Human Hepatocytes in Experimental Steatosis: Influence of Donor Sex and Sex Hormones
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Primary Human Hepatocytes
2.2. Cell Culture, Sex Hormone Treatment and Steatosis Induction
2.3. TAG Assay
2.4. FFA Assay
2.5. ApoB-100 Assay
2.6. BCA Assay
2.7. RNA Isolation, Reverse Transcription, and qPCR Analyses
2.8. Fluorescence Staining, Microscopy and Imaging Analysis
2.9. Statistical Analysis
3. Results
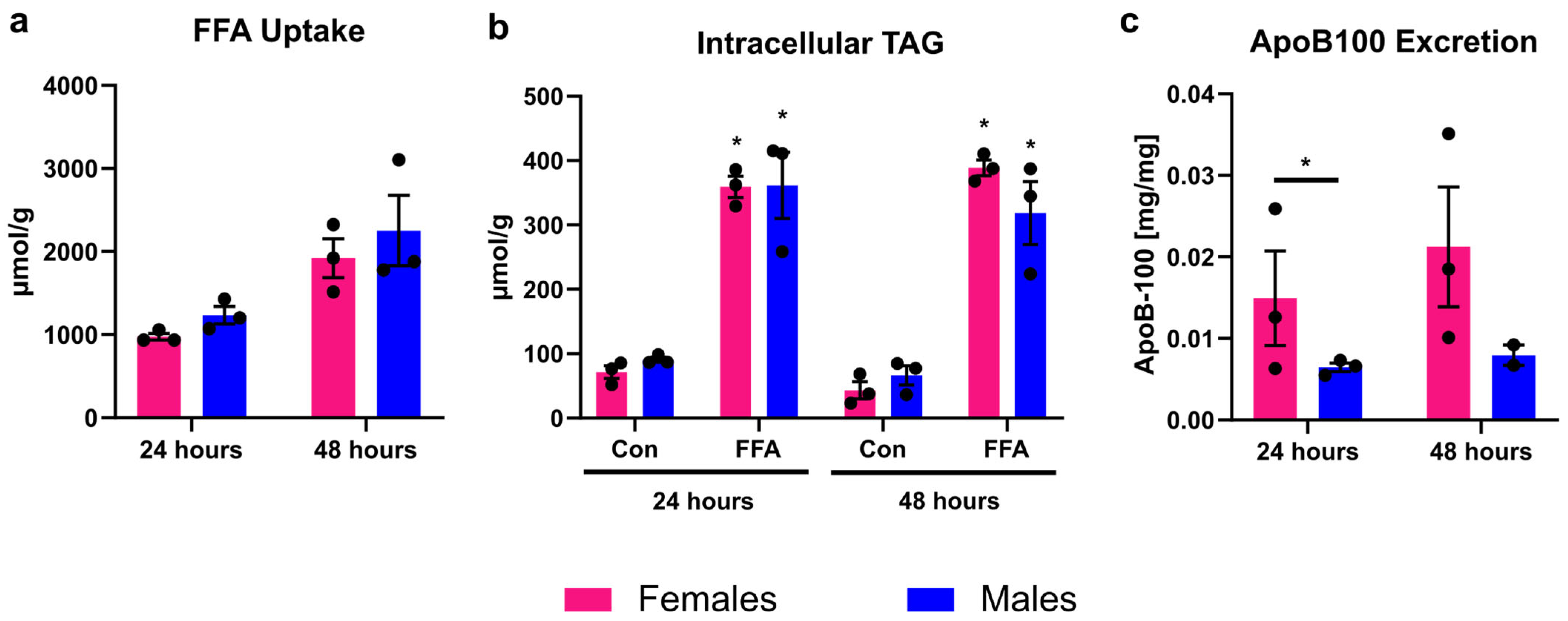
3.1. Male and Female PHHs Do Not Show Differences in Lipid Uptake or Storage, but in VLDL Excretion
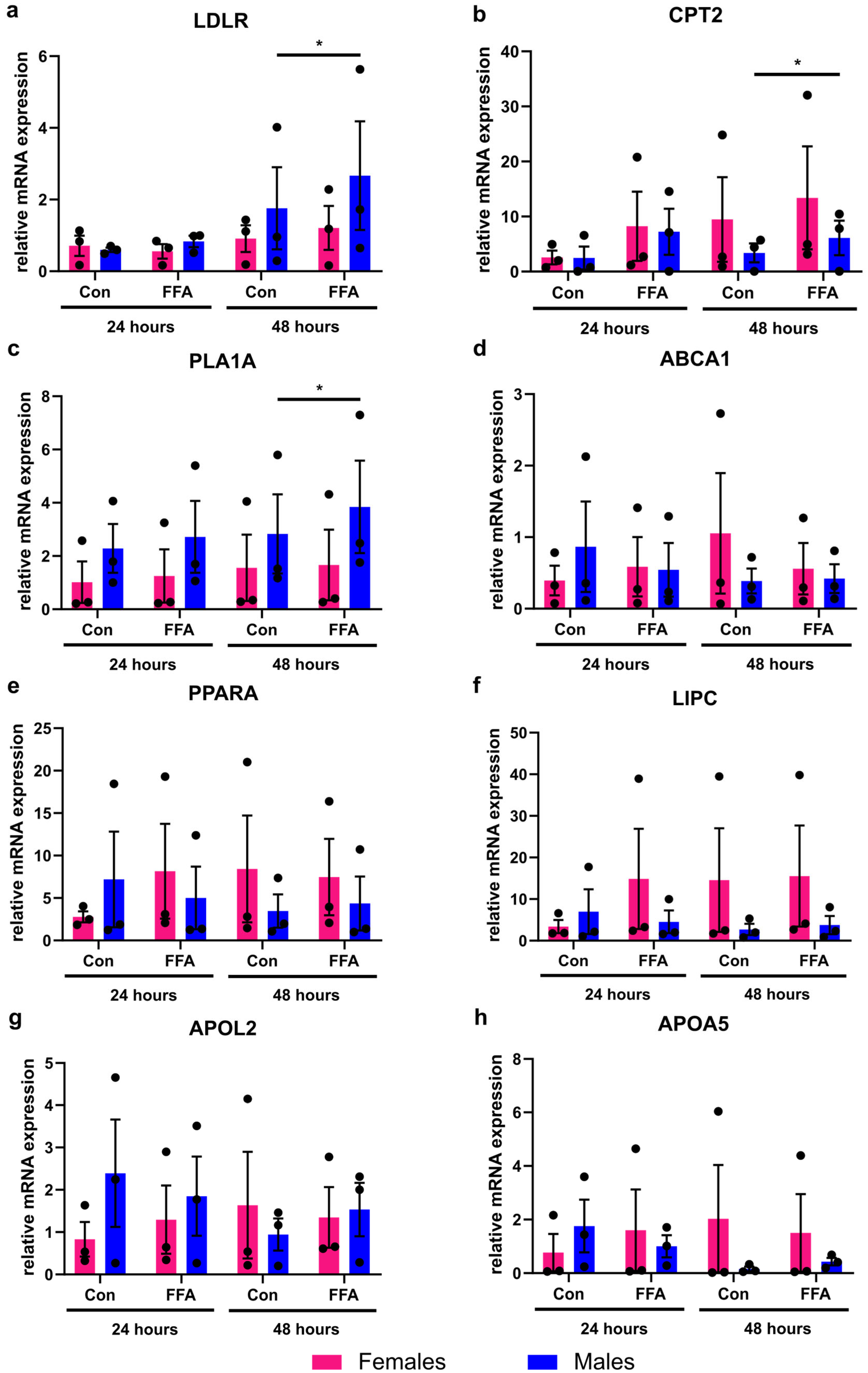
3.2. Only Male PHHs Show Increased Lipid Metabolism Gene Expression After Induction of Steatosis
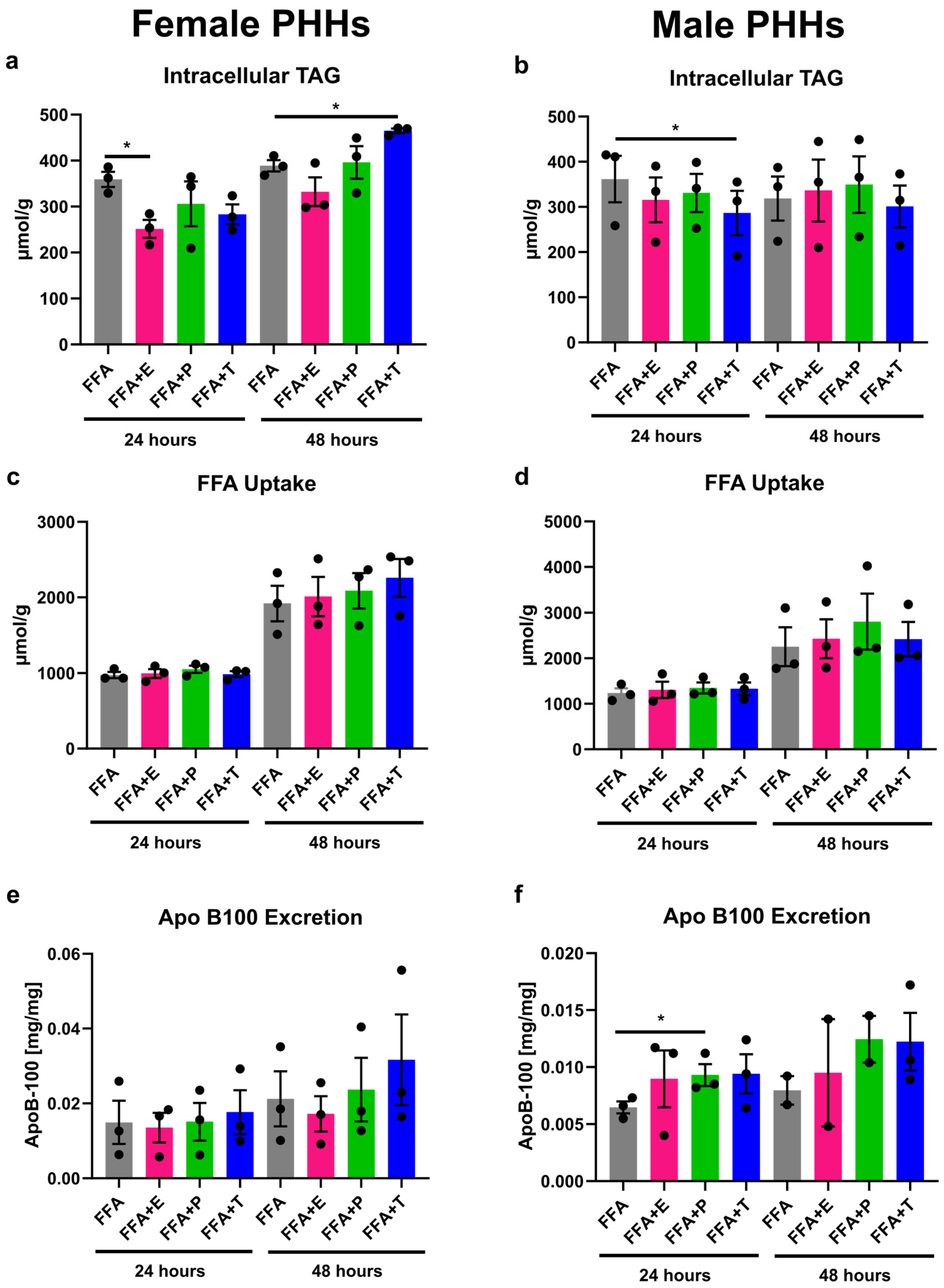
3.3. Sex Hormones Have Sex-Specific Influence on Lipid Storage and Excretion
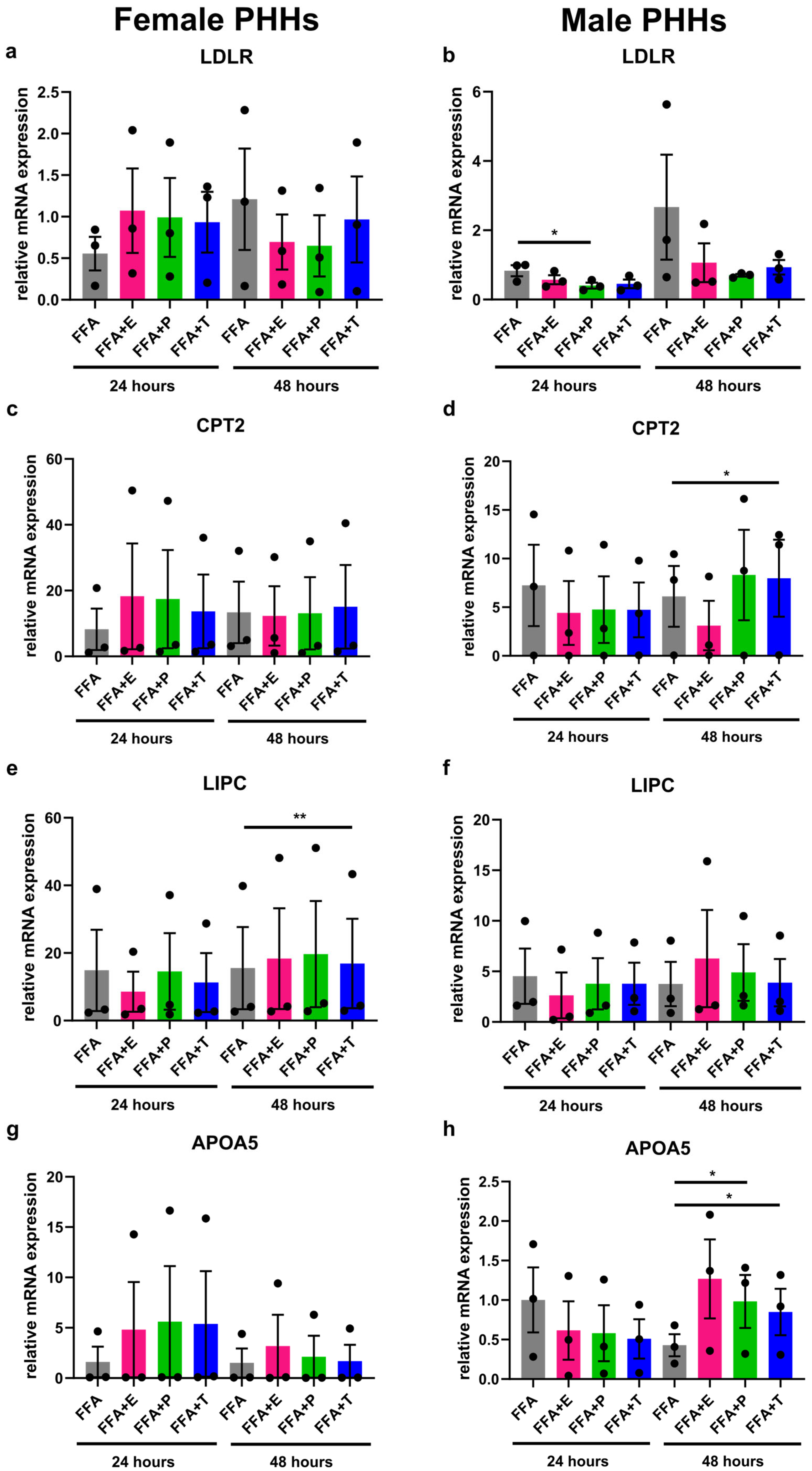
3.4. Sex Hormones Influence Lipid Metabolism Gene Expression Sex-Specifically Under Steatotic Conditions
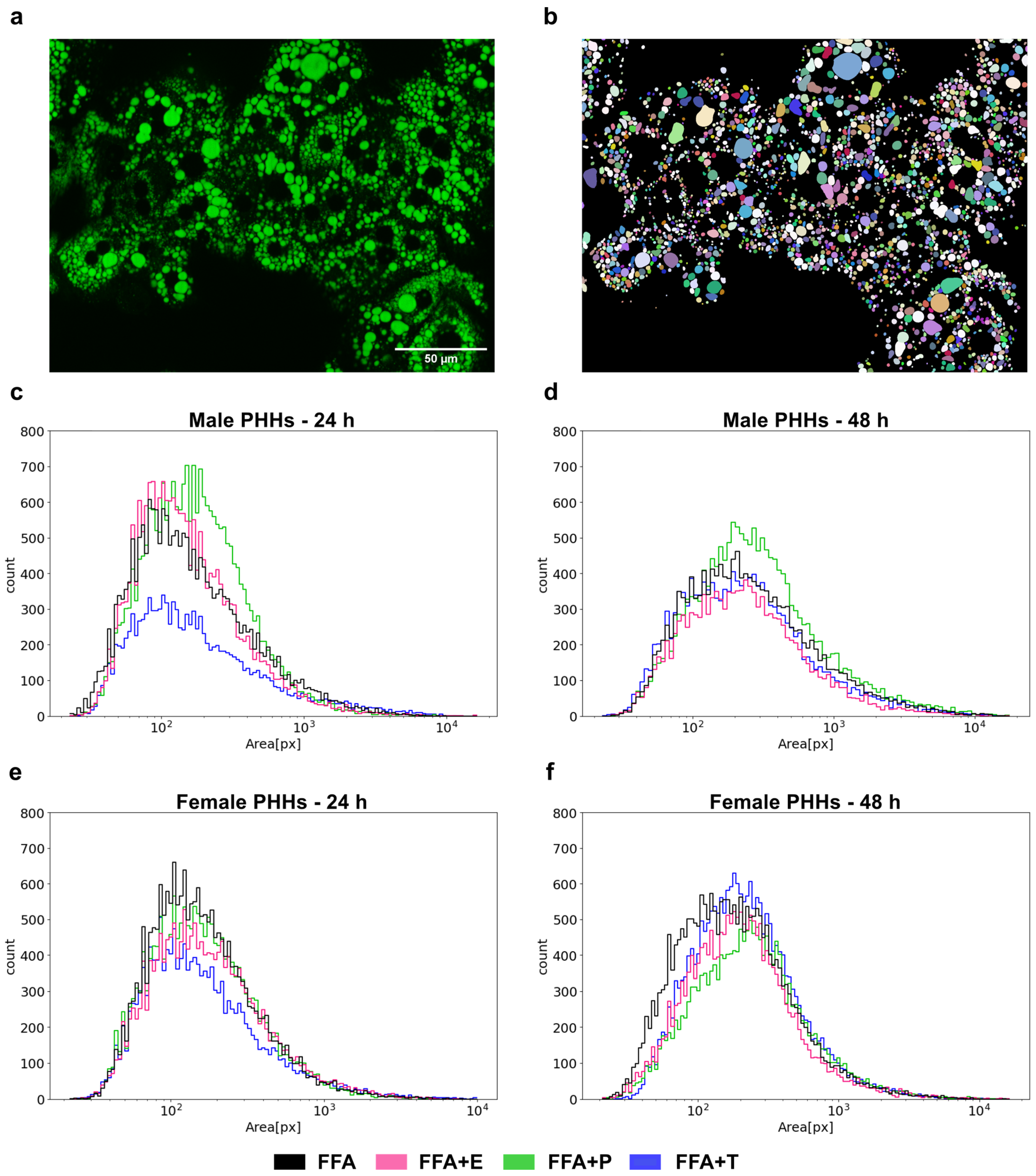
3.5. Lipid Droplet Formation Is Sex-Specifically Influenced by Sex Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette sub-family A member 1 |
| APOA5 | Apolipoprotein A-V |
| APOB-100 | Apolipoprotein B-100 |
| APOL2 | Apolipoprotein L2 |
| BCA | Bicinchoninic acid |
| BSA | Bovine serum albumin |
| CD36 | Fatty acid translocase (Cluster of Differentiation 36) |
| CPT2 | Carnitine palmitoyltransferase 2 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EEF2 | Eukaryotic translation elongation factor 2 |
| ERα | Estrogen receptor alpha |
| FFA | Free fatty acid |
| FBS | Fetal bovine serum |
| FATPs | Fatty acid transport proteins |
