Resistin as Modulator of Functional Activity of Phagocytes in Colostrum and Blood of Overweight and Obese Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sampling and Cell Isolation
2.3. Colostrum Sampling and Cell Isolation
2.4. Resistin Quantification
2.5. Treatment of Colostral and Blood Phagocytes
2.6. Escherichia Coli Strain
2.7. Superoxide Release Assay
2.8. CuZn-Superoxide Dismutase (SOD) Activity
2.9. Bactericidal Assay
2.10. Intracellular Ca2+ Release Determination
2.11. Statistical Analysis
3. Results
3.1. Resistin Levels
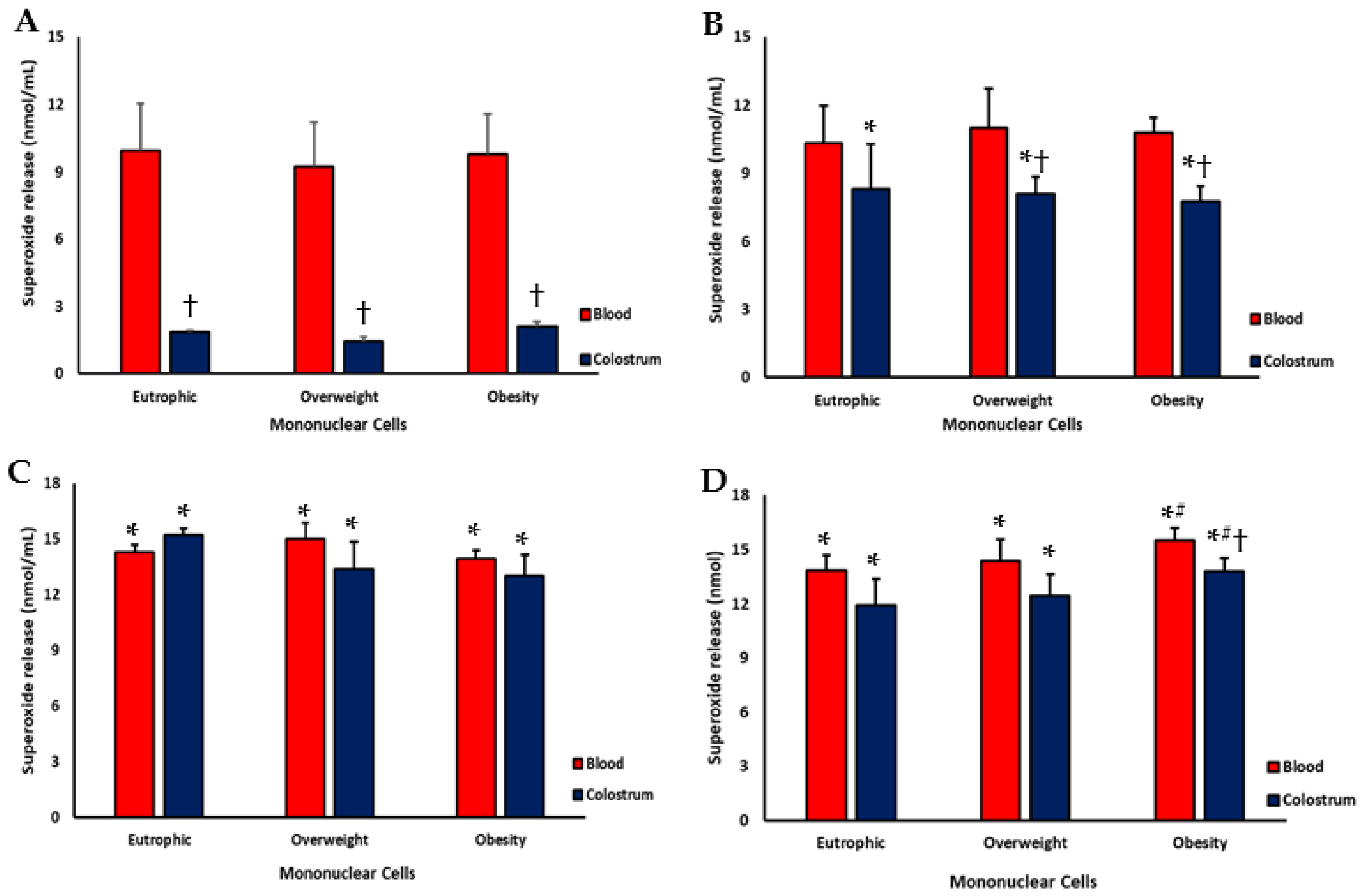
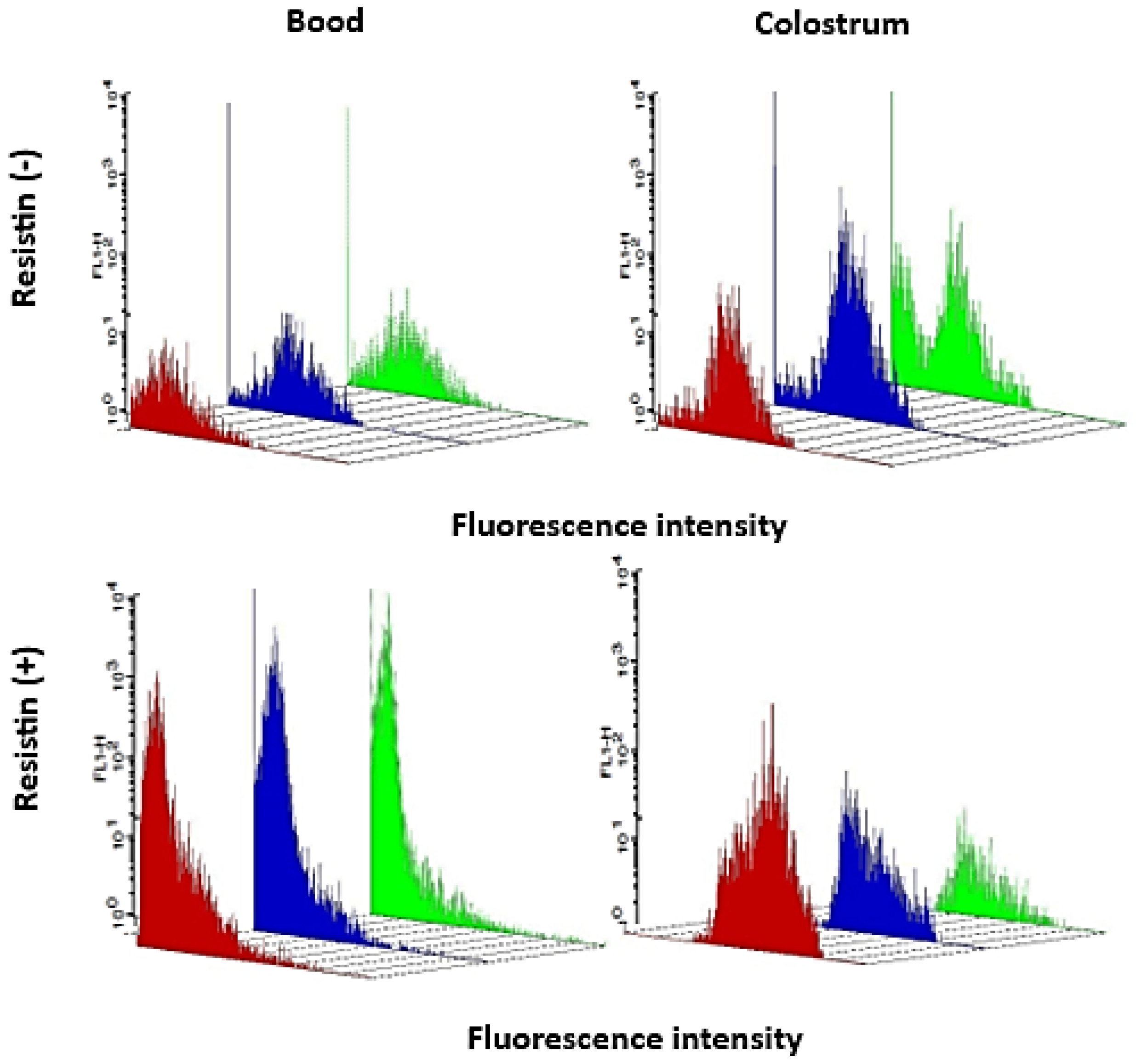
3.2. Superoxide Release
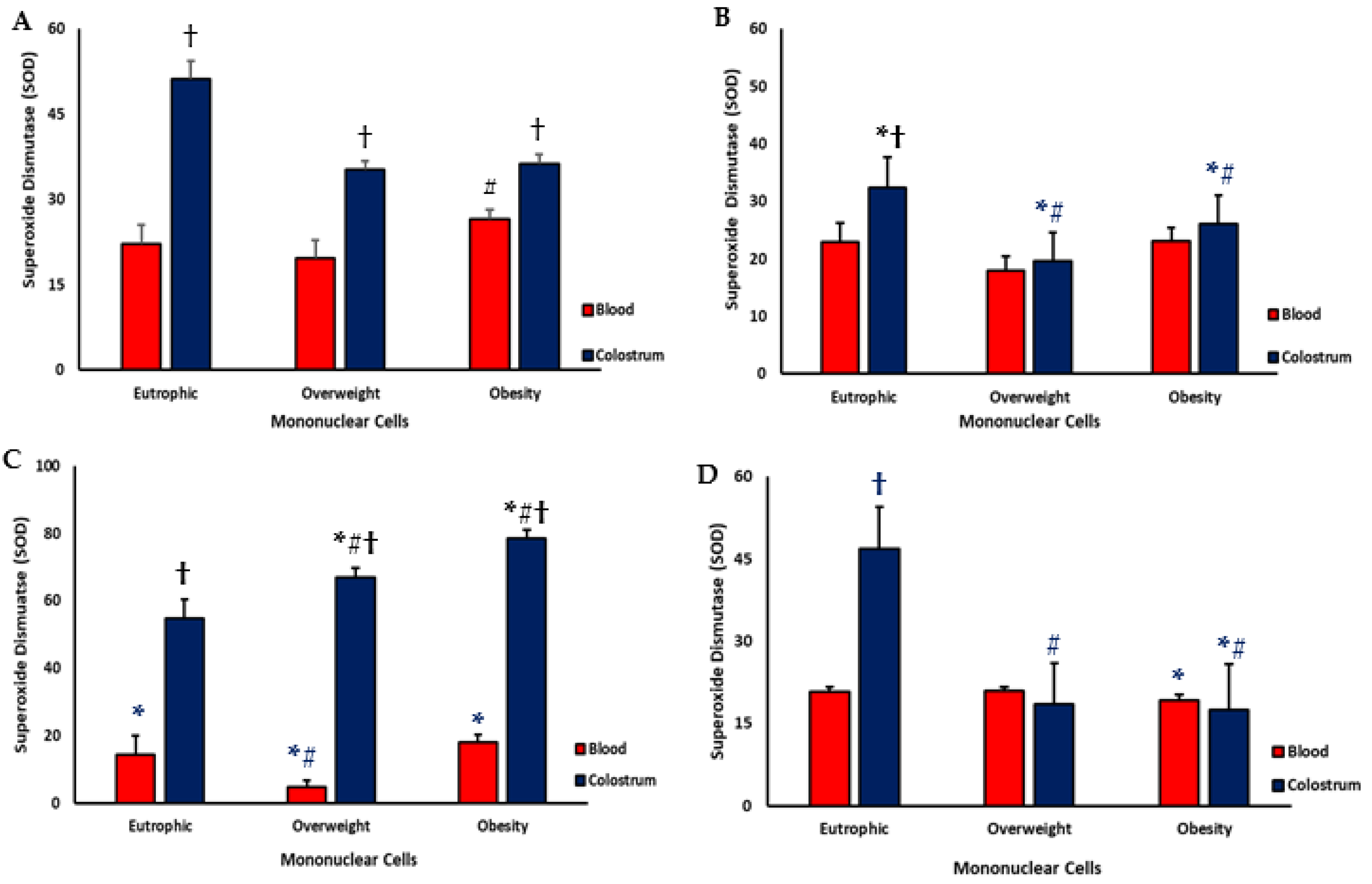
3.3. Superoxide Dismutase (SOD)
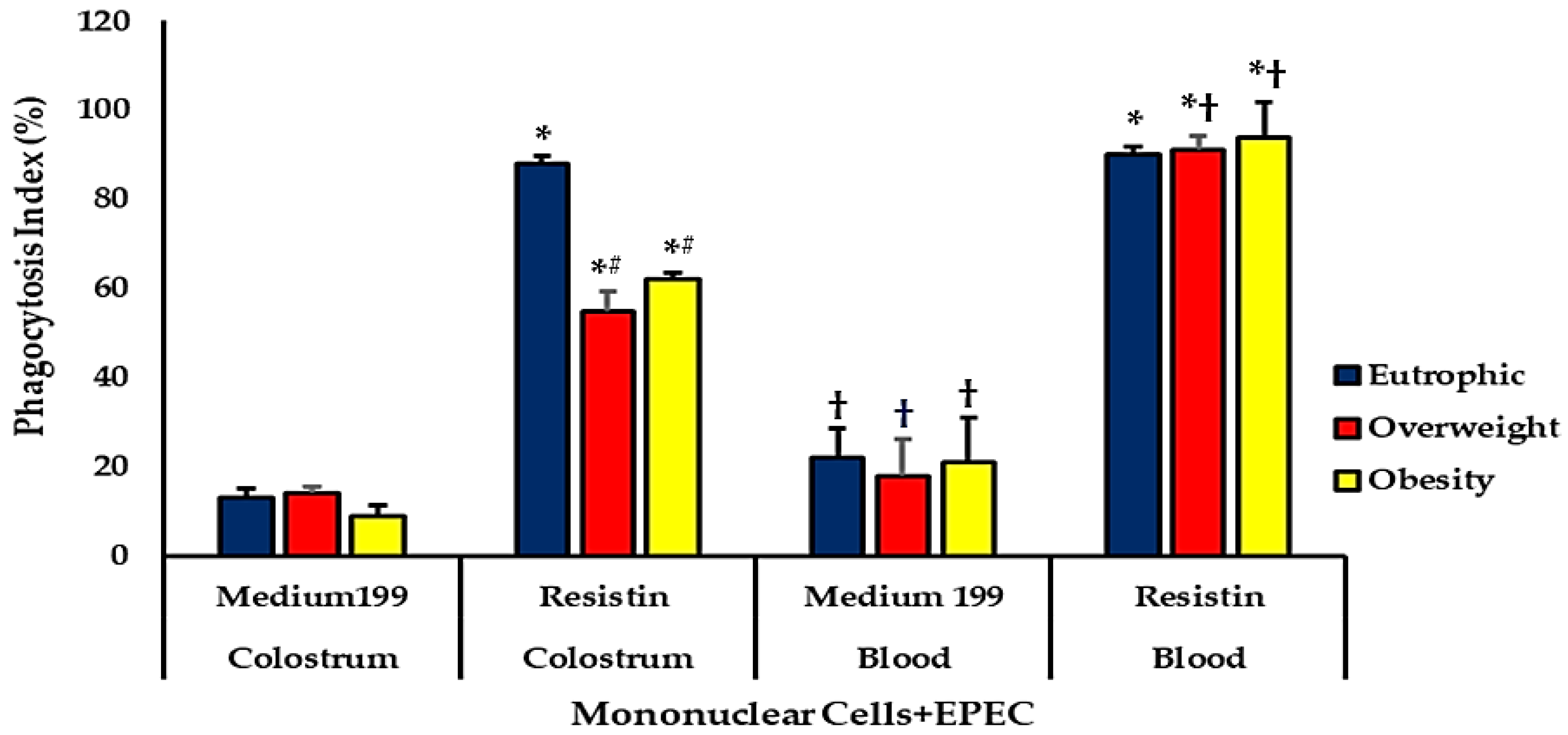
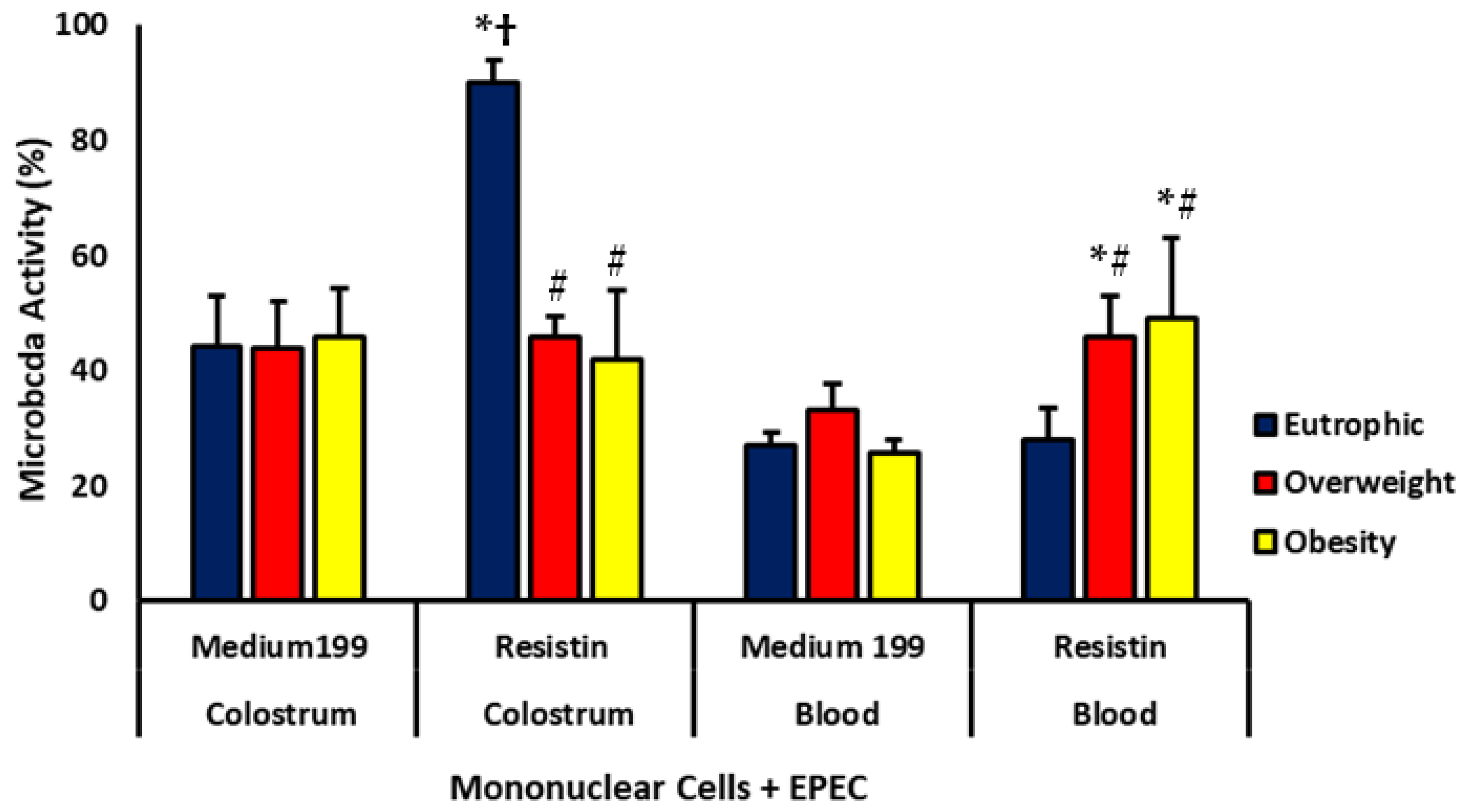
Phagocytosis and Microbicidal Activities of Blood and Colostrum Phagocytes
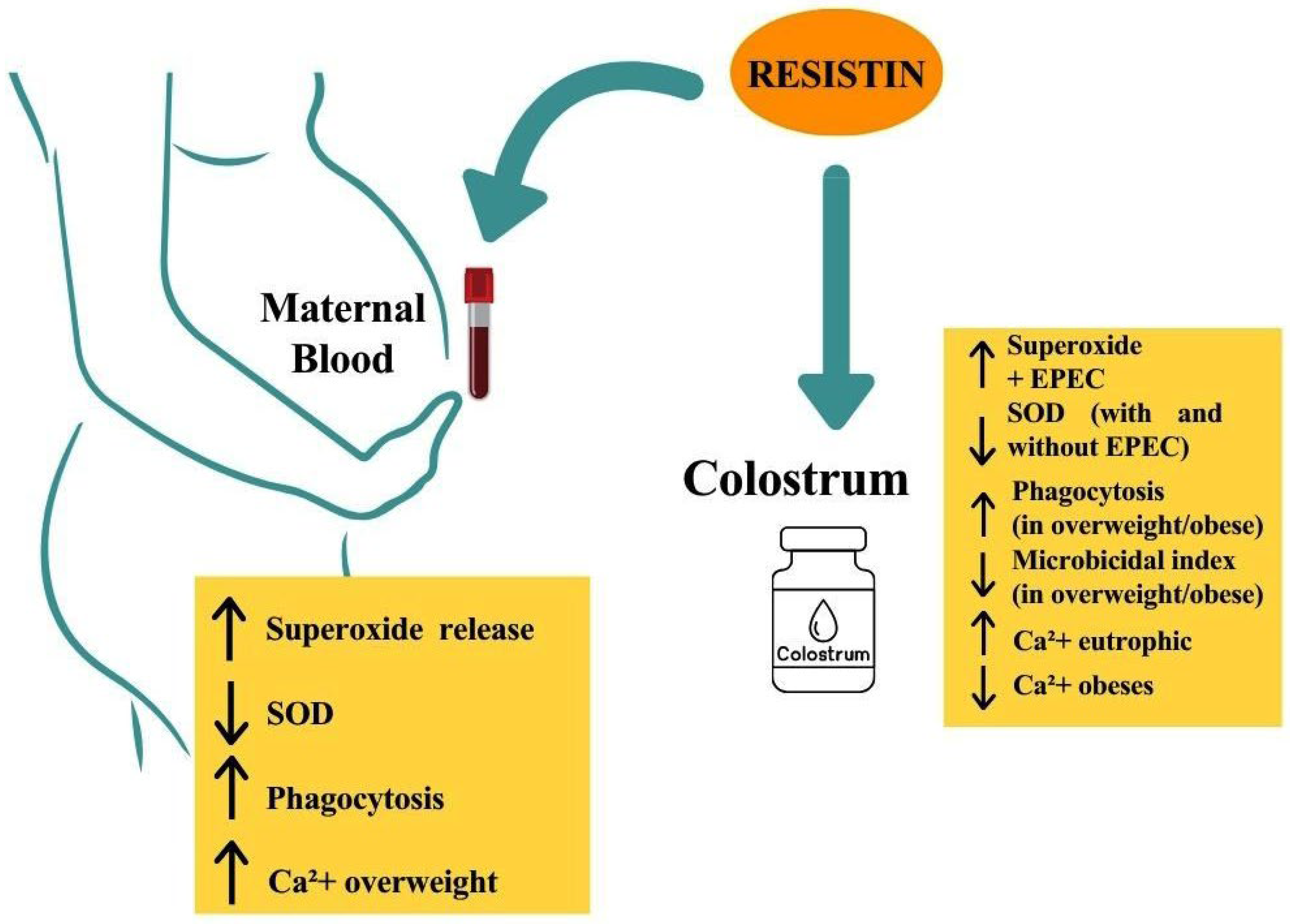
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, F.S.; Alsaykhan, A.M.; Almatrood, A.A. Obesity Prevalence and Its Impact on Maternal and Neonatal Outcomes in Pregnant Women: A Systematic Review. Cureus 2024, 16, e75262. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, E.; Deng, L.; Zhu, Y.; Lu, X.; Li, X.; Li, F.; Yan, Y.; Han, J.Y.; Li, Y.; et al. Immunological Roles for Resistin and Related Adipokines in Obesity-Associated Tumors. Int. Immunopharmacol. 2024, 142, 112911. [Google Scholar] [CrossRef] [PubMed]
- Çatli, G.; Dündar, N.O.; Dündar, B.N. Adipokines in breast milk: An update. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 192–201. [Google Scholar] [CrossRef]
