1. Introduction
The mandibular canal (MC) and inferior alveolar nerve (IAN) are critical anatomical structures encountered in a wide range of oral and maxillofacial surgical procedures. The MC originates at the mandibular foramen (MF) on the medial surface of the mandibular ramus and extends anteriorly to terminate at the mental foramen (MeF). It contains the inferior alveolar neurovascular bundle, which includes the IAN, inferior alveolar artery (IAA), and vein, providing innervation and vascular supply to the mandibular dentition, buccal gingiva, and lower lip [
1,
2].
Historically, limited awareness of anatomical variability within the MC led to a widespread assumption of structural uniformity. Clinical practice relied heavily on two-dimensional panoramic radiography (PR), which frequently failed to identify canal variants due to image distortion, structural superimposition, and poor spatial resolution [
3,
4,
5,
6]. The advent of cone-beam computed tomography (CBCT) and magnetic resonance imaging (MRI) has significantly improved the visualization of the MC, revealing substantial variation in its trajectory, branching patterns, and vertical position [
3,
7,
8]. This technological shift has prompted a paradigm change in surgical planning away from standardized approaches and toward individualized, variant-aware strategies [
9].
Several variant configurations, including bifid and trifid canals, retromolar canals, anterior loops (AL), and accessory foramina, have been increasingly reported in the literature and are now considered common rather than exceptional anatomical findings [
10,
11,
12]. These variations may impact procedural accuracy and are relevant for local anesthesia efficacy, bleeding risk, and postoperative neurosensory outcomes [
7,
11].
The aim of this review is to consolidate anatomical, radiological, and developmental data regarding MC and IAN variability and to examine the implications of such findings in the context of modern surgical practice. A classification-based framework is also proposed to facilitate systematic identification and documentation of MC variants. This is not a proposal of a new anatomical taxonomy; we apply an established classification in a pragmatic, imaging-led clinical workflow. This review delivers a focused, clinically oriented synthesis of MC variants that directly informs surgical planning in the posterior mandible. We outline a practical, variant-aware imaging workflow that begins with PR for screening, uses CBCT when predefined criteria are met, and reserves MRI for selected soft-tissue questions, in line with As Low As Reasonably Achievable (ALARA) and As Low As Diagnostically Acceptable (ALADA) principles. Prevalence data are contextualized by imaging modality and operational definitions (e.g., voxel size; AL thresholds), and implications are summarized for anesthesia, implant positioning, third-molar surgery, and osteotomies. We present the Landfald Clinical Framework (LCF) as a hypothesis-generating tool linking variant patterns to procedural modifications; formal reliability and validity testing remain necessary. Accordingly, the framework is used as a clinical synthesis tool rather than an anatomical reclassification Detailed step-by-step operative techniques and broad educational content are intentionally outside the scope of this article. We retained a single integrated review rather than split the content into separate articles because chairside decisions depend on the joint consideration of variant anatomy, modality choice, and procedural risk. To preserve clinical focus, we trimmed didactic/operative detail and emphasized imaging-led decision points.
2. Materials and Methods
2.1. Review Design and Protocol
We conducted a focused narrative review using structured methods to ensure transparency, traceability, and basic reproducibility appropriate for narrative reviews. Reporting follows PRISMA 2020 [
13] items where applicable to qualitative syntheses; no meta-analysis was planned. Predefined eligibility criteria, dual independent screening, standardized data extraction, and qualitative risk-of-bias considerations (referencing the JBI checklist for prevalence studies) are detailed in
Section 2.3,
Section 2.4,
Section 2.5,
Section 2.6 and
Section 2.7. Information sources and search dates are provided in
Section 2.2. Searches were limited to English. No prospective protocol was registered.
2.2. Information Sources and Search Strategy
We searched PubMed/MEDLINE and Scopus for human studies published from 1 January 2000 to 6 October 2025 (last search: 6 October 2025). Searches combined controlled vocabulary and free-text terms related to the MC, the inferior alveolar neurovascular bundle, and imaging modalities of interest (CBCT, MRI). Full, exact search strings and limits for both databases are provided in
Table S1 (Supplementary).PubMed (example search string):
(mandibular canal[Title/Abstract] OR “inferior alveolar nerve”[Title/Abstract] OR “bifid mandibular canal”[Title/Abstract] OR retromolar canal[Title/Abstract] OR “anterior loop”[Title/Abstract] OR “accessory mental foramen”[Title/Abstract]) AND (“cone-beam”[Title/Abstract] OR CBCT[Title/Abstract] OR “magnetic resonance”[Title/Abstract] OR MRI[Title/Abstract]) AND (humans[MeSH Terms] OR humans[Title/Abstract]).
Scopus (example search string):
TITLE-ABS-KEY(mandibular W/1 canal OR “inferior alveolar nerve” OR “bifid mandibular canal” OR “retromolar canal” OR “anterior loop” OR “accessory mental foramen”) AND TITLE-ABS-KEY(“cone-beam” OR CBCT OR “magnetic resonance” OR MRI) AND DOCTYPE(ar OR re) AND LANGUAGE(english) AND PUBYEAR > 1999.