| HDL | High-density lipoprotein |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| LD | Lipid droplet |
| LDLR | Low-density lipoprotein receptor |
| LIPC | Hepatic lipase |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MEM NEAA | Minimum essential medium non-essential amino acids |
| NP40 | Nonyl phenoxypolyethoxylethanol (Nonidet P-40) |
| PBS | Phosphate-buffered saline |
| PHHs | Primary human hepatocytes |
| PPARA | Peroxisome proliferator-activated receptor alpha |
| qPCR | Quantitative polymerase chain reaction |
| RPL13A | Ribosomal protein L13a |
| RPS18 | Ribosomal protein S18 |
| TAG | Triacylglycerol (triglyceride) |
| VLDL | Very-low-density lipoprotein |
| WME | William’s Medium E |
References
- Kan, C.; Zhang, K.; Wang, Y.; Zhang, X.; Liu, C.; Ma, Y.; Hou, N.; Huang, N.; Han, F.; Sun, X. Global burden and future trends of metabolic dysfunction-associated Steatotic liver disease: 1990–2021 to 2045. Ann. Hepatol. 2025, 30, 101898. [Google Scholar] [CrossRef]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open 2025, 8, e2454707. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef] [PubMed]
- Lau-Corona, D.; Suvorov, A.; Waxman, D.J. Feminization of Male Mouse Liver by Persistent Growth Hormone Stimulation: Activation of Sex-Biased Transcriptional Networks and Dynamic Changes in Chromatin States. Mol. Cell. Biol. 2017, 37, e00301-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Klein, K.; Sugathan, A.; Nassery, N.; Dombkowski, A.; Zanger, U.M.; Waxman, D.J. Transcriptional profiling of human liver identifies sex-biased genes associated with polygenic dyslipidemia and coronary artery disease. PLoS ONE 2011, 6, e23506. [Google Scholar] [CrossRef]