- Dalcin, L.D.L.; Fagundes-Triches, D.L.G.; de Queiroz, A.A.; Torres, A.H.F.; França, D.C.H.; Soares, T.A.; Ramos, L.C.S.; Souza Antônio, C.R.S.; Fujimori, M.; França, E.L.; et al. Resistin modulates the functional activity of colostral macrophages from mothers with obesity and diabetes. Biomedicines 2022, 10, 2332. [Google Scholar] [CrossRef]
- Gámez-Valdez, J.S.; Corona-Cervantes, K.; Sánchez-Salguero, E.S.; Alcorta-García, M.R.; López-Villaseñor, C.N.; Carballo-Castañeda, R.A.; Moreno-Ulloa, A.; Lara-Díaz, V.J.; Brunck, M.E.G.; Licona-Cassani, C. Analysis of human colostrum reveals differential co-occurrence networks of metabolites, microbiota and cytokines in maternal obesity. Food Funct. 2025, 16, 5900–5916. [Google Scholar] [CrossRef]
- Pereira, G.d.A.V.; Morais, T.C.; França, E.L.; Daboin, B.E.G.; Bezerra, I.M.P.; Pessoa, R.S.; de Quental, O.B.; Honório-França, A.C.; Abreu, L.C.d. Leptin, Adiponectin, and Melatonin Modulate Colostrum Lymphocytes in Mothers with Obesity. Int. J. Mol. Sci. 2023, 24, 2662. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Lima, M.C.; Lira, P.I.C.; Silva, G.A.P. Low birth weight and obesity: Causal or casual association? Rev. Paul. Pediatr. 2015, 33, 340–348. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Abrams, S. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Honorio-França, A.C.; Hara, C.C.P.; Ormonde, J.V.J.; Triches, G.N.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human milk lipidomics: Current techniques and methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.P. Protective effects of human milk antimicrobial peptides against bacterial infection. J. Pediatr. 2015, 91, 4–5. [Google Scholar] [CrossRef]
