Long noncoding RNAs and microRNAs in Endometriosis
Abstract
1. Introduction
- Retrograde menstruation—supported by peritoneal lesion distribution but does not explain distant sites.
- Lymphatic or hematogenous spread—underlies extra peritoneal implants, though evidence is limited.
- Coelomic metaplasia—highlights peritoneal cell plasticity, yet rarely demonstrated experimentally.
- Stem/progenitor cell theory—multipotent bone marrow and endometrial progenitor cells may differentiate ectopically.
- Müllerian remnants—embryonic rests may persist and aberrantly differentiate.
2. Noncoding RNAs: Classification, Mechanisms and Functions
2.1. Long Non-Coding RNAs
2.2. microRNAs
3. Long Noncoding RNAs in Endometriosis
3.1. The Molecular Pathways of lncRNAs in Endometriosis
3.1.1. lncRNAs That Recruit and Target Chromatin Remodeling or Transcriptional Regulatory Factors
3.1.2. lncRNAs with miRNA Sponging Functions
3.1.3. lncRNAs That Modulate Cellular Signaling Pathways
4. microRNAs in Endometriosis
The Pathological Role of miRNAs in Endometriosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| lncRNAs | Long non-coding RNAs |
| miRNAs | Micro RNAs |
| EMT | Epithelial–Mesenchymal Transition |
| Pol II | RNA Polymerase II |
| VEGF A | Vascular endothelial growth factor A |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| ESCs | Endometrial stromal cells |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
| ncRNAs | Noncoding RNAs |
References
- Rogers, P.A.; D‘Hooghe, T.M.; Fazleabas, A.; Giudice, L.C.; Montgomery, G.W.; Petraglia, F.; Taylor, R.N. Defining future directions for endometriosis research: Workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod. Sci. 2013, 20, 483–499. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Prapas, Y.; Goudakou, M.; Matalliotakis, I.; Kalogeraki, A.; Matalliotaki, C.; Panagiotidis, Y.; Ravanos, K.; Prapas, N. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod. Biomed. Online 2012, 25, 543–548. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C.; Guideline Committee. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935. [Google Scholar]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Update 2018, 24, 497–515. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118, Erratum in Nat. Rev. Mol. Cell Biol. 2021, 22, 159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gil, N.; Ulitsky, I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 2020, 21, 102–117. [Google Scholar] [PubMed]
