Abstract
Background: Sex differences in epidemiology and outcomes in atrial fibrillation (AF) are well documented, but their role in early detection and risk stratification in primary care remains unclear. Methods: This study used an observational, retrospective cohort design, including 9677 individuals identified as being at high risk (Quartile 4 of a validated prediction model) for developing atrial fibrillation, aged 65–95 years, and without prior AF or stroke in the Terres de l’Ebre health region (Catalonia, Spain). Incident AF and comorbidities prevalence were assessed from 1 January 2015 to 31 December 2024. Analyses compared sex-specific differences. Results: During follow-up, 3370 individuals (8.4%) developed AF, with higher incidence in men than women (9.9% vs. 7.0%, p < 0.001). In the high-risk subgroup (n = 9677), women had higher CHA2DS2-VA scores (4.10 vs. 3.84, p < 0.001) and greater prevalence of cognitive impairment (21.5% vs. 14.6%), while men more often presented with diabetes, ischemic cardiomyopathy, and peripheral vascular disease. Among new AF cases in this subgroup, men exhibited clustering of cardiometabolic conditions, whereas women showed higher cognitive decline. Conclusions: Distinct sex-specific patterns in comorbidity clustering and AF incidence were observed. These findings highlight the need for sex-tailored strategies for early AF detection and integrated risk management in primary care.
1. Introduction
Atrial fibrillation (AF) is one of the most commonly encountered heart conditions, with a broad impact on all health services across primary, secondary and tertiary care. The prevalence of AF is expected to double by 2050 because of the aging population, an increasing burden of comorbidities, improved awareness, and new technologies for detection [1]. The lifetime risk of AF is estimated to be 1 in 3–5 individuals over age 55 (37%; 95% CI 34.7–39.6%), and AF-related strokes are expected to rise by 34% in the coming decades. Current European guidelines highlight the need for early detection and structured, integrated management of AF [1,2,3].
AF is linked to multiple risk factors that contribute both to incident disease and increased risks of stroke, heart failure, cognitive decline, hospitalization, and all-cause mortality [4,5]. Sex differences have been consistently observed in AF, affecting pathogenesis, prevalence, clinical presentation, and outcomes [6,7]. Notably, women have a lower overall prevalence of AF but a higher risk of stroke [8,9]. They also tend to present at older ages, report more severe symptoms, and experience poorer quality of life and higher complication rates. Despite these findings, the implications of sex-specific differences for early detection, comorbidity clustering, and risk stratification remain insufficiently explored.
The Atrial Fibrillation Better Care (ABC) pathway [10] has demonstrated that comprehensive management—encompassing stroke prevention, symptom control, and cardiovascular risk optimization—reduces mortality and adverse events. Building on this, the 2024 European Society of Cardiology (ESC) guidelines propose the AF-CARE pathway (Comorbidity and risk factor management, Avoidance of stroke, Rate and rhythm control, and Evaluation and reassessment) as the central framework for AF management [1]. This patient-centered, multidisciplinary model emphasizes addressing underlying conditions and lifestyle factors, preventing thromboembolic events, optimizing rate and rhythm control, and ensuring continuous reassessment. However, most healthcare systems still adopt a reactive approach, with cardiology referral typically occurring only after disease onset or major complications such as stroke or heart failure. In contrast, Primary care is particularly well-positioned to implement a proactive and integrated approach through timely identification and intervention [11] on high-risk individuals before a serious problem arises.
Although several AF prediction scores have been developed [12,13,14,15,16] from primary care electronic health records, their discriminative performance across different clinical settings is unknown and they are not widely integrated into practice. Screening a high-risk population has improved detection and may provide an opportunity to address co-morbid conditions and prevent future cardiovascular outcomes like stroke. Optimal strategies and settings for atrial fibrillation screening remain undefined. A major gap is the lack of sex-specific risk assessment tools, despite well-documented differences between women and men. Personalized risk prediction for AF incidence, AF progression, and associated outcomes remains challenging. The aim of this study was to analyze sex differences in AF incidence and comorbidities in a large community-based cohort, with a focus on high-risk population.
2. Materials and Methods
2.1. Study Design and Setting
The study employed an observational, retrospective cohort design, including individuals identified as being at high risk (Quartile 4) for developing AF. This study analyzed data collected from 1 January 2015 to 31 December 2024. The research was undertaken within the framework of the project Gender Perspective on Cardiovascular Diseases in the Terres de l’Ebre (GECA-TE), part of a doctoral research program. The present work represents a substudy of patients included in previously published investigations [11,17,18,19], with the objective of identifying sex differences in the epidemiology of AF and cardiovascular health. Specifically, the study examined differences in epidemiological patterns, clinical presentation, risk factors, and outcomes within the geographical setting of the Terres de l’Ebre region (Figure S1). The study report followed the Strengthening the Reporting of Observational Studies in 104 Epidemiology (STROBE) guidelines. The research protocol was reviewed and approved by the Ethics Committee of the Jordi Gol University Institute of Primary Care Research (registration number 24/187-P; approval date 12 February 2025).
2.2. Study Scope
The study was carried out in the Terres de l’Ebre Health Region, located in southern Catalonia, Spain (Supplementary Materials). The region includes 178,112 inhabitants (49.6% women) distributed across 54 municipalities, with an average population density of 53.8 inhabitants/km2, compared with 241.8 inhabitants/km2 in Catalonia overall [20,21]. The population is characterized by advanced aging, with an aging index of 159.5, higher than that of Catalonia (131.3) and Spain (118.4) [22]. This index was calculated as the ratio between individuals aged ≥ 65 years and those aged < 15 years per 100 inhabitants. The population aged ≥ 65 years represented 31.1% of the total. The average per capita income was lower than that of Catalonia (77.4% vs. 100%) [23].