Reference lists of included articles and key reviews were scanned to identify additional relevant studies.
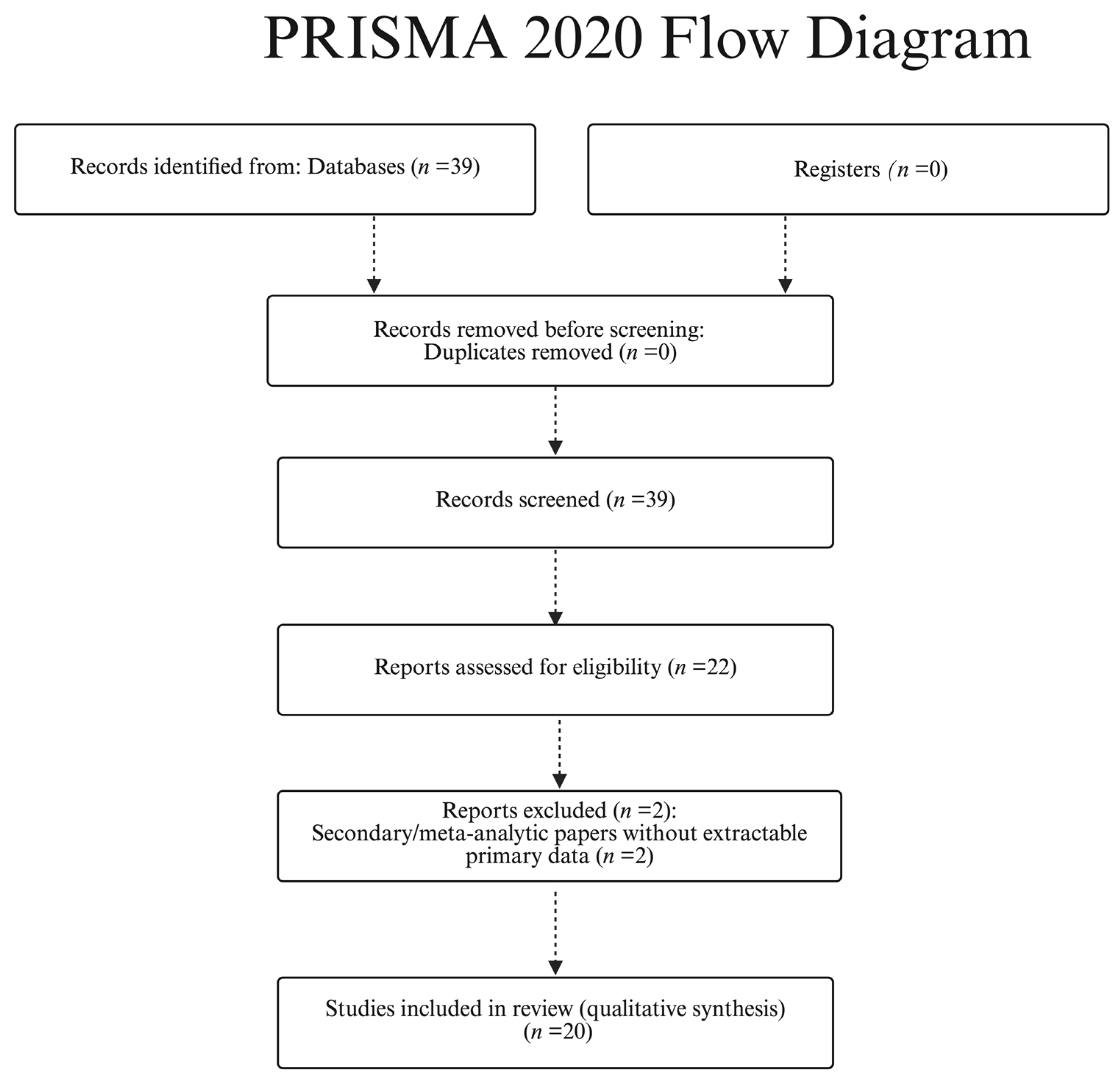
Study selection (PRISMA-style): We identified 39 records from PubMed/MEDLINE and Scopus (last search 6 October 2025). No duplicates were found; therefore, 39 titles/abstracts were screened and 22 full texts were assessed for eligibility. 20 studies were included in the qualitative synthesis (
Table 1). Full-text exclusions (
n = 2) were secondary/meta-analytic papers without extractable primary data; title/abstract exclusions (
n = 17) were pediatric-only cohorts without adult strata, case reports without generalizable measurements, or articles with unclear imaging parameters. See
Figure 1 (PRISMA flow diagram).
