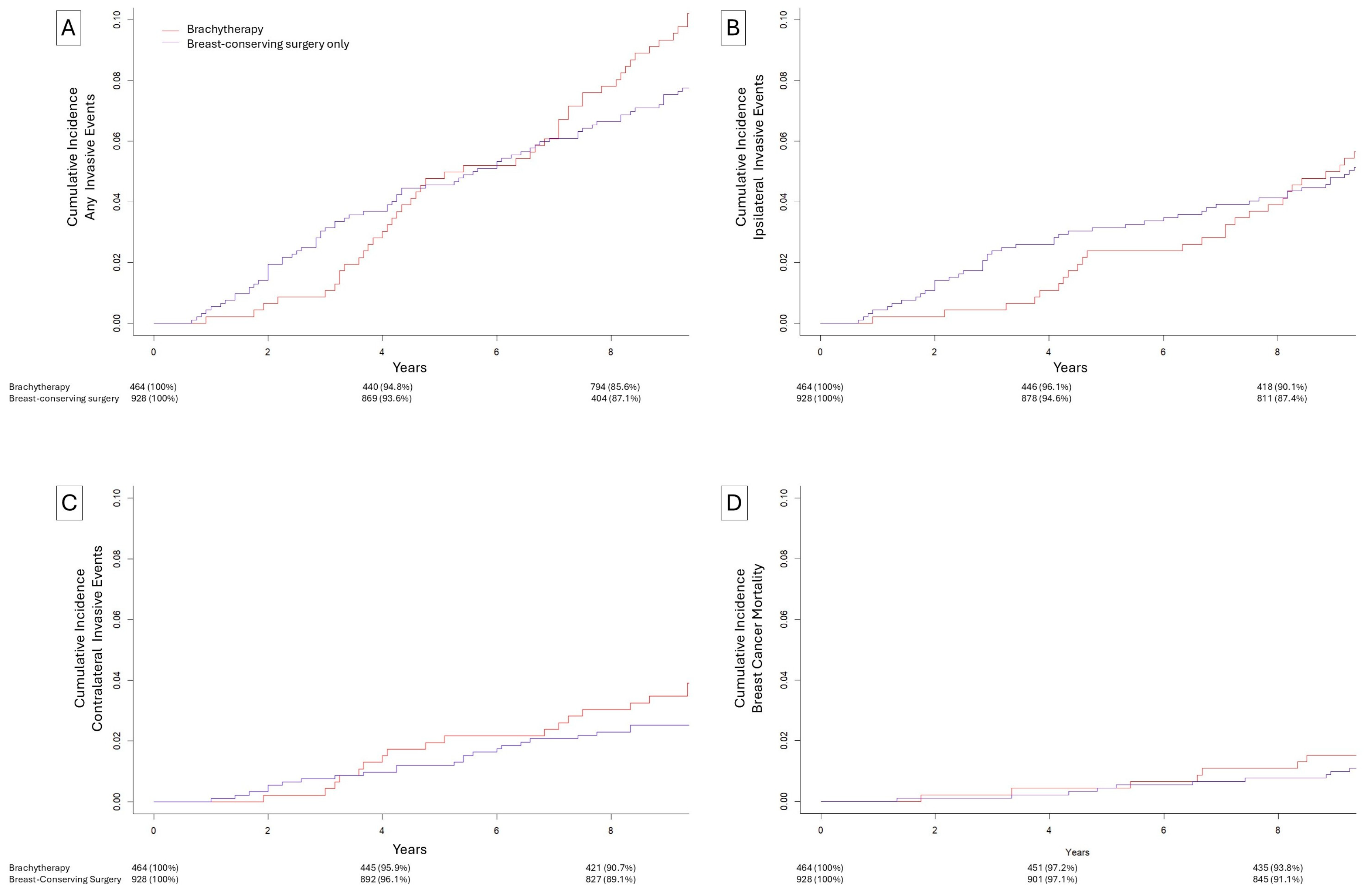
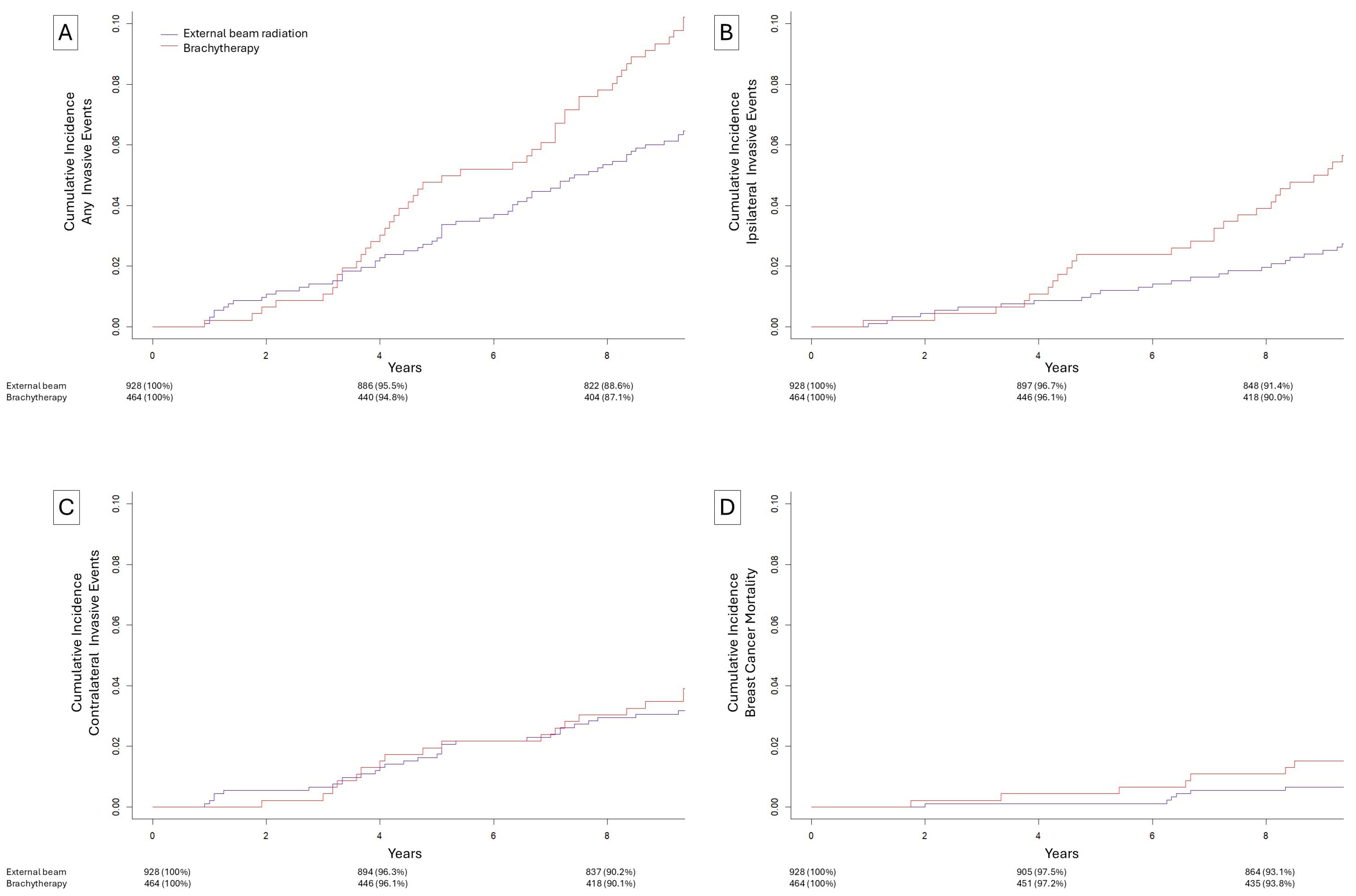
| Breast-Conserving Surgery with Brachytherapy (N = 464) | Breast-Conserving Surgery Only (N = 928) | Breast-Conserving Surgery with External-Beam Radiation (N = 928) | |
|---|---|---|---|
| Age | |||
| Median (IQR) | 60 (53–66) | 60 (53–67) | 60 (53–67) |
| Race | |||
| White | 386 (83.2%) | 767 (82.7%) | 771 (83.1%) |
| Black | 43 (9.3%) | 93 (10.0%) | 93 (10.0%) |
| Other | 35 (7.5%) | 68 (7.3%) | 64 (6.9%) |
| Grade * | |||
| Low | 46 (9.9%) | 92 (9.9%) | 92 (9.9%) |
| Intermediate | 208 (44.8%) | 416 (44.8%) | 416 (44.8%) |
| High | 161 (34.7%) | 322 (34.7%) | 322 (34.7%) |
| Undifferentiated | 49 (10.6%) | 98 (10.6%) | 98 (10.6%) |
| Hormone receptor status * | |||
| Positive | 368 (79.3%) | 736 (79.3%) | 736 (79.3%) |
| Negative | 96 (20.7%) | 192 (20.7%) | 192 (20.7%) |
| Size | |||
| Median (IQR) | 8 (4.75–14) | 7 (4–14) | 8 (4–15) |
| Adjuvant Radiation | |||
| None | 0 (0%) | 928 (100%) | 0 (0%) |
| Brachytherapy | 464 (100%) | 0 (0%) | 0 (0%) |
| External-beam | 0 (0%) | 0 (0%) | 928 (100%) |
| Adjuvant Endocrine | |||
| Received | 0 (0%) | 0 (0%) | 0 (0%) |
| Not received | 464 (100%) | 928 (100%) | 928 (100%) |
| Any Invasive Event | Ipsilateral Invasive Event | Contralateral Invasive Event | ||||
|---|---|---|---|---|---|---|
| sHR | p | sHR | p | sHR | p | |
| Age | ||||||
| ≤55 years | Ref | - | Ref | - | Ref | - |
| >55 years | 1.27 (1.52–3.72) | 0.24 | 0.97 (0.60–1.57) | 0.90 | 2.01 (0.97–4.16) | 0.06 |