- Crossley, M.P.; Bocek, M.; Cimprich, K.A. R-Loops as Cellular Regulators and Genomic Threats. Mol. Cell 2019, 73, 398–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Delás, M.J.; Hannon, G.J. lncRNAs in development and disease: From functions to mechanisms. Open Biol. 2017, 7, 170121. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, L.; Lin, R.; Ma, S.; Li, J.; Yang, S. A comprehensive overview of exosome lncRNAs: Emerging biomarkers and potential therapeutics in endometriosis. Front. Endocrinol. 2023, 14, 1199569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long non-coding RNAs in the regulation of gene expression: Physiology and disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Maligianni, I.; Yapijakis, C.; Nousia, K.; Bacopoulou, F.; Chrousos, G.P.P. Exosomes and exosomal noncoding RNAs throughout human gestation (Review). Exp. Ther. Med. 2022, 24, 582. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, X.; Zhu, Y.; Wang, C.; Jiang, Z.; Yang, N.; Wang, T.; Shu, L.; Xu, Y.; Sun, L. IGF2BP2 enhances LincRNA01116 stability via m6 A: A potential biomarker and therapeutic target for patients with preeclampsia. J. Cell Biochem. 2023, 124, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. Role of noncoding RNAs in the pathogenesis of endometriosis. Front. Oncol. 2020, 10, 1370. [Google Scholar] [CrossRef]
- Hudson, Q.J.; Proestling, K.; Perricos, A.; Kuessel, L.; Husslein, H.; Wenzl, R.; Yotova, I. The Role of Long Non-Coding RNAs in Endometriosis. Int. J. Mol. Sci. 2021, 22, 11425. [Google Scholar] [CrossRef]
- Nunez-Martinez, H.N.; Recillas-Targa, F. Emerging functions of lncRNA loci beyond the transcript itself. Int. J. Mol. Sci. 2022, 23, 6258. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.-P.; Yi, H.-C. DeepWalk-based method to predict lncRNA-miRNA associations via lncRNA-miRNA-disease-protein-drug graph. BMC Bioinf. 2022, 22 (Suppl. 12), 621. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Y.; Zhang, C.; Duan, C. Multiple roles of exosomal long noncoding RNAs in cancers. BioMed Res. Int. 2019, 2019, 1460572. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, H.; Yu, Q.; Xiao, W.; Wang, D.O. LncRNA-mediated DNA methylation: An emerging mechanism in cancer and beyond. J. Exp. Clin. Cancer Res. 2022, 41, 100. [Google Scholar] [CrossRef]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef]
- Sanbonmatsu, K. Getting to the bottom of lncRNA mechanism: Structure-function relationships. Mamm. Genome 2022, 33, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
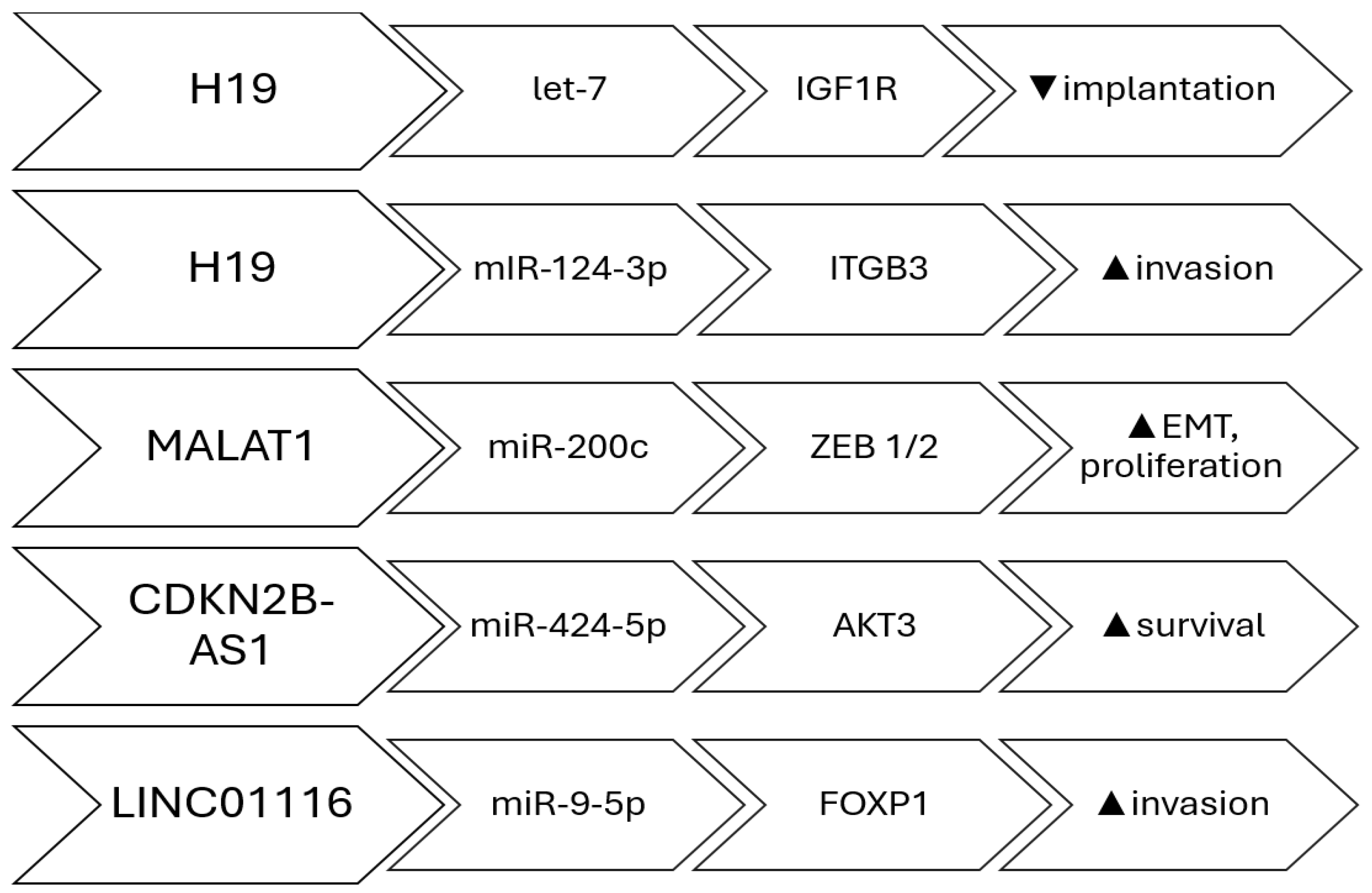