The territory comprises four counties and 11 primary care teams (EAPs), all managed by the Catalan Health Institute (ICS) under the authority of the Department of Health (CatSalut). Specialized care is provided at the reference hospital, Hospital Verge de la Cinta (Tortosa), also publicly managed by the ICS. The EAPs operate as independent clinical-functional units. Nearly all residents (99.2%) have an active digital health record in the Shared Health Record of Catalonia (HCC3), enabling continuous monitoring across all levels of care.
2.3. Data Collection and Information Sources
Data were extracted by the Information and Communication Technology Department from the CMBD (Minimum Basic Data Set), a clinical-administrative database that compiles detailed information on healthcare episodes, particularly demographic and clinical data, as well as information on patient procedures, such as diagnoses, treatments, length of stay, and mortality for use in health planning, evaluation, and research; and the Shared Health Record of Catalonia (HCC3), which integrates information from primary and specialist care, hospital admissions, prescriptions, and mortality records. Collected variables included demographic data, comorbidities (hypertension, diabetes, ischemic heart disease, peripheral vascular disease, chronic kidney disease, and cognitive impairment), AF diagnosis, and mortality. Risk scores, including the CHA2DS2-VA (as recommended in the 2024 ESC/EACTS Guidelines on AF) and the Charlson Comorbidity Index, as well as healthcare resource utilization. Diagnoses were coded according to the International Classification of Diseases, 10th Revision (ICD-10). All data were processed in encrypted format.
2.4. Study Population
The study population included 40,079 individuals without a prior diagnosis of AF (Figure 1, flowchart). The primary outcomes were sex-specific incidence of AF and cardiovascular comorbidities, cognitive decline, and all-cause mortality among individuals classified as high risk for AF. The study hypothesis was that incidence of these outcomes prior to AF diagnosis would not differ significantly between men and women.
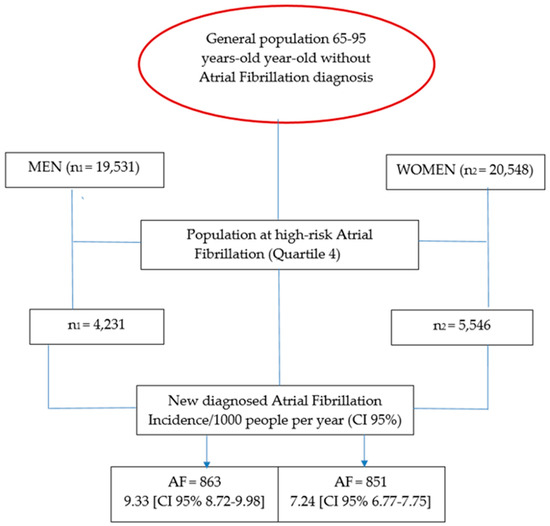
Figure 1.
Flow-chart schedule [12,24].
2.5. Inclusion and Exclusion Criteria
Inclusion criteria: Patients aged 65–95 years were included if they were in the highest quartile (Q4th) of AF risk according to a validated prediction model [12,24], had active medical records accessible through the HCC3/CMBD systems, had no prior diagnosis of AF, and were residents assigned to one of the region’s EAPs. The risk score used to stratify patients was developed and internally validated in a previous population-based cohort [12,24]. The model incorporates age, sex, hypertension, diabetes, vascular disease, heart failure, and body mass index, with predictive performance AUC = 0.78 (95% CI 0.75–0.81). The present study enrolled individuals in the top quartile (Q4) of this score, corresponding to those with ≥75th percentile of predicted AF risk.
Exclusion criteria: Patients with prior stroke to avoid reverse causality between previous cerebrovascular events and subsequent AF detection. Individuals with pacemakers or defibrillators to minimize detection bias associated with continuous rhythm monitoring. In addition, patients who lacked an AF risk index [12,24] or who resided outside the Terres de l’Ebre region were excluded.
2.6. Variables
Clinical events and comorbidities were systematically collected from primary care practices and supplemented through linkage with AI-driven electronic health databases. Follow-up extended until death, loss to follow-up, or 31 December 2024, whichever occurred first. AF diagnosis followed the European Society of Cardiology (ESC) guidelines. Upon AF diagnosis, the event was timestamped, and clinical data for all participants—both those who developed AF and those who did not—were extracted at the end of follow-up. This ensured consistency in data collection across groups.
Cardiovascular risk factors and comorbidities were identified using ICD-10 codes: cerebrovascular disease (I63, G45), heart failure (I50–I51), ischemic heart disease (I20–I25), hypertension (I10–I15), hypercholesterolemia (E78), diabetes mellitus (E10–E14), body mass index (BMI), chronic kidney disease (N18), and estimated glomerular filtration rate (eGFR, mL/min/1.73 m2). Only incident events were included, verified as occurring after the AF diagnosis. Clinical assessments included the Charlson Comorbidity Index, CHA2DS2-VA score, and Pfeiffer Short Mental Status Questionnaire. Biological sex, as registered in patient databases, was used for analysis. Vital status was recorded at the end of follow-up.
2.7. Statistical Analysis
Population characteristics were described using descriptive statistics. Continuous variables are presented as mean ± standard deviation if normally distributed, or median values otherwise; categorical variables are reported as counts and percentages. Student’s t-test was applied for continuous variables, and chi-square tests for categorical variables. Incidence rates were calculated as events per 1000 person-years of follow-up. Person-time was computed as the cumulative follow-up time from AF diagnosis until study end or censoring. Incidence rate ratios (IRRs) were calculated to compare incidence between groups, as these account for time at risk. Two-sided p-values < 0.05 were considered statistically significant. All analyses were performed using IBM SPSS Statistics, version 21.0.