- Masi, A.C.; da Silva, J.M.; Oliveira, R.S.; Carvalho, L.P.; Santos, M.F.; Almeida, T.C. Role of Breastfeeding in Disease Prevention. Fron. Pediatr. 2024, 11, 1086999. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Carvalho, M.P.S.M.; Isaac, L.; Trabulsi, L.R.; Carneiro-Sampaio, M.M.S. Colostral mononuclear phagocytes are able to kill Enteropathogenic Escherichia coli opsonized with colostral IgA. Scand. J. Immunol. 1997, 46, 59–66. [Google Scholar] [CrossRef]
- Novelli, E.L.; Rodríguez, N.L.; França, E.L.; Gebra, L.M.N.; Ribas, B.O. High dietary carbohydrate and pancreatic lesion. Braz. J. Med. Biol. Res. 1993, 26, 31–36. [Google Scholar]
- França, E.L.; Silva, V.A.; Volpato, R.M.J.; Silva, P.A.; Brune, M.F.S.S.; Honorio-França, A.C. Maternal anemia induces changes in immunological and nutritional components of breast milk. J. Matern.-Fetal Neonatal Med. 2013, 26, 1223–1227. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Morceli, G.; Rudge, M.V.C.; Calderon, I.D.M.P.; Honorio-França, A.C. The role of cytokines in the functional activity of phagocytes in blood and colostrum of diabetic mothers. Clin. Dev. Immunol. 2013, 2013, 590190. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, P.; Singh, A.K.; Gupta, V. Association of Adipokines with Insulin Resistance and Metabolic Syndrome Including Obesity and Diabetes. GHM Open 2023, 3, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Li, Z. Adipokines in Glucose and Lipid Metabolism. Adipocyte 2023, 12, 2202976. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.A.; Peeran, S.W.; Basheer, S.N.; Elhassan, A.; Alam, M.N.; Thiruneervannan, M. Role of resistin in various diseases with special emphasis on periodontal and periapical inflammation—A review. J. Pharm. Bioallied Sci. 2023, 15 (Suppl. 1), S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.M.R.; Barbosa, L.E.R. Adipose tissue-derived stem cells: A new approach to the treatment of Crohn’s disease-associated perianal fistulae. J. Coloproctol. 2018, 38, 240–245. [Google Scholar] [CrossRef]