- Ghazal, S.; McKinnon, B.; Zhou, J.; Mueller, M.; Men, Y.; Yang, L.; Mueller, M.; Flannery, C.; Huang, Y.; Taylor, H.S. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 2015, 7, 996–1003. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, J.; Tang, X.; Cui, H.; Zhang, Q.; Yang, Q. LncRNA-H19 regulates cell proliferation and invasion of ectopic endometrium by targeting ITGB3 via modulating miR-124-3p. Exp. Cell Res. 2019, 381, 215–222. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Zhong, Y.; Cai, M.; Gao, J.; Tan, C.; Han, X.; Guo, R.; Han, L. LncRNA H19 over-expression inhibited Th17 cell differentiation to relieve endometriosis through miR-342-3p/IER3 pathway. Cell Biosci. 2019, 9, 84. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Zhang, X.; Zhang, T.; Jiang, L.; Cao, L.; Zhou, Y.; Fang, X. Effects of CDKN2B-AS1 on cellular proliferation, invasion and AKT3 expression are attenuated by miR-424-5p in a model of ovarian endometriosis. Reprod. Biomed. Online 2021, 42, 1057–1066. [Google Scholar] [CrossRef]
- Cui, L.; Chen, S.; Wang, D.; Yang, Q. LINC01116 promotes proliferation and migration of endometrial stromal cells by targeting FOXP1 via sponging miR-9-5p in endometriosis. J. Cell Mol. Med. 2021, 25, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, Y.; Zhao, Y.; Xu, C.; Zhang, A.; Zhang, Q.; Wang, D.; He, J.; Hua, W.; Duan, P. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 251. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Xiong, W.; Li, N.; Liu, H.; He, H.; Li, Q.; Liu, Y.; Zhang, L. Estradiol promotes EMT in endometriosis via MALAT1/miR200s sponge function. Reproduction 2019, 157, 179–188. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Yu, Q.; Zhang, Y.; Yan, L.; Chen, Z.J. The estrogen-regulated lncRNA H19/miR-216a-5p axis alters stromal cell invasion and migration via ACTA2 in endometriosis. Mol. Hum. Reprod. 2019, 25, 550–561. [Google Scholar] [CrossRef]
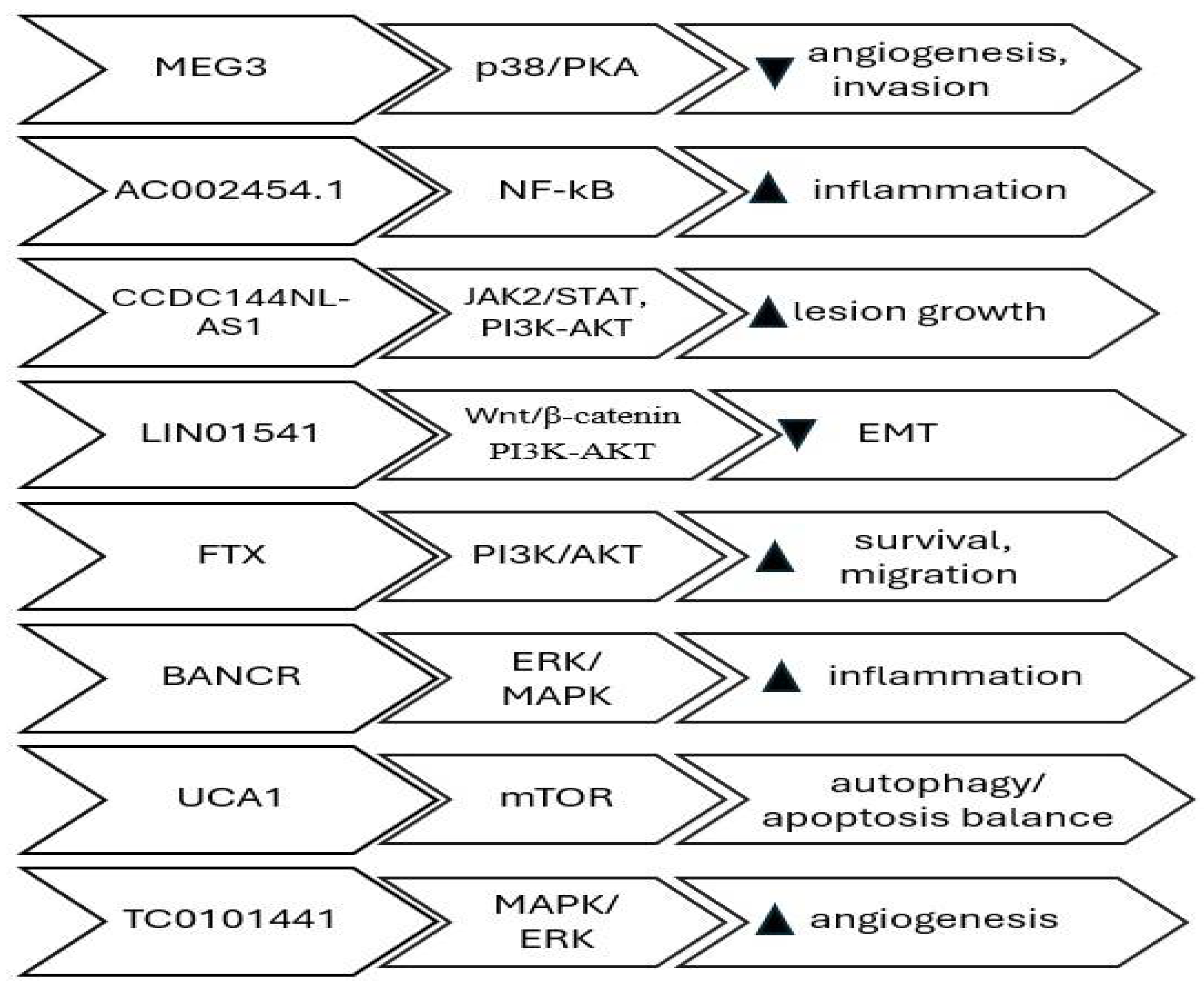
- Mai, H.; Xu, H.; Lin, H.; Wei, Y.; Yin, Y.; Huang, Y.; Huang, S.; Liao, Y. LINC01541 Functions as a ceRNA to Modulate the Wnt/beta-Catenin Pathway by Decoying miR-506-5p in Endometriosis. Reprod. Sci. 2021, 28, 665–674. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, M.; Wang, S.; Xiao, Y.; Wu, J.; Zhou, Y.; Fang, X. LINC01018 and SMIM25 sponged miR-182-5p in endometriosis revealed by the ceRNA network construction. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420976309. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Lu, D.; Feng, Y.; Xu, R.; Li, X.; Yin, C.; Xue, B.; Zhao, H.; Wang, S.; et al. LncRNA SNHG4 promotes the increased growth of endometrial tissue outside the uterine cavity via regulating c-Met mediated by miR-148a-3p. Mol. Cell Endocrinol. 2020, 514, 110887. [Google Scholar] [CrossRef]