3. Results
3.1. Overall Cohort
Table 1 provides a summary of the baseline characteristics of the study cohort, which consisted of 40,079 individuals between the ages of 65 and 95 years. Data are stratified by sex for the total population. During the study period, 3370 new cases of AF were diagnosed, resulting in an overall prevalence of 8.4%. A significant sex-based difference was noted, with a higher prevalence in men (9.9%) compared to women (7.0%) (p < 0.001). The prevalence of AF in the territory is showed in Figure S2 (Supplementary Materials). The average age was slightly higher for women (77.6 ± 6.63 years) than for men (77.28 ± 6.56 years), a difference that was also statistically significant (p < 0.001). Significant sex-related differences were observed across several comorbidities. Men had a higher prevalence of diabetes mellitus (29.5% vs. 21.9%), peripheral vascular disease (10.2% vs. 3.9%), and ischemic cardiomyopathy (10.6% vs. 4.5%). Conversely, women exhibited a higher prevalence of dyslipidemia (51.7% vs. 43.0%) and dementia or cognitive impairment (10.8% vs. 7.4%). These differences highlight the distinct comorbidity profiles between sexes within this older cohort.

Table 1.
Baseline characteristics of the study population according to sex (n = 40,079).
3.2. High-Risk for Atrial Fibrillation Subgroup (Quartile 4)
Table 2 presents the characteristics of the high-risk subgroup, defined as individuals in the fourth quartile of AF risk (n = 9677). There is a higher prevalence of classic cardiovascular risk factors associated with a heightened AF risk in both sexes and showed sex-specific differences. Within this subgroup, men exhibited a higher mean CHA2DS2-VA score compared with women (4.10 ± 0.97 vs. 3.84 ± 0.88, p < 0.001). Men also demonstrated higher prevalence of new-onset AF (20.4% vs. 15.6%), heart failure (29.2% vs. 26.0%), diabetes mellitus (53.4% vs. 44.3%), stroke (10.8% vs. 9.1%), vascular peripheral disease (24.2% vs. 11.4%), and ischemic cardiomyopathy (26.0% vs. 13.1%). Conversely, women in the high-risk cohort had a higher prevalence of dementia or cognitive impairment (21.5% vs. 14.6%) and dyslipidemia (57.0% vs. 49.1%).

Table 2.
Baseline characteristics by sex in the population at high risk of AF (quartile 4).
3.3. New-Onset AF in the High-Risk AF (Quartile 4) Subgroup
Table 3 presents a sex-stratified comparison of clinical characteristics for individuals in the highest risk quartile based on the incidence of new-onset AF. The data reveals that 20.4% of men and 15.62% of women developed AF during the follow-up period. 563 individuals were excluded from the analysis due to a lack of available data. The table shows significant differences between men who developed AF and those who did not, with those developing AF having a higher CHA2DS2-VA score (4.43 vs. 4.04), higher prevalence of heart failure, stroke, and chronic kidney disease. Among women, those who developed AF exhibited higher CHA2DS2-VA score (4.18 ± 0.9 vs. 3.80 ± 0.8), higher prevalence of heart failure, stroke, vascular peripheral disease, ischemic cardiomyopathy, and chronic kidney disease. They also showed higher Charlson comorbidity index values (2.30 ± 1.4 vs. 1.90 ± 1.38), more hospital visits (0.65 ± 1.5 vs. 0.38 ± 1.2), and greater polypharmacy (8.84 ± 5.2 vs. 7.54 ± 4.9).

Table 3.
Comparative characteristics by sex of high-risk individuals (quartile 4) without AF versus with new-onset AF.
Comparing sex-specific differences within the Q4th-AF subgroup, men exhibited higher CHA2DS2-VA and Charlson comorbidity index scores, along with a greater prevalence of diabetes mellitus, peripheral vascular disease, ischemic cardiomyopathy, and obstructive sleep apnea. In contrast, women presented with higher body mass index and Pfeiffer scores, as well as a greater prevalence of dementia and cognitive impairment. Among all individuals with new-onset AF (n = 863, 20.4%), the prevalence of several comorbidities was higher in the new-onset AF group, including heart failure (55.2% vs. 22.5%), ischemic cardiomyopathy (27.2% vs. 25.9%), peripheral vascular disease (25.0% vs. 23.9%), and chronic kidney disease (41.6% vs. 33.1%). Furthermore, characteristics were significantly more prevalent compared to those without AF: higher CHA2DS2-VA scores (4.43 ± 0.9 vs. 4.04 ± 0.9), higher Charlson comorbidity index scores (2.83 ± 1.4 vs. 2.48 ± 1.5), and a higher number of active medications (8.8 ± 4.8 vs. 6.76 ± 4.8).
In summary, new-onset AF was associated with a substantial comorbidity burden in both sexes, with men exhibiting a predominance of cardiometabolic risk factors and vascular disease, and women more frequently presenting with cognitive impairment. The percentage of individuals with heart failure, vascular peripheral disease and ischemic cardiomyopathy was significantly higher in both men and women who developed new AF compared to their counterparts who did not.
3.4. Clinical Outcomes
Table 4 shows the incidence of cardiovascular events and rate ratios by sex. The data are stratified by AF status within the Q4th, with event rates expressed per 1000 person-years. Among individuals with a new AF diagnosis, men had a higher incidence rate for ischemic heart disease and peripheral artery disease. Women with new-onset AF, however, had a higher rate of chronic kidney disease. No significant differences were detected between the sexes for stroke, heart failure, cognitive impairment, or all-cause mortality in this group. Conversely, among individuals without AF, women had a higher incidence rate of both cognitive impairment and mortality compared to men.

Table 4.
Sex-specific incidence of cardiovascular comorbidities and incidence rate ratios.