- Suwaydi, M.A.; Gridneva, Z.; Perrella, S.L.; Wlodek, M.E.; Lai, C.T.; Geddes, D.T. Human Milk Metabolic Hormones: Analytical Methods and Current Understanding. Int. J. Mol. Sci. 2021, 22, 8708. [Google Scholar] [CrossRef]
- Savino, F.; Sardo, A.; Rossi, L.; Benetti, S.; Savino, A.; Silvestro, L. Adiponectin, resistin, and leptin in breast milk and their relationship to maternal nutritional status and infant growth. Eur. J. Clin. Nutr. 2012, 66, 1083–1089. [Google Scholar] [CrossRef]
- Froń, A.; Tomecka, P.; Orczyk-Pawiłowicz, M. Impact of Maternal Overweight and Obesity on Adipokines During Pregnancy and Lactation. Int. J. Mol. Sci. 2025, 26, 9757. [Google Scholar] [CrossRef]
- Park, H.K.; Ahima, R.S. Resistin in rodents and humans. Diabetes Metab. J. 2013, 37, 404–414. [Google Scholar] [CrossRef]
- Andreas, N.J.; Hyde, M.J.; Gale, C.; Parkinson, J.R.; Jeffries, S.; Holmes, E.; Modi, N. Effect of maternal body mass index on hormones in breast milk: A systematic review. PLoS ONE 2014, 9, e115043. [Google Scholar] [CrossRef]
- Enstad, S.; Cheema, S.; Thomas, R.; Fichorova, R.N.; Martin, C.R.; O’Tierney-Ginn, P.; Wagner, C.L.; Sen, S. The impact of maternal obesity and breast milk inflammation on developmental programming of infant growth. Eur. J. Clin. Nutr. 2021, 75, 180–188. [Google Scholar] [CrossRef]
- Taouis, M.; Benomar, Y. Is resistin the master link between inflammation and inflammation-related chronic diseases? Mol. Cell. Endocrinol. 2021, 533, 111341. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Dena-Beltrán, J.L.; Ruiz-Herrera, X.; Ocampo-Ruiz, A.L.; Martínez de la Escalera, G.; Clapp, C.; Macotela, Y. Obesity-derived alterations in the lactating mammary gland: Focus on prolactin. Mol. Cell. Endocrinol. 2023, 559, 111810. [Google Scholar] [CrossRef]