- Feng, Y.; Tan, B.Z. LncRNA MALAT1 inhibits apoptosis of endometrial stromal cells through miR-126-5p-CREB1 axis by activating PI3K-AKT pathway. Mol. Cell Biochem. 2020, 475, 185–194. [Google Scholar] [CrossRef]
- Wang, L.; Xing, Q.; Feng, T.; He, M.; Yu, W.; Chen, H. SNP rs710886 A > G in long noncoding RNA PCAT1 is associated with the risk of endometriosis by modulating expression of multiple stemness-related genes via microRNA-145 signaling pathway. J. Cell Biochem. 2020, 121, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sha, L.; Huang, L.; Yang, S.; Zhou, Q.; Luo, X.; Shi, B. LINC00261 functions as a competing endogenous RNA to regulate BCL2L11 expression by sponging miR-132-3p in endometriosis. Am. J. Transl. Res. 2019, 11, 2269–2279. [Google Scholar] [PubMed] [PubMed Central]
- Liu, Y.; Ma, J.; Cui, D.; Fei, X.; Lv, Y.; Lin, J. LncRNA MEG3-210 regulates endometrial stromal cells migration, invasion and apoptosis through p38 MAPK and PKA/SERCA2 signalling via interaction with Galectin-1 in endometriosis. Mol. Cell Endocrinol. 2020, 513, 110870. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Du, Y.; Liu, Y.; Xiong, X. Long non-coding RNA MALAT1 mediates hypoxia-induced pro-survival autophagy of endometrial stromal cells in endometriosis. J. Cell Mol. Med. 2019, 23, 439–452. [Google Scholar] [CrossRef]
- Mai, H.; Wei, Y.; Yin, Y.; Huang, S.; Lin, H.; Liao, Y.; Liu, X.; Chen, X.; Shi, H.; Liu, C.; et al. LINC01541 overexpression attenuates the 17beta-Estradiol-induced migration and invasion capabilities of endometrial stromal cells. Syst. Biol. Reprod. Med. 2019, 65, 214–222. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Zheng, T.T.; Tang, X.Y.; Zhang, Y.; Hua, K.Q. The Exosomal Long Noncoding RNA aHIF is Upregulated in Serum from Patients with Endometriosis and Promotes Angiogenesis in Endometriosis. Reprod. Sci. 2019, 26, 1590–1602. [Google Scholar] [CrossRef]
- Yotova, I.; Hudson, Q.J.; Pauler, F.M.; Proestling, K.; Haslinger, I.; Kuessel, L.; Perricos, A.; Husslein, H.; Wenzl, R. LINC01133 Inhibits Invasion and Promotes Proliferation in an Endometriosis Epithelial Cell Line. Int. J. Mol. Sci. 2021, 22, 8385. [Google Scholar] [CrossRef]