4. Discussion
This study focuses on individuals at high risk of developing AF, a population in which cardiovascular comorbidities are frequent, often interrelated and challenging to distinguish [3,25]. Consistent with prior evidence, men developed AF earlier and more frequently, while women presented later with a higher comorbidity burden [26,27]. However, evidence is lacking regarding potential sex-related differences among individuals at high risk of developing AF. This study aimed to characterize sex-specific clinical patterns within individuals already identified as high-risk for AF, rather than to identify new predictors. The analysis explores heterogeneity within the upper quartile of a validated prediction model, complementing prior work on risk development. Addressing this gap could refine risk stratification, screening, and preventive strategies. Table S1 provides a summary of sex differences in patients at high risk of AF (quartile 4) and new AF. These sex-related differences should not be interpreted as causal or as direct consequences of AF, but rather as two complementary patterns:
Intra-sex differences: Comorbidities are largely comparable across sexes, although men exhibit higher mortality rates after diagnosis AF and women in Quartile 4. Within each sex, individuals with AF may differ considerably from those without AF, primarily due to variations in comorbidity profiles and their associated outcomes. Many of these factors are already incorporated into clinical prediction scores [12,13,14,15,16]. Reinforcing their systematic use in primary care—together with comprehensive prevention strategies, opportunistic screening initiatives, and targeted management of comorbidities—could represent a pragmatic approach to mitigating AF risk and improving long-term outcomes [28].
Inter-sex differences: Men and women differ in their comorbidity profiles. Beyond these differences, sex itself influences AF incidence, clinical presentation, and prognosis. Biological mechanisms, including hormonal regulation, atrial remodeling, and disparities in healthcare utilization, may interact with social determinants such as health-seeking behavior and unequal access to treatment [29]. These interactions may partly account for the observed disparities in anticoagulation, rhythm control, and clinical outcomes. Future research directed toward the development and validation of sex-specific risk scores could enhance the understanding of these mechanisms and support follow-up strategies tailored to sex-specific risks [30].
Within high-risk individuals, intra-sex comparisons revealed that AF cases carried a greater comorbidity load than non-AF counterparts, reinforcing the value of systematic use of prediction scores in primary care alongside prevention and opportunistic screening. Inter-sex comparisons showed distinct comorbidity patterns: women more often had hypertension, cognitive impairment, dyslipidemia, and mortality, whereas men more often had diabetes, vascular disease, ischemic cardiomyopathy, OSAHS, and higher Charlson index. Incident AF in both sexes was associated with heart failure, stroke, chronic kidney disease, and OSAHS. Excess mortality was more pronounced in men.
Age remained a major determinant, with AF incidence rising linearly [31,32]. In the very elderly, AF is frequently asymptomatic or manifests with nonspecific symptoms, which may delay diagnosis and consequently heighten the risk of adverse outcomes and reduce survival [33]. Additionally, older patients may experience a lower benefit from rhythm control strategies, such as antiarrhythmic medications and ablation techniques. Findings from European studies showed the ablation procedures for atrial fibrillation were less frequently performed in women [34]. The presence of frailty and cognitive decline can further complicate management by affecting medication adherence and the patient’s capacity to engage in treatment decisions. These two conditions collectively underscore the importance of early AF diagnosis and the management of associated vascular comorbidities.
Within the 4th Quartile, women exhibited a higher prevalence of cognitive impairment and greater mortality compared to men. However, these differences were not statistically significant once an atrial fibrillation diagnosis was established. Differences in sex for prior cardiovascular disease prevalence are well established major risk factors for incident AF [35,36]. The cumulative risk of developing AF was higher in men than in women. The presence of microvascular disease, particularly in those with type 2 diabetes, was independently associated with higher risk of incident atrial fibrillation [37]. Attributable risk over time for atrial fibrillation associated with higher body mass index and most cardiovascular factors included in the CHA2DS2-VA score have been previously described and incorporated into AF risk scores [24,38,39]. The significant increase in heart failure prevalence following an atrial fibrillation diagnosis warrants attention, as the co-occurrence of these conditions is associated with a worse prognosis, particularly for women, who face increased mortality and greater symptom burden compared to men [40]. AF is a known risk factor for cognitive impairment and dementia, and changes like brain atrophy independent of clinical stroke. High-risk individuals, particularly women, also face an increased risk of these conditions, making screening for AF and cognitive impairment a crucial preventive measure for this population [41,42].
The overall age-adjusted mortality rates (AAMRs) for AF-related deaths among adults aged ≥ 65 years showed a steady increase between 1999 and 2020. This rise was accompanied by higher AAMRs among men for conditions frequently associated with an AF diagnosis, including coronary artery disease, heart failure, cognitive impairment, obstructive sleep apnea, and metabolic syndrome [43,44,45]. These conditions and their sex-specific prevalence highlight the substantial mortality burden associated with AF, particularly through its contribution to stroke, heart failure, and cognitive impairment [46]. Thus, AF poses a significant risk for premature mortality. The early identification of high-risk subgroups is critical for implementing targeted preventive strategies and optimizing care for individuals most likely to benefit.
Emerging evidence indicates that an intrinsically prothrombotic atrial substrate may precede the onset of atrial fibrillation and contribute to thromboembolic events independently of the arrhythmia itself. Notably, the incidence of Major Adverse Cardiovascular Events increases progressively from individuals at low risk of atrial fibrillation to those at high risk, with a significantly higher incidence in patients with established AF [19,28]. These patients also frequently present with multiple comorbidities that exert a substantial impact on prognosis and therefore require careful clinical attention. Reflecting this evolving understanding, updated guidelines now conceptualize AF as a continuum that necessitates stage-specific strategies, with particular emphasis on and the comprehensive management of comorbidities and anticoagulation [1,5]
Early detection of AF is paramount because it enables early initiation of treatment that can lower the risk of adverse cardiovascular outcomes, particularly in older patients > 75 or with a CHA2DS2-VA score ≥ 2 and cardiovascular conditions or those with existing comorbidities, as demonstrated by the EAST-AFNET 4 study [47]. Photoplethysmography devices have demonstrated significant utility in the screening and detection of new cases of AF among high-risk patient populations. Studies have shown promising results, with a new AF diagnosis rate of approximately 1 in 10–14 screened individuals, though this rate is contingent on the specific device and patient cohort [3,11,48]. The use of this screening methodology could potentially diagnose a substantial number of additional AF cases. Based on our analysis, the consistent application of these strategies could have led to the identification of up to 600 additional cases of atrial fibrillation, which would supplement the 1724 cases diagnosed during the study period. This finding highlights the substantial potential for proactive screening and early detection of AF diagnosis and underscores a critical need for evaluating and integrating risk prediction tools within primary care. A more targeted approach creates a more efficient care pathway. Once a high-risk patient is identified, they can be directly routed to confirmatory diagnostic tests, which reduces delays and improves the continuity of care [1,10].