- Morais, T.C.; Abreu, L.C.; Quental, O.B.; Pessoa, R.S.; Fujimori, M.; Daboin, B.E.G.; França, E.L.; Honorio-França, A.C. Obesity as an inflammatory agent can cause cellular changes in human milk due to the actions of the adipokines leptin and adiponectin. Cells 2019, 8, 519. [Google Scholar] [CrossRef]
- Silva, C.G.; Luz, V.F.; Nunes, V.L.; Verzoto, A.B.M.; Cotrim, A.C.M.; dos Santos, W.B.; França, E.L.; Honorio-França, A.C. Colostrum-Derived Melatonin Plus PEG Microspheres Modulate the Oxidative Metabolism of Human Colostrum Phagocytes. Metabolites 2025, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a novel host defense peptide of innate immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Kim, J.; Kim, B.-K.; Seo, H.J.; Kim, J.-Y.; Lee, J.-E.; Lee, J.; You, J.; Jin, S.; Kim, H.-S. Resistin regulates inflammation and insulin resistance in humans via the endocannabinoid system. Research 2024, 7, 0326. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Vargas, R.; Reizes, O. The impact of obesity and adipokines on breast and gynecologic malignancies. Ann. N. Y. Acad. Sci. 2022, 1518, 131–150. [Google Scholar] [CrossRef]
- Wilson, R.M.; Messaoudi, I. The impact of maternal obesity during pregnancy on offspring immunity. Mol. Cell. Endocrinol. 2015, 418, 134–142. [Google Scholar] [CrossRef]
- Morais, T.C.; Honorio-França, A.C.; Fujimori, M.; Quental, O.B.; Pessoa, R.S.; França, E.L.; Abreu, L.C. Melatonin action on the activity of phagocytes from the colostrum of obese women. Medicina 2019, 55, 625. [Google Scholar] [CrossRef]