2.3. Eligibility Criteria
Inclusion criteria
Human studies (imaging or anatomical) reporting prevalence or measurable features of MC-related variants (e.g., bifid/trifid canals, retromolar canal (RMC), AL presence/length, AMF, high-positioned canal).
Imaging context explicitly stated, including at minimum modality and acquisition parameters (e.g., CBCT voxel size; for MRI, field strength and sequences).
Original data (observational cohorts, cadaveric series, imaging audits), or systematic reviews with extractable primary data (used cautiously, avoiding double-counting).
Exclusion criteria
2.4. Study Selection
Two reviewers independently screened titles/abstracts and then full texts against the eligibility criteria. Disagreements were resolved by consensus. When necessary, uncertainty about duplicate cohorts or overlapping recruitment periods was resolved by preferring the study with larger sample size, clearer imaging parameters, and/or more complete reporting.
2.5. Data Extraction
A standardized extraction form captured the following:
Study identifiers: first author, year, country, design, sample size, population characteristics.
Imaging details: modality (PR/CBCT/MRI), CBCT voxel size and reconstruction parameters; MRI field strength and sequences (e.g., T1/T2, DESS, DTI where applicable).
Operational definitions/thresholds: criteria used to define each variant (e.g., definition of bifid canal; AL measurement method and threshold), coordinate planes used for measurements.
Outcomes: prevalence (%) and/or morphometrics (e.g., AL length), inter-/intra-observer agreement if reported, and reported clinical implications (anesthesia failure, implant safety margins, third-molar risk, osteotomy planning).
Extraction was performed independently by two reviewers; discrepancies were reconciled by discussion, consulting the full text and figures as needed.
2.6. Outcomes and Definitions (A Priori)
Primary outcomes: prevalence of MC variants (BMC/TMC, RMC/RMF, AL, AMF, high-positioned canal). Secondary outcomes: AL length, MC-to-crest height (mm), inter-/intra-observer agreement. Operational definitions/thresholds are consolidated in
Box 1 and applied in
Table 1 (per-study) and
Table 2 (decision keys);
Section 7.1 lists point-of-use thresholds. Strata by voxel size/MRI sequence were extracted separately.
Box 1. Operational definitions & thresholds (applied across
Table 1 and
Table 2).
General confirmation rules (CBCT).
A variant is present when a corticated tract/foramen is visible in ≥2 orthogonal planes with adequate quality.
Measurements on MPR with perpendicular calipers; report in mm (1 decimal).
Discrepancies resolved by consensus between ≥2 readers.
Imaging triggers (selection logic).
PR for screening → selective CBCT when the result will change management (implant site in variant-rich zones; uncertain mental foramen [MeF] position; suspected anterior loop; retromolar surgery; sagittal split/ramus procedures; discordant PR).
MRI (3 T) reserved for soft-tissue pathway questions (bundle course; perineural pathology) when CBCT is inconclusive.
Variant-specific definitions.
BMC/TMC—duplication/splitting of the corticated MC forming ≥2 distinct tracts. Presence: identifiable in ≥2 planes (axial + coronal/sagittal). Report: configuration (bifid/trifid), limb diameter(s), course.
RMC/RMF—corticated accessory canal to a retromolar foramen posterior to the last molar. Presence: RMF and connecting tract in ≥2 planes. Key measures: MeF–RMF distance; RMF–crest height; canal width.
AL (IAN)—anterior extension of the IAN beyond the mental foramen (MeF) before looping posteriorly to exit. Presence: pathway consistent with IAN trajectory in ≥2 planes. Measure: loop length (mm) along the neurovascular course.
HMC—reduced vertical MC–crest clearance at the planned site. Measure: shortest perpendicular distance from MC roof to the alveolar crest in the target region.
AMF—separate corticated foramen distinct from MeF with an independent tract. Presence: discrete cortical rim and measurable separation from MeF (report bilaterality). Measure: foramen diameter(s), MeF–AMF distance, depth to crest.
Decision thresholds (point-of-use).
AL: clinically relevant when ≥1–3 mm anterior to MeF (measure along IAN trajectory on MPR in ≥2 planes).
HMC: MC–crest ≤ 4–5 mm denotes a high-risk corridor at the planned site.
BMC/TMC & RMC/RMF: limb/tract ≥ ~1 mm or intersecting the planned osteotomy/implant path ⇒ modify plan/re-route.
AMF: avoid lateral drilling along the AMF axis; margins individualized from CBCT.
Reader methodology & quality control.
Document voxel size (CBCT) and sequence/field (MRI); minimize metal artifacts; use consistent windowing.
2.7. Risk of Bias and Certainty Considerations
Given the observational nature and imaging heterogeneity, we qualitatively assessed risks related to the following: selection bias (clinic- vs. population-based cohorts), imaging bias (voxel size, reconstruction kernels, slice thickness, MRI sequence choice and artifacts), definitional bias (operational thresholds for AL and canal duplication), and measurement bias (lack of blinded dual reading or inter-observer metrics). Findings are synthesized with explicit attention to these sources of heterogeneity; no quantitative pooling was attempted.