The findings demonstrate sex-specific differences in cardiovascular profiles, which are associated with variations in the incidence of atrial fibrillation and are consistent with previous reports describing greater comorbidity burden and worse outcomes in women with AF. Nevertheless, several potentially relevant factors were not considered such as AF-related fibrotic remodeling [49], emerging sex-specific factors [50,51] social determinants of health [52], and AF burden [53]. There is limited evidence on how sex differences in atrial electrical remodeling directly contribute to the observed disparities in risk [7,54]. It remains unclear whether these sex differences are related to intrinsic biological disparities in atrial remodeling or by a greater burden of comorbidities in one sex versus the other.
The main strengths of this study include its community-based design, large sample size, and long follow-up period, which enhance the external validity of our findings and reflect real-world primary care practice. While most large-scale studies derive from hospital or registry data, our findings suggest that sex-specific differences are also evident in primary care populations and may influence preventive strategies. The longitudinal design (2015–2024) further strengthens the relevance of our results, allowing assessment of temporal changes in risk factor management. Limitations include its retrospective design, lack of detailed information on pharmacological treatment, socioeconomic status, AF burden, and fibrotic remodeling, which may influence AF risk. Although sex and comorbidities form part of the variables included in the risk score, our subgroup analysis examined residual heterogeneity within the highest-risk quartile and the persistence of sex-specific differences within this stratum suggests that factors beyond the model’s weighting—such as cognitive decline or differential healthcare use—may further modulate AF incidence. Eventually multivariable modeling could improve robustness, but we opted for univariate analyses, because the high-risk subgroup was defined using a composite score that already integrates the main confounding variables. Further adjustment would have introduced collinearity and model instability. Despite these limitations, the findings support the recommendations of the AF-CARE Guidelines, particularly regarding the importance of early detection and management of comorbidities, highlighting the need for tailored strategies to address sex-specific risk patterns.
These findings should be interpreted within the context of a cohort defined by an internally risk score and primarily serve to generate hypotheses for sex-specific preventive strategies rather than to infer causality or establish universal risk gradients. Given the rapidly increasing global burden of atrial fibrillation, our findings highlight the need for a targeted public health strategy, particularly within the primary care setting. Our study suggests three core objectives to modify the impact of this condition: Enhancing Awareness of Atrial Fibrillation, Implementing Proactive Screening Strategies, and Targeting personalized Modifiable Risk Factors.
5. Conclusions
Sex differences were evident within this large population-based cohort. Men exhibited higher comorbidity clustering, particularly cardiometabolic disorders, while women more often presented with cognitive decline and greater comorbidity burden at diagnosis. These results underline the importance of adopting a sex-sensitive approach to AF prevention, early detection, and integrated management within primary care. These results reinforce guideline recommendations for risk stratification, tailored strategies for early AF detection and comprehensive management of comorbidities, supporting the implementation of sex-specific strategies to optimize patient outcomes, and contribute to more equitable healthcare.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13112814/s1: Table S1. Summary of sex differences in patients at high-risk of AF (quartile 4) and new AF. Figure S1. Spanish map and the territory of Terres de l’Ebre. Figure S2. Prevalence of Atrial Fibrillation in the territory Terres de l’Ebre (May 2025).
Author Contributions
Conceptualization, J.L.C.-E., A.P.-T., S.R.-V. and J.L.-N.; methodology, J.L.C.-E., A.P.-T. and S.R.-V.; software, J.L.C.-E., A.P.-T. and P.M.-B.; validation, J.L.C.-E., A.P.-T. and S.R.-V.; formal analysis, J.L.C.-E., A.P.-T., S.R.-V. and P.M.-B.; investigation, A.H.-P., J.C.-Q., E.M.-S., T.F.-A. and A.P.-T.; resources, J.L.C.-E., A.P.-T., E.M.-S., J.C.-Q., T.F.-A. and S.R.-V.; data curation, J.L.C.-E. and A.P.-T.; writing—original draft preparation, J.L.C.-E., A.P.-T. and S.R.-V.; writing—review and editing, J.L.C.-E., A.P.-T. and S.R.-V.; visualization, A.H.-P., J.C.-Q., E.M.-S., T.F.-A. and P.M.-B.; supervision, J.L.C.-E. and S.R.-V.; project administration, A.P.-T.; funding acquisition, A.P.-T. and J.L.C.-E. All authors have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Rovira I Virgili University (URV) and received a pre-doctoral grant (File number: PREDOC_ECO-24/11 and funding code: 7Z25/024-1) within the framework of the “Public Call for the Pursuit of a Doctorate by Healthcare Professionals in the Primary Care Setting of the ICS,” issued by IDIAP Jordi Gol in collaboration with the Catalan Health Institute.
Institutional Review Board Statement
The study was approved by the Ethical Committee of Jordi Gol University Institute of Primary Care Research (protocol code 24/187-P, approval date 12 February 2025).
Informed Consent Statement
Not applicable (retrospective study using anonymized records).
Data Availability Statement
The data supporting the findings of this study are not currently publicly available but can be requested from the authors upon reasonable request. These data will be available through an institutional repository following the public defense of the corresponding PhD thesis.