- Wang, H.; Ni, C.; Xiao, W.; Wang, S. Role of lncRNA FTX in invasion, metastasis, and epithelial-mesenchymal transition of endometrial stromal cells caused by endometriosis by regulating the PI3K/Akt signaling pathway. Ann. Transl. Med. 2020, 8, 1504. [Google Scholar] [CrossRef]
- Zhu, M.B.; Chen, L.P.; Hu, M.; Shi, Z.; Liu, Y.N. Effects of lncRNA BANCR on endometriosis through ERK/MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6806–6812. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wan, Y.; Feng, Z.; Liu, D.; Ouyang, L.; Li, Y.; Liu, K. Long Noncoding RNA UCA1 Is Related to Autophagy and Apoptosis in Endometrial Stromal Cells. Front. Oncol. 2020, 10, 618472. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaukat, A.; Zhang, H.; Yang, Y.F.; Li, H.X.; Li, G.Y.; Liu, Y.N.; Liang, C.; Kang, J.W.; Li, S.C.; et al. Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses. Int. J. Mol. Sci. 2024, 25, 13150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in endometriosis: Biological function and emerging biomarker candidates. Biol. Reprod. 2019, 100, 1135–1146. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yan, L.; Liang, G.; Zhu, C.; Wang, Y.; Ji, S.; He, C.; Sun, J.; Zhang, J. Exosomal microRNAs in tubal fluid may be involved in damage to tubal reproductive function associated with tubal endometriosis. Reprod. Biomed. Online 2023, 47, 103249. [Google Scholar] [CrossRef]
- Teague, E.M.; Print, C.G.; Hull, M.L. The role of microRNAs in endometriosis and associated reproductive conditions. Hum. Reprod. Update 2010, 16, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Fehlmann, T.; Ludwig, N.; Backes, C.; Meese, E.; Keller, A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 2016, 13, 1084–1088. [Google Scholar] [CrossRef]
- Wang, S.; Cao, Z.; Wu, Q.; Dong, H. A Comparative Analysis and Verification of Differentially Expressed MiRNAs Could Provide New Insights for the Treatment of Endometritis in Yaks. Pak. Vet. J. 2023, 43, 486–492. [Google Scholar] [CrossRef]
- Antonio, L.G.L.; Meola, J.; Rosa-e-Silva, A.C.J.d.S.; Nogueira, A.A.; Candido dos Reis, F.J.; Poli-Neto, O.B.; Rosa-e-Silva, J.C. Altered Differential Expression of miRNAs Related to Adhesion and Apoptosis Pathways in Patients with Different Phenotypes of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4434. [Google Scholar] [CrossRef]
- Braicu, O.-L.; Budisan, L.; Buiga, R.; Jurj, A.; Achimas-Cadariu, P.; Pop, L.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. miRNA expression profiling in formalin-fixed paraffin-embedded endometriosis and ovarian cancer samples. OncoTargets Ther. 2017, 10, 4225–4238. [Google Scholar] [CrossRef]