2.8. Synthesis Methods
Data are presented in comparative tables by variant and stratified by modality and imaging parameters (e.g., voxel size categories; MRI field/sequences). Narrative synthesis highlights how operational definitions and acquisition settings influence the reported ranges. On this basis, we derive a variant-aware imaging workflow (PR for screening; CBCT when predefined criteria are met; MRI reserved for specific soft-tissue questions) aligned with ALARA/ALADA principles.
2.9. Deviations from Protocol
No prospective registration was performed. After initial scoping, we narrowed the review to posterior mandibular planning (anesthesia, implants, third molars, osteotomies) and framed the Landfald concept explicitly as a hypothesis-generating framework requiring future validation.
3. Results
This section synthesizes findings from included imaging and anatomic studies with attention to heterogeneity in CBCT voxel size, operational definitions (e.g., confirmation in ≥2 planes for bifid canals), and thresholds for the AL. Given these differences, no meta-analysis was performed. Study-level parameters, definitions, and prevalence ranges are compiled in
Table 1.
To contextualize prevalence claims without duplicating tabular detail, we summarize ranges by imaging modality, acquisition parameters, and operational definitions; per-study values and criteria appear in
Table 1.
Typical ranges by modality/threshold. BMC: PR cohorts often report <1% due to superimposition; CBCT typically yields single- to low double-digit values in adult, non-targeted cohorts when voxel size is ~0.2–0.3 mm and confirmation is made in ≥2 orthogonal planes. TMC: ≈1–2% in CBCT series, sensitive to definitional criteria. RMC: CBCT ~6–25% depending on population and criteria; PR detects substantially fewer. AL: presence and reported prevalence are threshold-dependent; length commonly 1–3 mm on CBCT (plane/definition influence estimates); margins should be individualized from patient-specific CBCT. AMF: often missed on PR; adult CBCT cohorts commonly ≈10–12% under rigorous definitions. HMC: definition-dependent; MC–crest ≤4–5 mm at the target site indicates limited vertical clearance and higher risk. MRI (3T): selective soft-tissue tract visualization; pooled “prevalence” is not appropriate.
3.1. Study Selection and Characteristics (PRISMA-Lite)
A qualitative synthesis included imaging and anatomic studies (PR/CBCT/MRI) published between 2000 and 2025. The primary sources of heterogeneity were CBCT voxel size (≈0.12–0.40 mm), operational definitions of variants (e.g., confirmation in ≥2 planes for bifid canals), thresholds for the AL (see
Box 1). No meta-analysis was performed due to inconsistency in definitions and acquisition parameters (see
Table 1 for study-level detail).
3.2. Imaging Parameters Overview
PR served as an initial screening tool. CBCT substantially increased detectability of bony variants (e.g., BMC, RMC, AMF) and enabled reproducible morphometrics (e.g., AL length). MRI at 3 T (including DTI where applicable) was used selectively to delineate the inferior alveolar neurovascular bundle in soft tissue when clinical questions extended beyond CBCT’s capability (see
Table 1).
Figure 2 illustrates how CBCT MPR delineates the canal course and allows direct measurement of crest–MC clearance, which PR cannot provide.
3.3. Bifid/Trifid Mandibular Canal (BMC/TMC)
Across CBCT cohorts, BMC prevalence typically ranged from single-digit to low double-digit percentages, with extremes explained by definition and voxel size. Trifid canals were uncommon (≈1–2%). PR consistently underestimated canal branching compared with CBCT. Clinical implications for local anesthesia, osteotomy planning, and hemostasis are summarized in
Table 2; study-level prevalence and definitions appear in
Table 1. In practice, axial CBCT is useful for screening bifid or retromolar branches and for bilateral comparison (
Figure 3).
3.4. Retromolar Canal (RMC)
RMC was identified on CBCT from low to mid-double-digit rates depending on population and criteria, with representative cohorts reporting frequencies around the mid-teens to mid-twenties. The presence of an accessory neurovascular bundle in the retromolar triangle increases the risk of bleeding and sensory disturbance; planning and intraoperative modifications are outlined in
Table 2. Individual study frequencies and morphometrics are provided in
Table 1.
3.5. Anterior Loop (AL) of the Mental Nerve
In implantology, the AL of the mental nerve is often analyzed as a standalone topic; we retain it here to enable cross-variant comparison within a single, clinically oriented workflow. AL detection and measured lengths varied with the applied threshold (commonly ≥1–3 mm) and the measurement plane. In clinical practice, the safety margin in the premolar (3–5) region should be individualized from CBCT rather than assumed universally. Threshold-specific AL data are listed in
Table 1, and recommended surgical adjustments are summarized in
Table 2.