Acknowledgments
We are grateful to the clinical and administrative staff of the participating centers and the Information and Communication Technology Department (Jesus Carot-Domenech). During the preparation of this study, the authors used AI (chat.openai.com) to improve exclusively the language editing purposes. The authors have reviewed and edited the output and take full responsibility for the content of this publication. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | Atrial Fibrillation |
| ABC pathway | Atrial fibrillation Better Care |
| ESC | European Society of Cardiology |
| AF-CARE | AF Comorbidity Avoidance of stroke Rate and rhythm control Evaluation |
| EAPs | Primary Care teams |
| ICS | Catalan Health Institute |
| HCC3 | Shared Health records of Catalonia |
| CMBD | Minimum Basic Data set |
| IRRs | Incidence Rate Ratios |
| AAMRs | Age-Adjusted Mortality Rates |
| EAST-AFNET study | Early Treatment of Atrial Fibrillation for Stroke Prevention Trial |
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414, Erratum in Eur. Heart J. 2025, 46, ehaf306. https://doi.org/10.1093/eurheartj/ehaf306. [Google Scholar] [CrossRef]
- Linz, D.; Hermans, A.; Tieleman, R.G. Early atrial fibrillation detection and the transition to comprehensive management. EP Europace 2021, 23 (Suppl. S2), ii46–ii51. [Google Scholar] [CrossRef]
- Svennberg, E.; Freedman, B.; Andrade, J.G.; Anselmino, M.; Biton, Y.; Boriani, G.; Brandes, A.; Buckley, C.M.; Cameron, A.; Clua-Espuny, J.L.; et al. Recent-onset atrial fibrillation: Challenges and opportunities. Eur. Heart J. 2025, ehaf478. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H. Managing high-risk atrial fibrillation patients with multiple comorbidities. Int. J. Arrhythm. 2023, 24, 4. [Google Scholar] [CrossRef]
- Riesgo, A.; Sant, E.; Benito, L.; Hoyo, J.; Miró, O.; Mont, L.; Bragulat, E.; Coll-Vinent, B. Sex differences in the treatment of patients with atrial fibrillation: Population-based study in a local health district. Rev. Esp. Cardiol. 2011, 64, 233–236. [Google Scholar] [CrossRef]
- Odening, K.E.; Deiß, S.; Dilling-Boer, D.; Didenko, M.; Eriksson, U.; Nedios, S.; Ng, F.S.; Roca Luque, I.; Sanchez Borque, P.; Vernooy, K.; et al. Mechanisms of sex differences in atrial fibrillation: Role of hormones and differences in electrophysiology, structure, function, and remodelling. EP Europace 2019, 21, 366–376. [Google Scholar] [CrossRef]
- Jeong, J.H.; Choi, J.I. A ‘Gender Paradox’ of Female as a Stroke Risk in Atrial Fibrillation: Do Women Live Longer Than Men? Korean Circ. J. 2022, 52, 604–605. [Google Scholar] [CrossRef]
- Nakamizo, T.; Misumi, M.; Takahashi, T.; Kurisu, S.; Matsumoto, M.; Tsujino, A. Female “Paradox” in Atrial Fibrillation—Role of Left Truncation Due to Competing Risks. Life 2023, 13, 1132. [Google Scholar] [CrossRef]
- Lip, G. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Clua-Espuny, J.L.; Hernández-Pinilla, A.; Gentille-Lorente, D.; Muria-Subirats, E.; Forcadell-Arenas, T.; de Diego-Cabanes, C.; Ribas-Seguí, D.; Diaz-Vilarasau, A.; Molins-Rojas, C.; Palleja-Millan, M.; et al. Evidence Gaps and Lessons in the Early Detection of Atrial Fibrillation: A Prospective Study in a Primary Care Setting (PREFATE Study). Biomedicines 2025, 13, 119. [Google Scholar] [CrossRef]
- Abellana, R.; Gonzalez-Loyola, F.; Verdu-Rotellar, J.M.; Bustamante, A.; Palà, E.; Clua-Espuny, J.L.; Montaner, J.; Pedrote, A.; Del Val-Garcia, J.L.; Ribas Segui, D.; et al. Predictive model for atrial fibrillation in hypertensive diabetic patients. Eur. J. Clin. Investig. 2021, 51, e13633. [Google Scholar] [CrossRef]
- Alonso, A.; Krijthe, B.P.; Aspelund, T.; Stepas, K.A.; Pencina, M.J.; Moser, C.B.; Sinner, M.F.; Sotoodehnia, N.; Fontes, J.D.; Janssens, A.C.; et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The CHARGE-AF consortium. J. Am. Heart Assoc. 2013, 2, e000102. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Soliman, E.Z.; Chambless, L.E.; Crow, R.; Ambrose, M.; Alonso, A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am. J. Cardiol. 2011, 107, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Wang, L.; Zhang, T.; Gao, H.; Pastori, D.; Liang, Z.; Lip, G.Y.H.; Wang, Y. The mC2HEST Score for Incident Atrial Fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Adv. 2025, 4, 101521. [Google Scholar] [CrossRef] [PubMed]
- Segan, L.; Canovas, R.; Nanayakkara, S.; Chieng, D.; Prabhu, S.; Voskoboinik, A.; Sugumar, H.; Ling, L.H.; Lee, G.; Morton, J.; et al. New-onset atrial fibrillation prediction: The HARMS2-AF risk score. Eur. Heart J. 2023, 44, 3443–3452. [Google Scholar] [CrossRef]
- Clua-Espuny, J.L.; Panisello-Tafalla, A.; Lucas-Noll, J.; Muria-Subirats, E.; Forcadell-Arenas, T.; Carrera-Ortiz, J.M.; Molto-Balado, P.; Clua-Queralt, J.; Fusté-Anguera, I.; Reverte-Vilarroya, S.; et al. Stroke Risk Stratification in Incident Atrial Fibrillation: A Sex-Specific Evaluation of CHA2DS2-VA and CHA2DS2-VASc. J. Cardiovasc. Dev. Dis. 2025, 12, 259. [Google Scholar] [CrossRef]
- Lucas-Noll, J.; Clua-Espuny, J.L.; Carles-Lavila, M.; Solà-Adell, C.; Roca-Burgueño, Í.; Panisello-Tafalla, A.; Gavaldà-Espelta, E.; Queralt-Tomas, L.; Lleixà-Fortuño, M. Sex Disparities in the Direct Cost and Management of Stroke: A Population-Based Retrospective Study. Healthcare 2024, 12, 1369. [Google Scholar] [CrossRef]
- Moltó-Balado, P.; Reverté-Villarroya, S.; Monclús-Arasa, C.; Balado-Albiol, M.T.; Baset-Martínez, S.; Carot-Domenech, J.; Clua-Espuny, J.L. Heart Failure and Major Adverse Cardiovascular Events in Atrial Fibrillation Patients: A Retrospective Primary Care Cohort Study. Biomedicines 2023, 11, 1825. [Google Scholar] [CrossRef]
- Pla de Salut de la Regió Sanitària Terres de l’Ebre 2021–2025; Direcció General de Planificació i Recerca en Salut: Tortosa, Spain, 2022; Available online: https://scientiasalut.gencat.cat/handle/11351/7964 (accessed on 5 December 2024).