| Race | ||||||
| Non-Black | Ref | - | Ref | - | Ref | - |
| Black | 2.37 (1.52–3.72) | <0.001 | 2.71 (1.56–4.70) | <0.001 | 1.00 (0.34–2.84) | 1.00 |
| Grade | ||||||
| Low/Int | Ref | - | Ref | - | Ref | - |
| High/Undiff | 1.07 (0.74–1.54) | 0.73 | 1.06 (0.66–1.72) | 0.81 | 0.75 (0.41–1.37) | 0.35 |
| Size | ||||||
| ≤10 mm | Ref | - | Ref | - | Ref | - |
| >10 mm | 0.95 (0.66–1.37) | 0.79 | 0.95 (0.59–1.51) | 0.81 | 0.75 (0.40–1.43) | 0.39 |
| HR Status | ||||||
| HR Positive | Ref | - | Ref | - | Ref | - |
| HR Negative | 1.09 (0.70–1.70) | 0.70 | 1.34 (0.78–2.31) | 0.29 | 1.00 (0.47–2.14) | 1.00 |
| Treatment | ||||||
| BCS | Ref | - | Ref | - | Ref | - |
| BCS + Brachy 0–3 years | 0.28 (0.10–0.81) | 0.02 | 0.19 (0.04–0.81) | 0.03 | 0.29 (0.04–2.34) | 0.24 |
| BCS + Brachy 3–10 years | 1.82 (1.20–2.76) | 0.005 | 1.66 (0.96–2.86) | 0.07 | 1.92 (0.53–0.98) | 0.06 |
| Any Invasive Event | Ipsilateral Invasive Event | Contralateral Invasive Event | ||||
|---|---|---|---|---|---|---|
| sHR | p | sHR | p | sHR | p | |
| Age | ||||||
| ≤55 years | Ref | - | Ref | - | Ref | - |
| >55 years | 1.00 (0.67–1.47) | 0.98 | 0.72 (0.41–1.24) | 0.23 | 1.11 (0.62–1.99) | 0.73 |
| Race | ||||||
| Non-Black | Ref | - | Ref | - | Ref | - |
| Black | 2.44 (1.54–3.88) | <0.001 | 2.78 (1.47–5.24) | 0.002 | 1.55 (0.70–3.43) | 0.28 |
| Grade | ||||||
| Low/Int | Ref | - | Ref | - | Ref | - |
| High/Undiff | 1.30 (0.88–1.92) | 0.19 | 1.65 (0.91–2.99) | 0.10 | 0.81 (0.45–1.45) | 0.48 |
| Size | ||||||