- Fernandes, R.T.S.; França, E.L.; Fagundes, D.L.G.; Fujimori, M.; Marchi, P.G.F.; Massmann, P.F.; Tozetti, I.A.; Honorio-França, A.C. Nanodoses of melatonin induce apoptosis in human breast cancer cells co-cultured with colostrum cells. Biointerface Res. Appl. Chem. 2019, 9, 4416–4423. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Deng, H.; Sun, S.; Dai, Z.; Sun, Y. Intermittent high glucose exacerbates the aberrant production of adiponectin and resistin through mitochondrial superoxide overproduction in adipocytes. J. Mol. Endocrinol. 2010, 44, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, J.; Lü, J.M.; Chai, H.; Wang, X.; Lin, P.H.; Yao, Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H193–H201. [Google Scholar] [CrossRef] [PubMed]
- Menzaghi, C.; Marucci, A.; Antonucci, A.; De Bonis, C.; Ortega Moreno, L.; Salvemini, L.; Copetti, M.; Trischitta, V.; Di Paola, R. Suggestive evidence of a multi-cytokine resistin pathway in humans and its role on cardiovascular events in high-risk individuals. Sci. Rep. 2017, 7, 44337. [Google Scholar] [CrossRef]
- Patel, L.; Buckels, A.C.; Kinghorn, I.J.; Murdock, P.R.; Holbrook, J.D.; Plumpton, C.; Macphee, C.H.; Smith, S.A. Resistin is expressed in human macrophages and induces proinflammatory cytokines. Biochem. Biophys. Res. Commun. 2003, 300, 472–476. [Google Scholar] [CrossRef]
- Carrichon, L.; Picciocchi, A.; Debeurme, F.; Defendi, F.; Beaumel, S.; Jesaitis, A.J.; Dagher, M.C.; Stasia, M.J. Characterization of superoxide overproduction by the D-LoopNox4-Nox2 cytochrome b558 in phagocytes—Differential sensitivity to calcium and phosphorylation events. Biochim. Biophys. Acta Biomembr. 2011, 1808, 78–90. [Google Scholar] [CrossRef]
- Hernandes, M.R.G.; Moraes, L.C.A.; Ribeiro, E.B.; Fagundes, D.L.G.; Honorio-França, A.C.; França, E.L. In vitro immunomodulatory effects of microemulsions with levamisole delivery systems on blood phagocytes interacting with Giardia lamblia. Parasitol. Int. 2017, 66, 299–304. [Google Scholar] [CrossRef]