- Della Torre, S.; Mitro, N.; Meda, C.; Lolli, F.; Pedretti, S.; Barcella, M.; Ottobrini, L.; Metzger, D.; Caruso, D.; Maggi, A. Short-Term Fasting Reveals Amino Acid Metabolism as a Major Sex-Discriminating Factor in the Liver. Cell Metab. 2018, 28, 256–267.e5. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, X.; Antonson, P.; Gustafsson, J.-Å.; Li, Z. Genomics of sex hormone receptor signaling in hepatic sexual dimorphism. Mol. Cell. Endocrinol. 2018, 471, 33–41. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Zhang, N.; Lyu, Y.; Zhang, X.-F. Influence of Sex in the Development of Liver Diseases. Semin. Liver Dis. 2025, 45, 15–32. [Google Scholar] [CrossRef]
- Lefebvre, P.; Staels, B. Hepatic sexual dimorphism—Implications for non-alcoholic fatty liver disease. Nat. Rev. Endocrinol. 2021, 17, 662–670. [Google Scholar] [CrossRef]
- Rocha, A.L.L.; Faria, L.C.; Guimarães, T.C.M.; Moreira, G.V.; Cândido, A.L.; Couto, C.A.; Reis, F.M. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: Systematic review and meta-analysis. J. Endocrinol. Investig. 2017, 40, 1279–1288. [Google Scholar] [CrossRef]
- Gild, P.; Cole, A.P.; Krasnova, A.; Dickerman, B.A.; von Landenberg, N.; Sun, M.; Mucci, L.A.; Lipsitz, S.R.; Chun, F.K.-H.; Nguyen, P.L.; et al. Liver Disease in Men Undergoing Androgen Deprivation Therapy for Prostate Cancer. J. Urol. 2018, 200, 573–581. [Google Scholar] [CrossRef]