3.6. High-Positioned Mandibular Canal (HMC)
A reduced vertical distance from the MC to the alveolar crest was reported with definition-dependent frequency and was more likely in resorbed, edentulous mandibles in several series. Clinically, HMC narrows the osteotomy corridor and may necessitate shorter implants, altered trajectories, or navigation support. See
Table 1 for study definitions/metrics and
Table 2 for procedural adaptations.
3.7. Accessory Mental Foramen (AMF) and Accessory Canals
AMF was more commonly detected on CBCT than on PR. Because AMF represents additional exits of the mental neurovascular bundle, lateral cortical drilling and corticotomy trajectories should be planned to avoid these paths. Prevalence and imaging criteria are compiled in
Table 1; risk and technique modifications are presented in
Table 2.
3.8. Synthesis: Variant-Aware Imaging Workflow (PR → CBCT → Selective MRI)
We do not advocate routine CBCT for all posterior mandibular procedures; instead, CBCT is obtained when predefined criteria are met and when results are reasonably expected to change management, consistent with ALARA/ALADA. CBCT triggers (examples): equivocal or suspicious PR; planned implant near the mental foramen with uncertain AL; reduced MC–crest distance at the target site (e.g., ≤4–5 mm); PR/clinical suspicion of BMC/RMC/AMF; complex third-molar relation to MC; repeated IAN block failures; unexplained or persistent neurosensory symptoms.
PR for routine screening in straightforward cases without red flags.
CBCT when planning implants in the premolar region (3–5), when the canal is close to the planned osteotomy/implant, when BMC/RMC/AMF is suspected, after repeated IAN block failures, or for complex mandibular third-molar surgery (see
Table 2 for how variants alter practice).
MRI (selective) for soft-tissue questions about the inferior alveolar bundle or when CBCT does not explain persistent neurosensory symptoms.
All imaging decisions were aligned with ALARA/ALADA principles. Study-specific thresholds, parameters, and outcomes are consolidated in
Table 1; associated clinical adjustments appear in
Table 2.
3.9. Clinical Box: Anesthesia & MC Variants—Box 2
To translate variant-aware findings into practice, the following clinical box highlights anesthesia-related red flags and procedure-modifying implications; actionable pairings are summarized in
Table 2.
Box 2. Anesthesia & MC Variants.
| Checkpoint | Cue/Red Flag | Action (Concise) | See |
| IANB failures | Repeated/atypical response | Map variants on CBCT | Table 2 |
| Suspected BMC/TMC/AMF | PR hint or clinical pattern | Targeted CBCT; adjust plan | Table 2 |
| Premolar region (3–5) | Margin depends on AL | Measure AL; individualize margin | Table 2 |
| High-positioned MC (HMC) | Short MC–crest distance | Shorter implant; altered angle | Table 2 |
| Third-molar/posterior surgery | Possible RMC | Modify flap; prepare hemostasis | Table 2 |
| Discordant findings | Atypical paresthesia | CBCT to map variant anatomy | Table 2 |
| Anesthesia adjuncts | Persistent difficulty | Consider intraosseous/intraligamentary | Table 2 |
| Documentation/consent | Variant identified | Record baseline & postop sensibility | Table 2 |
3.10. Imaging Workflow
Building on the above, the variant-aware imaging workflow specifies when PR suffices, when CBCT is decision-changing, and when selective MRI adds value, in line with ALARA/ALADA (see
Figure 4 and
Table 2). Accordingly, CBCT use is indication-driven rather than universal, aligned with ALARA/ALADA stewardship and consistent with recent imaging-led anatomical frameworks [
30,
31,
32].
3.11. Landfald Clinical Framework
We next outline the LCF, a hypothesis-generating tool linking common variants to imaging steps and procedural adjustments, with pragmatic thresholds and illustrative vignettes; it is not proposed as a new standard (see
Table 2). Thresholds and confirmation rules are summarized in
Box 1.
Illustrative clinical vignettes showing LCF-guided changes in implant planning (AL) and third-molar surgery (RMC) are provided in
Section 7.4.
Validation statement: The LCF requires formal validation—dual-reader reliability (kappa/ICC), construct/criterion validity against surgical findings, and external replication, before any consideration as a standard.
Figure 4 depicts the overall imaging workflow; variant–technique pairings are consolidated in
Table 2. Operational thresholds (
Box 1) are applied at the target site—with confirmation in ≥2 orthogonal planes—and guide decision-changing imaging and patient-specific margins within the LCF.
A proposed roadmap for validating the framework—covering inter-reader reliability, construct validity, and external replication- is outlined in
Box 3.
Box 3. alidation plan for the LCF.
(i) Inter-reader reliability. Two independent readers, blinded to each other and outcomes; calibration on a small training set (not analyzed), then repeated reads of a random subset after a wash-out. Report κ (categorical calls:
BMC/RMC/AMF/HMC/AL presence) and ICC(2,1) (linear measures: AL length; MC–crest clearance), each with 95% CIs and number of cases/segments.