- McFadden, M.J.; Reynolds, M.B.; Michmerhuizen, B.C.; Ólafsson, E.B.; Marshall, S.M.; Davis, F.A.; Schultz, T.L.; Iwawaki, T.; Sexton, J.Z.; O’Riordan, M.X.D.; et al. IRE1α promotes phagosomal calcium flux to enhance macrophage fungicidal activity. Cell Rep. 2025, 44, 115694. [Google Scholar] [CrossRef]
- Dewitt, S.; Green, J.; Laffafian, I.; Lewis, K.J.; Hallett, M.B. Intraphagosomal Free Ca2+ Changes during Phagocytosis. Int. J. Mol. Sci. 2024, 25, 4254. [Google Scholar] [CrossRef]
- Zhao, C.W.; Song, W.X.; Liu, B.; Gao, Y.H.; Ding, L.; Huang, Y.F.; Qi, X. Resistin induces chemokine and matrix metalloproteinase production via CAP1 receptor and activation of p38-MAPK and NF-κB signalling pathways in human chondrocytes. Clin. Exp. Rheumatol. 2022, 40, 501–513. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef]

| Parameters | Eutrophic (n = 28) | Overweight (n = 27) | Obese (n= 27) |
|---|---|---|---|
| Age (years) | 29.5 ± 5.1 | 28.6 ± 4.5 | 31.7 ± 4.8 |
| Gestational age (weeks) | 39.8 ± 2.6 | 37.8 ± 3.5 | 37.2 ± 3.5 |
| BMI-1 | 23.7 ± 2.1 | 27.2 ± 3.1 | 34.7 ± 5.1 |
| BMI-2 | 33.6 ± 4.2 | 38.2 ± 4.8 | 41.1 ± 4.5 |
| Fasting glucose | 85.5 ± 4.6 | 92 ± 5.7 | 94.7 ± 7.5 |
| Hypertension (%) | 0 | 5 (18%) | 7 (26%) |
| Diabetes (%) | 0 | 0 | 0 |
| Physical exercise (%) | 11 (39%) | 9 (33%) | 11 (41%) |
| MN Cells Incubated | Sample | Eutrophic | Overweight | Obesity |
|---|---|---|---|---|
| Fluorescence Intensity [%] | ||||
| PBS | Blood | 27.0 ± 2.2 | 23.3 ± 2.1 | 25.8 ± 2.5 |
| Colostrum | 25.9 ± 1.8 | 29.5 ± 3.0 † | 29.9 ± 2.8 | |
| Resistin | Blood | 30.2 ± 3.5 | 29.3 ± 3.5 * | 20.8 ± 2.5 # |