- Gatzios, A.; Rombaut, M.; Buyl, K.; De Kock, J.; Rodrigues, R.M.; Rogiers, V.; Vanhaecke, T.; Boeckmans, J. From NAFLD to MAFLD: Aligning Translational In Vitro Research to Clinical Insights. Biomedicines 2022, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Smiriglia, A.; Lorito, N.; Serra, M.; Perra, A.; Morandi, A.; Kowalik, M.A. Sex difference in liver diseases: How preclinical models help to dissect the sex-related mechanisms sustaining NAFLD and hepatocellular carcinoma. iScience 2023, 26, 108363. [Google Scholar] [CrossRef] [PubMed]
- Seidemann, L.; Lippold, C.P.; Rohm, C.M.; Eckel, J.C.; Schicht, G.; Matz-Soja, M.; Berg, T.; Seehofer, D.; Damm, G. Sex hormones differently regulate lipid metabolism genes in primary human hepatocytes. BMC Endocr. Disord. 2024, 24, 135. [Google Scholar] [CrossRef] [PubMed]
- Damm, G.; Schicht, G.; Zimmermann, A.; Rennert, C.; Fischer, N.; Kießig, M.; Wagner, T.; Kegel, V.; Seehofer, D. Effect of glucose and insulin supplementation on the isolation of primary human hepatocytes. EXCLI J. 2019, 18, 1071–1091. [Google Scholar] [CrossRef]
- Seidemann, L.; Prinz, S.; Jan-Constantin, S.; Götz, C.; Seehofer, D.; Damm, G. Optimization of extracellular matrix for primary human hepatocyte cultures using mixed collagen-Matrigel matrices. EXCLI J. 2023, 22, 12–34. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Donato, M.T.; Martínez-Romero, A.; Jiménez, N.; Castell, J.V.; O’Connor, J.-E. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Weigert, M.; Schmidt, U. Nuclei Instance Segmentation and Classification in Histopathology Images with Stardist. In Proceedings of the 2022 IEEE International Symposium on Biomedical Imaging Challenges (ISBIC), Kolkata, India, 28–31 March 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–4, ISBN 978-1-6654-5172-7. [Google Scholar]