- Idescat. Indicadors Demogràfics i de Territori. Estructura per Edats, Envelliment i Dependència. Comarques i Aran; Institut d’Estadística de Catalunya: Barcelona, Spain, 2025; Available online: http://www.idescat.cat/pub/?id=inddt&n=915&by=com (accessed on 5 December 2024).
- Generalitat de Catalunya. Projeccions de Població Principals Resultats 2013–2051; Institut d’Estadística de Catalunya: Barcelona, Spain, 2008; Available online: https://www.idescat.cat/serveis/biblioteca/docs/cat/pp2021-2041pr.pdf (accessed on 5 December 2024).
- Idescat. Anuario Estadístico de Cataluña. Renda Familiar Disponible Bruta. Índex. Comarques i Aran, i Àmbits; Institut d’Estadística de Catalunya: Barcelona, Spain, 2024; Available online: http://www.idescat.cat/pub/?id=aec&n=941 (accessed on 5 December 2024).
- Muria-Subirats, E.; Clua-Espuny, J.L.; Ballesta-Ors, J.; Lorman-Carbó, B.; Lechuga-Durán, I.; Fernández-Sáez, J.; Pla-Farnós, R. Incidence and Risk Assessment for Atrial Fibrillation at 5 Years: Hypertensive Diabetic Retrospective Cohort. Int. J. Environ. Res. 2020, 17, 3491. [Google Scholar] [CrossRef]
- Wu, G.; Wu, J.; Lu, Q.; Cheng, Y.; Mei, W. Association between cardiovascular risk factors and atrial fibrillation. Front. Cardiovasc. Med. 2023, 10, 1110424. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, K.P.; Chen, L.Y.; Norby, F.L.; Soliman, E.Z.; Koton, S.; Alonso, A. Thirty-Year Trends in the Incidence of Atrial Fibrillation: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e023583. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Van Gelder, I.C.; Desteghe, L.; EHRA-PATHS Investigators. ESC and EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: The Horizon 2020 EHRA-PATHS project. Eur. Heart J. 2022, 43, 1450–1452. [Google Scholar] [CrossRef]
- Westerman, S.; Wenger, N. Gender Differences in Atrial Fibrillation: A Review of Epidemiology, Management, and Outcomes. Curr. Cardiol. Rev. 2019, 15, 136–144. [Google Scholar] [CrossRef]
- Piccini, J.P.; Simon, D.N.; Steinberg, B.A.; Thomas, L.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; Hylek, E.; Kowey, P.R.; Reiffel, J.A.; et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results From the ORBIT-AF Registry. JAMA Cardiol. 2016, 1, 282–291. [Google Scholar] [CrossRef]
- Roten, L.; Goulouti, E.; Lam, A.; Elchinova, E.; Nozica, N.; Spirito, A.; Wittmer, S.; Branca, M.; Servatius, H.; Noti, F.; et al. Age and Sex Specific Prevalence of Clinical and Screen-Detected Atrial Fibrillation in Hospitalized Patients. J. Clin. Med. 2021, 10, 4871. [Google Scholar] [CrossRef]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef]
- Romiti, G.; Corica, B.; Mei, D.A.; Bisson, A.; Boriani, G.; Olshansky, B.; Chao, T.-F.; Huisman, M.V.; Proietti, M.; Lip, G.Y.H.; et al. Patterns of comorbidities in patients with atrial fibrillation and impact on management and long-term prognosis: An analysis from the Prospective Global GLORIA-AF Registry. BMC Med. 2024, 22, 151. [Google Scholar] [CrossRef]
- Volgman, A.S.; Benjamin, E.J.; Curtis, A.B.; Fang, M.C.; Lindley, K.J.; Naccarelli, G.V.; Pepine, C.J.; Quesada, O.; Vaseghi, M.; Waldo, A.L.; et al. American College of Cardiology Committee on Cardiovascular Disease in Women. Women and atrial fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 2793–2807. [Google Scholar] [CrossRef]
- Ball, J.; Carrington, M.J.; Wood, K.A.; Stewart, S. Women versus men with chronic atrial fibrillation: Insights from the Standard versus Atrial Fibrillation spEcific managemenT studY (SAFETY). PLoS ONE 2013, 8, e65795. [Google Scholar] [CrossRef]
- Potpara, T.S.; Marinkovic, J.M.; Polovina, M.M.; Stankovic, G.R.; Seferovic, P.M.; Ostojic, M.C.; Lip, G.Y. Gender-related differences in presentation, treatment and long-term outcome in patients with first-diagnosed atrial fibrillation and structurally normal heart: The Belgrade atrial fibrillation study. Int. J. Cardiol. 2012, 161, 39–44. [Google Scholar] [CrossRef]