| Colostrum | 34.2 ± 2.9 * | 25.4 ± 2.4 | 16.4 ± 3.6 *# | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antônio, C.R.S.S.; Pereira, E.P.; França, D.C.H.; de Marchi, P.G.F.; França, E.C.H.; de Magalhães Neto, A.M.; Ribeiro, E.B.; Fagundes-Triches, D.L.G.; Honorio-França, A.C.; França, E.L. Resistin as Modulator of Functional Activity of Phagocytes in Colostrum and Blood of Overweight and Obese Mothers. Biomedicines 2025, 13, 2815. https://doi.org/10.3390/biomedicines13112815
Antônio CRSS, Pereira EP, França DCH, de Marchi PGF, França ECH, de Magalhães Neto AM, Ribeiro EB, Fagundes-Triches DLG, Honorio-França AC, França EL. Resistin as Modulator of Functional Activity of Phagocytes in Colostrum and Blood of Overweight and Obese Mothers. Biomedicines. 2025; 13(11):2815. https://doi.org/10.3390/biomedicines13112815
Chicago/Turabian StyleAntônio, Carla Roberta Silva Souza, Elisia Possidônea Pereira, Danielle Cristina Honorio França, Patricia Gelli Feres de Marchi, Emanuelle Carolina Honorio França, Anibal Monteiro de Magalhães Neto, Elton Brito Ribeiro, Danny Laura Gomes Fagundes-Triches, Adenilda Cristina Honorio-França, and Eduardo Luzía França. 2025. "Resistin as Modulator of Functional Activity of Phagocytes in Colostrum and Blood of Overweight and Obese Mothers" Biomedicines 13, no. 11: 2815. https://doi.org/10.3390/biomedicines13112815
APA StyleAntônio, C. R. S. S., Pereira, E. P., França, D. C. H., de Marchi, P. G. F., França, E. C. H., de Magalhães Neto, A. M., Ribeiro, E. B., Fagundes-Triches, D. L. G., Honorio-França, A. C., & França, E. L. (2025). Resistin as Modulator of Functional Activity of Phagocytes in Colostrum and Blood of Overweight and Obese Mothers. Biomedicines, 13(11), 2815. https://doi.org/10.3390/biomedicines13112815