- van Rossum, G. The Python Language Reference, Release 3.0.1 [Repr.]; Python Software Foundation; SoHo Books: Hampton, NH, USA; Redwood City, CA, USA, 2010; ISBN 1441412697. [Google Scholar]
- Waskom, M. Seaborn: Statistical data visualization. JOSS 2021, 6, 3021. [Google Scholar] [CrossRef]
- Canbay, A.; Bechmann, L.; Gerken, G. Lipid metabolism in the liver. Z. Gastroenterol. 2007, 45, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Rennert, C.; Heil, T.; Schicht, G.; Stilkerich, A.; Seidemann, L.; Kegel-Hübner, V.; Seehofer, D.; Damm, G. Prolonged Lipid Accumulation in Cultured Primary Human Hepatocytes Rather Leads to ER Stress than Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 7097. [Google Scholar] [CrossRef] [PubMed]
- Seidemann, L.; Krüger, A.; Kegel-Hübner, V.; Seehofer, D.; Damm, G. Influence of Genistein on Hepatic Lipid Metabolism in an In Vitro Model of Hepatic Steatosis. Molecules 2021, 26, 1156. [Google Scholar] [CrossRef]
- Stilkerich, A.; Schicht, G.; Seidemann, L.; Hänsel, R.; Friebel, A.; Hoehme, S.; Seehofer, D.; Damm, G. Cell Homeostasis or Cell Death-The Balancing Act Between Autophagy and Apoptosis Caused by Steatosis-Induced Endoplasmic Reticulum (ER) Stress. Cells 2025, 14, 449. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.W. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: Possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G194–G198. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, D.; Zhou, S.L.; Kokkotou, E.; Berk, P.D. Sex differences in hepatic fatty acid uptake reflect a greater affinity of the transport system in females. Am. J. Physiol. 1992, 263, G380–G385. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, N.; Rico-Bautista, E.; Fisher, R.M.; Wu, X.; Cheung, L.; Flores-Morales, A.; Tybring, G.; Norstedt, G.; Tollet-Egnell, P. Female-predominant expression of fatty acid translocase/CD36 in rat and human liver. Endocrinology 2004, 145, 1972–1979. [Google Scholar] [CrossRef]