(ii) Construct/criterion validity. Test convergence with intra-operative findings and patient outcomes. Predefine decision-change endpoints (e.g., implant size/trajectory change; altered flap/osteotomy; avoidance of nerve injury). Evaluate performance of operational thresholds (AL ≥ 1–3 mm; HMC ≤ 4–5 mm, confirmed in ≥2 planes) against surgical margins/neuropraxia outcomes.
(iii) External replication. Prospective multi-center registry using standard data fields (modality, voxel size, plane confirmation, variant codes, thresholds, decision changes, complications). Report adherence to ALARA/ALADA triggers and patient-level outcomes.
4. Embryological and Developmental Basis of Variants
Anatomical variants of the MC and IAN originate from embryological transformations involving Meckel’s cartilage, which is derived from the first pharyngeal arch. Meckel’s cartilage becomes apparent around the fifth embryonic week (Carnegie stage 13; approximately day 32), acting as a cartilaginous scaffold for mandibular development. Ossification of the mandible begins adjacent to this cartilage around gestational week 10 [
33,
34].
During fetal development, Meckel’s cartilage undergoes partial regression, with only residual structures persisting postnatally as the malleus and sphenomandibular ligament. The embryonic IAN develops from multiple nerve bundles originating from neural crest cells. Incomplete fusion of these bundles during ossification may result in BMC or TMC [
33,
35,
36]. Likewise, the RMC likely represents a persistent pathway of secondary nerve branches supplying developing dentition or masticatory muscle insertions.
Vascular development also plays a critical role in variant formation. Embryonic vascular channels penetrating the mandibular bone can persist as accessory foramina, such as accessory mental or lingual foramina. A notable example is the “conduct of Serres”, a temporary fetal canal that transmits the embryonic vein of Serres; in some cases, this structure persists postnatally as an accessory MC [
37,
38].
This developmental perspective allows logical categorization of variants into
- (1)
nerve-splitting variants (e.g., bifid and trifid canals);
- (2)
vascular-based variants (e.g., accessory foramina);
- (3)
positional growth variants (e.g., high-positioned MC).
These should be considered normal developmental outcomes rather than pathological anomalies. These developmental mechanisms are summarized in
Table 3, which categorizes major anatomical variants of the MC and IAN according to their embryological origins and associated clinical implications.
5. Imaging Modalities and Detection of Variants
5.1. PR Versus CBCT
Traditional PR has significant limitations in accurately detecting MC anatomical variations. Earlier studies relying exclusively on panoramic imaging reported very low prevalences typically below 1% for bifid MC, largely due to anatomical superimposition and the inherent distortion of two-dimensional imaging [
4,
5].
Figure 5 illustrates why PR can be equivocal for canal variants; in such scenarios, CBCT confirmation is recommended if it would alter the treatment plan.
The advent of CBCT has markedly improved detection sensitivity. Its three-dimensional capability eliminates structural overlap, enabling accurate assessment of BMC, TMC, AL, RMC, and accessory foramina, which are often missed on PR [
8,
12]. For detailed prevalence data and diagnostic implications see
Table 1 and
Table 2.
Given these considerations, CBCT should be obtained selectively—when predefined, decision-changing criteria are met (see
Figure 4): suspected canal variants (BMC/TMC/RMC/AMF) on PR or clinical grounds; implant planning in the premolar region (3–5) where AL length influences safety margins; a short MC–crest distance at the planned osteotomy/implant site; repeated IANB failure; or complex mandibular third-molar surgery. This approach aligns with ALARA/ALADA and current evidence [
25,
39].
5.2. Magnetic Resonance Imaging (MRI) and Advanced Techniques
MRI, especially at higher field strengths such as 3T, complements CBCT by visualizing the neurovascular bundle (soft-tissue tract) within the MC region. Unlike CBCT, MRI can detect subtle soft-tissue structures, including branches of the IAN, even without corresponding distinct bony canals. Krasny [
11] utilized 3T MRI to identify multiple nerve branches within apparently single canals, offering explanations for certain unexplained anesthetic failures or neuropathies encountered clinically.
Recent MRI studies further illustrate this selectivity. Öçbe and Borahan [
7] demonstrated accessory-branch soft-tissue tract visualization in question-driven MRI examinations; because MRI does not depict corticated bony canals in the CBCT sense and these cohorts are selectively indicated, population-level “prevalence” figures are not appropriate and are not directly comparable to CBCT estimates. Furthermore, emerging diffusion tensor imaging tractography methods have shown significant promise for detailed nerve mapping, potentially enhancing preoperative planning accuracy by visualizing precise nerve trajectories [
20]. Nevertheless, rigorous anatomical validation through cadaveric studies remains necessary before widespread clinical adoption.
However, MRI’s clinical utility is currently limited by higher costs, longer scanning times, limited availability, and sensitivity to metallic artifacts, restricting its routine dental use. Thus, MRI is recommended selectively, primarily when complex nerve anatomy is suspected or when CBCT findings are inconclusive.