| ≤10 mm | Ref | - | Ref | - | Ref | - |
| >10 mm | 0.70 (0.46–1.06) | 0.09 | 0.60 (0.32–1.11) | 0.10 | 0.88 (0.48–1.59) | 0.67 |
| HR Status | ||||||
| HR Positive | Ref | - | Ref | - | Ref | - |
| HR Negative | 1.19 (0.75–1.88) | 0.46 | 1.65 (0.87–3.14) | 0.12 | 0.83 (0.39–1.78) | 0.63 |
| Treatment | ||||||
| BCS + External | Ref | - | Ref | - | Ref | - |
| BCS + Brachy 0–3 years | 0.61 (0.20–1.87) | 0.39 | 0.65 (0.13–3.23) | 0.60 | 0.33 (0.04–2.75) | 0.31 |
| BCS + Brachy 3–10 years | 1.73 (1.15–2.59) | 0.009 | 2.20 (1.22–3.95) | 0.009 | 1.36 (0.73–2.51) | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Keefe, T.J.; Prionas, N.D.; Wallace, A.M. Relative Effects of Brachytherapy and Beam Radiation for DCIS on Subsequent Invasive Events. Biomedicines 2025, 13, 2823. https://doi.org/10.3390/biomedicines13112823
O’Keefe TJ, Prionas ND, Wallace AM. Relative Effects of Brachytherapy and Beam Radiation for DCIS on Subsequent Invasive Events. Biomedicines. 2025; 13(11):2823. https://doi.org/10.3390/biomedicines13112823
Chicago/Turabian StyleO’Keefe, Thomas J., Nicolas D. Prionas, and Anne M. Wallace. 2025. "Relative Effects of Brachytherapy and Beam Radiation for DCIS on Subsequent Invasive Events" Biomedicines 13, no. 11: 2823. https://doi.org/10.3390/biomedicines13112823
APA StyleO’Keefe, T. J., Prionas, N. D., & Wallace, A. M. (2025). Relative Effects of Brachytherapy and Beam Radiation for DCIS on Subsequent Invasive Events. Biomedicines, 13(11), 2823. https://doi.org/10.3390/biomedicines13112823