- Dixon, J.L.; Furukawa, S.; Ginsberg, H.N. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J. Biol. Chem. 1991, 266, 5080–5086. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Patterson, B.W.; Klein, S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am. J. Clin. Nutr. 2003, 77, 573–579. [Google Scholar] [CrossRef]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef]
- Meyer, J.; Teixeira, A.M.; Richter, S.; Larner, D.P.; Syed, A.; Klöting, N.; Matz-Soja, M.; Gaul, S.; Barnikol-Oettler, A.; Kiess, W.; et al. Sex differences in diet-induced MASLD—Are female mice naturally protected? Front. Endocrinol. 2025, 16, 1567573. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Barjesteh, F.; Azedi, F.; Alipourfard, I.; Rezaei, Z.; Bahreini, E. Comparative analysis of β-Estradiol and testosterone on lipid droplet accumulation, and regulatory protein expression in palmitate/oleate-induced fatty HepG2 cells. BMC Gastroenterol. 2025, 25, 263. [Google Scholar] [CrossRef]
- Nasiri, M.; Nikolaou, N.; Parajes, S.; Krone, N.P.; Valsamakis, G.; Mastorakos, G.; Hughes, B.; Taylor, A.; Bujalska, I.J.; Gathercole, L.L.; et al. 5α-Reductase Type 2 Regulates Glucocorticoid Action and Metabolic Phenotype in Human Hepatocytes. Endocrinology 2015, 156, 2863–2871. [Google Scholar] [CrossRef]