5.3. Capabilities and Limitations of Imaging Modalities
Each imaging technique presents unique strengths and limitations in evaluating MC variants. CBCT excels in demonstrating detailed bony anatomy with excellent spatial resolution and minimal distortion, reliably depicting accessory canals larger than approximately 0.1–0.2 mm in diameter. However, CBCT may miss smaller neural branches lacking distinct bony delineation. In contrast, MRI excels in soft-tissue differentiation, clearly depicting nerves and small vascular structures even in the absence of a distinct bony canal. However, MRI is disadvantaged by lower spatial resolution for bone, limited accessibility, higher costs, and susceptibility to artifacts.
Given these considerations, a recommended clinical imaging protocol involves:
Step 1: PR screening—routine, straightforward cases without red flags.
Step 2: Selective CBCT—obtain only when predefined, decision-changing criteria are met (suspected BMC/TMC/RMC/AMF; premolar region 3–5 where AL affects margins; short MC–crest distance at the planned site; repeated IANB failure; complex M3) (see
Figure 4).
Step 3: Selective MRI (3T)—reserved for unresolved soft-tissue questions when the result would change management.
5.4. Clinical Implications of Radiological Findings
The accurate identification of MC and IAN variants using advanced imaging modalities has profound clinical and medicolegal implications. Conventional PR, despite its historical utility in screening, consistently underestimates anatomical variability due to low spatial resolution and structural superimposition, with BMC detection rates rarely exceeding 1% [
4,
5].
By contrast, CBCT markedly enhances diagnostic sensitivity. In adult cohorts, typical BMC frequencies on CBCT fall in the single-digit to low double-digit range; higher values reported in some series reflect finer voxel sizes and stricter operational definitions, whereas PR detects substantially fewer. Trifid canals remain uncommon (≈1–2%) [
3,
8,
22,
25].
MRI, especially at 3 Tesla, complements CBCT by enabling direct visualization of soft-tissue structures, including IAN branches and neurovascular pathways, even in the absence of visible bony canals. Studies using high-resolution MRI have identified accessory neural branches within apparently single canals, providing explanations for anesthesia failure and unexpected neuropathy [
7,
11]. Moreover, advanced techniques such as DTI offer promising yet still experimental nerve-mapping capabilities, warranting further anatomical validation before clinical adoption [
20].
Clinically, preoperative identification of variants such as BMC, AL, accessory foramina, or HMC allows for tailored surgical strategies, thereby reducing the risks of nerve trauma, hemorrhage, and postoperative complications. These findings inform decisions regarding surgical access, implant angulation, anesthetic technique, and flap design.
Furthermore, thorough radiological evaluation and documentation play a crucial role in medicolegal risk mitigation. From a medicolegal perspective, advanced imaging—particularly CBCT and, in selected indications, MRI—should be obtained when it is reasonably expected to change management in high-risk mandibular regions, consistent with ALARA/ALADA principles.
In summary, CBCT should be the primary modality for variant detection and surgical planning, while MRI serves as a valuable adjunct in complex cases. Their combined use supports safer, variant-aware oral and maxillofacial surgery, improving patient outcomes and medico-legal defensibility. The distinct diagnostic capacities of various imaging modalities used in assessing MC variants are summarized in
Table 4, which contrasts their sensitivity, tissue visualization capabilities, radiation profiles, and clinical limitations. This structured overview facilitates optimal modality selection depending on the anatomical and surgical context.
6. Surgical and Clinical Implications
This section translates variant-aware imaging into domain-specific procedural adaptations for local anesthesia, implantology, mandibular third-molar surgery, and osteotomies. Consolidated, procedure-level guidance—together with decision-change imaging triggers (PR → selective CBCT; MRI reserved for soft-tissue pathway questions)—is summarized in
Table 5 (Domain-specific adaptations by variant). For a rapid, variant-first view of risk → mechanism → imaging trigger → planning, see
Table 2 (Quick Clinical Keys); per-study thresholds and parameters remain in
Table 1.
6.1. Local Anesthesia and Nerve Blocks
Undiagnosed MC variations are a common cause of failed IAN blocks. Bifid MCs or accessory branches near the mandibular foramen may result in incomplete anesthesia, as conventional IAN blocks may only affect the main nerve trunk, leaving accessory branches intact [
10]. Advanced imaging, particularly CBCT, improves detection of such variants [
3,
21], while MRI may reveal subtle branching patterns not visible on conventional imaging [
7,
11]. This justifies its selective use in patients with recurrent IANB failures. In such cases, clinicians should consider alternative anesthetic approaches such as Gow-Gates, Akinosi-Vazirani, or targeted infiltration at accessory foramina sites [
19,
40]. See
Table 5 for variant-specific anesthesia adaptations and decision-change triggers.