- Nothnick, W.B. MicroRNAs and endometriosis: Distinguishing drivers from passengers in disease pathogenesis. Semin. Reprod. Med. 2017, 35, 173–180. [Google Scholar] [CrossRef]
- Ohlsson Teague, E.M.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Mutlu, L.; Grechukhina, O.; Taylor, H.S. Circulating microRNAs as potential biomarkers for endometriosis. Fertil. Steril. 2015, 103, 1252–1260. [Google Scholar] [CrossRef]
- Jia, S.-Z.; Yang, Y.; Lang, J.; Sun, P.; Leng, J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum. Reprod. 2013, 28, 322–330. [Google Scholar]
- Ramón, L.A.; Braza-Boïls, A.; Gilabert-Estellés, J.; Gilabert, J.; España, F.; Chirivella, M.; Estellés, A. MicroRNAs expression in endometriosis and their relation to angiogenic factors. Hum. Reprod. 2011, 26, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Wang, C.C.; Wu, M.H.; Yang, S.H.; Li, Y.H.; Tsai, S.J. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 2012, 97, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Braza-Boïls, A.; Marí-Alexandre, J.; Gilabert, J.; Sánchez-Izquierdo, D.; España, F.; Estellés, A.; Gilabert-Estellés, J. MicroRNA expression profile in endometriosis: Its relation to angiogenesis and fibrinolytic factors. Hum. Reprod. 2014, 29, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.G.; Mamillapalli, R.; Taylor, H.S. Low body mass index in endometriosis is promoted by hepatic metabolic gene dysregulation in mice. Biol. Reprod. 2016, 95, 115. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zhao, Z.; Gao, B.; Meng, L.; Feng, X. miR-146b level and variants is associated with endometriosis related macrophages phenotype and plays a pivotal role in the endometriotic pain symptom. Taiwan. J. Obstet. Gynecol. 2019, 58, 401–408. [Google Scholar]
- Meng, X.; Liu, J.; Wang, H.; Chen, P.; Wang, D. MicroRNA-126-5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis. Mol. Cell. Endocrinol. 2019, 494, 110486. [Google Scholar]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 79–89. [Google Scholar]
- Viganò, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, X.; Wu, D.; Deng, M.; Miao, J.; Jin, Z. Down-regulation of exosomal miR-214-3p targeting CCN2 contributes to endometriosis fibrosis and the role of exosomes in the horizontal transfer of miR-214-3p. Reprod. Sci. 2020, 28, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, Y.-X.; Chen, Y.-Y. miRNA-34a-5p downregulation of VEGFA in endometrial stem cells contributes to the pathogenesis of endometriosis. Mol. Med. Rep. 2017, 16, 8259–8264. [Google Scholar] [CrossRef] [PubMed][Green Version]