- Jeong, K.J.; Mukae, M.; Lee, S.R.; Kim, S.-Y.; Kim, S.H.; Cho, Y.-E.; An, B.-S.; Ko, J.-W.; Kwun, H.-J.; Baek, I.-J.; et al. Progesterone increases hepatic lipid content and plasma lipid levels through PR- B-mediated lipogenesis. Biomed. Pharmacother. 2024, 172, 116281. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, S.; Li, Y.; Zhou, J.; Wang, K.; Chen, N.; Li, Z. Associations of sex-related and thyroid-related hormones with risk of metabolic dysfunction-associated fatty liver disease in T2DM patients. BMC Endocr. Disord. 2024, 24, 84. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Miao, G.; Zhang, W.; Shi, H.; Lai, P.; Xu, Y.; Zhang, L.; Chen, G.; Han, Y.; Zhao, Y.; et al. Depletion of ApoA5 aggravates spontaneous and diet-induced nonalcoholic fatty liver disease by reducing hepatic NR1D1 in hamsters. Theranostics 2024, 14, 2036–2057. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.; Gillian-Daniel, D.L.; Tebon, A.; Wang, L.; Barrett, P.H.; Attie, A.D. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Investig. 2000, 105, 521–532. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, P.; Qin, Y.-Y.; Wang, L.; Yao, D.-F. Mitochondrial carnitine palmitoyltransferase-II dysfunction: A possible novel mechanism for nonalcoholic fatty liver disease in hepatocarcinogenesis. World J. Gastroenterol. 2023, 29, 1765–1778. [Google Scholar] [CrossRef]
- Tikkanen, M.J.; Nikkilä, E.A. Regulation of hepatic lipase and serum lipoproteins by sex steroids. Am. Heart J. 1987, 113, 562–567. [Google Scholar] [CrossRef]
- Deeb, S.S.; Zambon, A.; Carr, M.C.; Ayyobi, A.F.; Brunzell, J.D. Hepatic lipase and dyslipidemia: Interactions among genetic variants, obesity, gender, and diet. J. Lipid Res. 2003, 44, 1279–1286. [Google Scholar] [CrossRef]