- Kaze, A.D.; Yuyun, M.F.; Fonarow, G.C.; Echouffo-Tcheugui, J.B. Burden of Microvascular Disease and Risk of Atrial Fibrillation in Adults with Type 2 Diabetes. Am. J. Med. 2022, 135, 1093–1100.e2. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Vinayagamoorthy, M.; Gencer, B.; Ng, C.; Pester, J.; Cook, N.R.; Lee, I.M.; Buring, J.; Manson, J.E.; Albert, C.M. Sex Differences in Atrial Fibrillation Risk: The VITAL Rhythm Study. JAMA Cardiol. 2022, 7, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Li, Z.; O’Brien, E.C.; Pritchard, J.; Chew, D.S.; Bunch, T.J.; Mark, D.B.; Nabutovsky, Y.; Greiner, M.A.; Piccini, J.P. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm. 2021, 18, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Yee Chen, L.; et al. Atrial Fibrillation and Dementia: A Report From the AF-SCREEN International Collaboration. Circulation 2022, 145, 392–409, Erratum in Circulation 2022, 145, e842. https://doi.org/10.1161/CIR.0000000000001067. [Google Scholar] [CrossRef]
- Passey, S.; Patel, J.; Patail, H.; Aronow, W. Association of Atrial Fibrillation and Cognitive Dysfunction: A Comprehensive Narrative Review of Current Understanding and Recent Updates. J. Clin. Med. 2024, 13, 5581. [Google Scholar] [CrossRef]
- Hassan, I.N.; Ibrahim, M.; Yaqub, S.; Ibrahim, M.; Abdalla, H.; Aljaili, G.; Osman, W.; Abuassa, N.; Ashraf, H.; Shoukat, M. Trends in Atrial Fibrillation-Related Mortality Among Older Adults With Obstructive Sleep Apnea in the United States, 1999–2020. Clin. Cardiol. 2025, 48, e70178. [Google Scholar] [CrossRef]
- Sohail, M.U.; Batool, R.M.; Saad, M.; Waqas, S.A.; Noushad, M.A.; Sohail, M.O.; Bates, M.; Ahmed, R.; Ripley, D. Trends in Mortality Related to Atrial Fibrillation and Dementia in Older Adults in the United States: A 2000–2020 Analysis. J. Cardiovasc. Electrophysiol. 2025, 36, 1234–1243. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, L.; Ali, S.M.E.; Ashraf, S.; Bhimani, S.; Kumar, S.; Raja, A.; Bs, A.; Ayalew, B.D.; Malik, M.H.B.A. Trends in United States mortality among patients with atrial fibrillation/flutter related heart failure (1999–2024): Disparities by gender, race/ethnicity and region. BMC Cardiovasc. Disord. 2025, 25, 558. [Google Scholar] [CrossRef] [PubMed]
- van den Dries, C.J.; van Doorn, S.; Rutten, F.H.; Oudega, R.; van de Leur, S.J.C.M.; Elvan, A.; Oude Grave, L.; Bilo, H.J.G.; Moons, K.G.M.; Hoes, A.W.; et al. Integrated management of atrial fibrillation in primary care: Results of the ALL-IN cluster randomized trial. Eur. Heart J. 2020, 41, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Dickow, J.; Kany, S.; Roth Cardoso, V.; Ellinor, P.T.; Gkoutos, G.V.; Van Houten, H.K.; Kirchhof, P.; Metzner, A.; Noseworthy, P.A.; Yao, X.; et al. Outcomes of Early Rhythm Control Therapy in Patients with Atrial Fibrillation and a High Comorbidity Burden in Large Real-World Cohorts. Circ. Arrhythm. Electrophysiol. 2023, 16, e011585. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.A. Different by Design: Heterogeneity in Models of Risk Prediction and Clinical Decision Support in Screening for Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2025, 18, e012281. [Google Scholar] [CrossRef]
- Veen, D.; Ye, Z.; van Schie, M.S.; Knops, P.; Kavousi, M.; Vos, L.; Yildirim, V.; Taverne, Y.J.H.J.; de Groot, N.M.S. Sex differences in atrial potential morphology. Int. J. Cardiol. Heart Vasc. 2025, 56, 101597. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Salehi Omran, S.; Leppert, M. Female-Specific Risk Factors in Cardiovascular Disease: Important or Superfluous? Circ. Cardiovasc. Qual. Outcomes 2024, 17, e011666. [Google Scholar] [CrossRef]
- Houle, J.; Proietti, M.; Raparelli, V.; Atzema, C.L.; Norris, C.M.; Abrahamowicz, M.; Lip, G.Y.; Boriani, G.; Pilote, L. Gendered social determinants of health and risk of major adverse outcomes in atrial fibrillation. Eur. J. Intern. Med. 2025, 135, 83–90. [Google Scholar] [CrossRef]
- Tan, J.L.; Johnson, L.; Dziubinski, M.; Napiorkowski, N.; Witkowska, O.; Slusarczyk, M.E.; Healey, J.S.; Russo, A.M. Sex differences in presentation of atrial fibrillation: Findings from 30-day ambulatory monitoring in real-world practice. Am. Heart J. Plus 2022, 22, 100208. [Google Scholar] [CrossRef]
- Duarte, F.; Silva-Teixeira, R.; Aguiar-Neves, I.; Almeida, J.G.; Fonseca, P.; Monteiro, A.V.; Oliveira, M.; Gonçalves, H.; Ribeiro, J.; Caramelo, F.; et al. Sex differences in atrial remodeling and atrial fibrillation recurrence after catheter ablation. Heart Rhythm. 2025, 22, e563–e571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).