| lncRNA | Mechanism/Target | Biological Role |
|---|---|---|
| H19 | Regulates IGF1R via let-7 sponging | Reduces stromal proliferation |
| CDKN2B-AS1 | Regulates AKT3 by binding miR-424-5p | Promotes invasion in ovarian endometriosis |
| LINC01116 | Targets FOXP1 via miR-9-5p sponging | Promotes stromal cell proliferation and migration |
| MALAT1 | Epigenetic regulation through ZEB1/ZEB2 activation | Enhances EMT and migration |
| LINC01541 | Wnt/β-catenin and TGF-β/Smad | Reduces EMT and proliferation |
| AC002454.1 | NF-κB signaling | Promotes inflammation and cytokine release |
| CCDC144NL-AS1 | STAT3/JAK2 and PI3K-AKT | Enhances lesion proliferation |
| TC0101441 | MAPK/ERK cascade | Promotes angiogenesis and migration |
| MEG3-210 | p38/PKA pathway | Inhibits angiogenesis and invasion |
| FTX | PI3K/AKT | Supports proliferation and survival |
| BANCR | ERK/MAPK | Regulates inflammatory cytokines |
| UCA1 | mTOR axis | Balances autophagy and apoptosis |
| miRNA | Target/Pathway | Biological Effect |
|---|---|---|
| miR-145 | Cytoskeletal and proliferation genes | Inhibits proliferation |
| miR-200 family | ZEB1/ZEB2 (EMT regulators) | Suppresses EMT |
| miR-146b | IRF5 | Modulates inflammation and pain |
| miR-126-5p | BCAR3 | Enhances cell migration/invasion |
| miR-34a-5p | VEGFA | Regulates angiogenesis |
| miR-214-3p | CCN2 | Reduces fibrosis |
| let-7b | p53 pathway | Regulates cell cycle |
| miR-20a | ERK and angiogenesis genes | Alters angiogenesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhaxhiri, E.; Debeljak, M.; Trebušak Podkrajšek, K.; Ban Frangež, H. Long noncoding RNAs and microRNAs in Endometriosis. Biomedicines 2025, 13, 2777. https://doi.org/10.3390/biomedicines13112777
Muhaxhiri E, Debeljak M, Trebušak Podkrajšek K, Ban Frangež H. Long noncoding RNAs and microRNAs in Endometriosis. Biomedicines. 2025; 13(11):2777. https://doi.org/10.3390/biomedicines13112777
Chicago/Turabian StyleMuhaxhiri, Edi, Maruša Debeljak, Katarina Trebušak Podkrajšek, and Helena Ban Frangež. 2025. "Long noncoding RNAs and microRNAs in Endometriosis" Biomedicines 13, no. 11: 2777. https://doi.org/10.3390/biomedicines13112777
APA StyleMuhaxhiri, E., Debeljak, M., Trebušak Podkrajšek, K., & Ban Frangež, H. (2025). Long noncoding RNAs and microRNAs in Endometriosis. Biomedicines, 13(11), 2777. https://doi.org/10.3390/biomedicines13112777