- Jones, D.R.; Schmidt, R.J.; Pickard, R.T.; Foxworthy, P.S.; Eacho, P.I. Estrogen receptor-mediated repression of human hepatic lipase gene transcription. J. Lipid Res. 2002, 43, 383–391. [Google Scholar] [CrossRef]
| Donor | Sex | Age | BMI [kg/m2] | Steatosis 1 | Diagnosis |
|---|---|---|---|---|---|
| FD1 | Female | 46 | 23 | None | Sarcoma metastasis |
| FD2 | Female | 57 | 21 | 1% | iCCA |
| FD3 | Female | 65 | 22 | None | Sarcoma metastasis |
| MD1 | Male | 46 | 22 | None | Echinococcosis |
| MD2 | Male | 47 | 31 | None | Chologenic abscess |
| MD3 | Male | 66 | 25 | 10% | CRLM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidemann, L.; Rohm, C.M.; Stilkerich, A.; Hänsel, R.; Götz, C.; Seehofer, D.; Damm, G. Human Hepatocytes in Experimental Steatosis: Influence of Donor Sex and Sex Hormones. Biomedicines 2025, 13, 2770. https://doi.org/10.3390/biomedicines13112770
Seidemann L, Rohm CM, Stilkerich A, Hänsel R, Götz C, Seehofer D, Damm G. Human Hepatocytes in Experimental Steatosis: Influence of Donor Sex and Sex Hormones. Biomedicines. 2025; 13(11):2770. https://doi.org/10.3390/biomedicines13112770
Chicago/Turabian StyleSeidemann, Lena, Carolin Marie Rohm, Anna Stilkerich, René Hänsel, Christina Götz, Daniel Seehofer, and Georg Damm. 2025. "Human Hepatocytes in Experimental Steatosis: Influence of Donor Sex and Sex Hormones" Biomedicines 13, no. 11: 2770. https://doi.org/10.3390/biomedicines13112770
APA StyleSeidemann, L., Rohm, C. M., Stilkerich, A., Hänsel, R., Götz, C., Seehofer, D., & Damm, G. (2025). Human Hepatocytes in Experimental Steatosis: Influence of Donor Sex and Sex Hormones. Biomedicines, 13(11), 2770. https://doi.org/10.3390/biomedicines13112770