6.2. Third-Molar Surgery
Surgical extraction of mandibular third molars poses a significant risk for IAN injury, especially in the presence of RMCs or HMC. Undiagnosed RMC variants can cause unexpected intraoperative hemorrhage and postoperative neuropathy if transected during flap elevation or bone removal [
12,
16]. Similarly, an HMC located close to tooth apices limits surgical space, increasing the risk of nerve stretch or compression injuries [
2,
28]. Preoperative CBCT imaging significantly improves the detection of these variations [
8,
12,
25], facilitating tailored surgical techniques, such as coronectomy to avoid nerve entrapment or modified flap designs to circumvent accessory nerve branches [
3,
39]. When proximity is high, prioritize conservative ostectomy, consider coronectomy, and plan flap/suture strategy to avoid accessory foramina and the retromolar foramen (see
Table 5).
Figure 6 shows a sagittal CBCT with an impacted third molar and the MC in close proximity, illustrating why CBCT mapping can alter the choice of approach (e.g., coronectomy, modified flap).
6.3. Dental Implantology
Accurate identification of MC and IAN variations is critical during posterior mandibular implant planning. Variants such as an HMC restrict vertical bone availability, increasing the risk of nerve injury during osteotomies [
26]. AL of the mental nerve extending ≥3 mm anterior to the MeF require larger safety margins to avoid nerve impingement or injury [
18,
24]. Reported AL prevalence and measured length vary with imaging modality, voxel size, measurement plane and operational definition; consequently, implant safety margins in the premolar (3–5) region should be individualized from patient-specific CBCT measurements rather than set as a fixed universal rule. When the loop cannot be delineated with confidence, a more conservative posteriorization or a shorter fixture—and, where appropriate, guided surgery—are prudent. Maintain a conservative vertical and horizontal safety margin to the canal and mental foramen/loop; adjust implant length, trajectory, or site accordingly. Any uncertainty about loop length or course should trigger a posterior shift or shorter fixture (see
Table 5). Additionally, accessory mental foramina may contain small nerve branches leading to localized numbness or hemorrhage if intersected during implant osteotomies [
11,
19]. Buccolingual bifid canal variants can complicate lateral drilling or ridge modifications due to unpredictable nerve positions [
41,
42]. Thus, preoperative CBCT imaging is essential for precise planning of implant length, angulation, and position [
1,
8].
6.4. Oncologic and Reconstructive Surgery
In mandibular resections and reconstructive procedures, precise knowledge of MC anatomy is essential. Unrecognized BMC or TMC variants increase the risk of unintended nerve transection or excessive bleeding during segmental osteotomies [
3,
22]. Similarly, accessory foramina and RMC may serve as conduits for postoperative bleeding or infection [
37,
38]. Preoperative CBCT is vital for mapping these anatomical variants, while MRI may offer additional soft-tissue insights in complex cases [
7,
11]. Accurate imaging allows for surgical plan adjustments that preserve nerve integrity and minimize vascular injury. In orthognathic surgeries such as sagittal split ramus osteotomies, medial or lingual deviation of the MC substantially increases the risk of nerve injury, necessitating careful modification of osteotomy techniques [
27,
28].
6.5. Postoperative Complications and Medicolegal Considerations
Undiagnosed MC and IAN variants frequently contribute to postoperative complications such as prolonged paresthesia, dysesthesia, and neuroma formation. Partial injury to bifid or accessory nerve branches during extractions or osteotomies may result in persistent neuropathy or chronic pain [
22,
39]. Similarly, injury to accessory foramina can lead to localized numbness or vascular complications. From a medicolegal perspective, advanced imaging, particularly CBCT and, in selected indications, MRI—should be obtained when it is reasonably expected to change management in high-risk mandibular regions, consistent with ALARA/ALADA principles [
1]. Failure to identify anatomical variants may expose clinicians to liability, especially when preventable complications occur. Structured, variant-aware documentation templates can support consistent preoperative risk assessment and postoperative follow-up. Postoperative follow-up should include active screening for atypical neurosensory symptoms, with targeted CBCT or MRI when variant anatomy is suspected. Early detection can guide management strategies, including focused nerve stimulation and neuromodulation [
7,
20]. The clinical consequences of anatomical variants of the MC and IAN vary according to the surgical domain and variant type. These implications are summarized in
Table 5, which outlines the variant-specific risks, recommended imaging modalities, and procedural precautions relevant to contemporary oral and maxillofacial practice. For medicolegal clarity, clinicians should document the indication and decision-change trigger for imaging (e.g., equivocal PR, proximity below threshold, suspected AL).
7. LCF Clinical Framework and Workflow
7.1. Rationale and Scope
Earlier descriptive systems—particularly those relying on PR—offered limited practical guidance for risk assessment and planning: BMC were often underreported (<1%) due to superimposition and 2D distortion [
20,
35]. With cross-sectional imaging, visualization improved substantially, revealing higher rates of BMC/TMC, RMC/RMF and accessory foramina, and more intricate IAN branching [
2,
6,
18,
26,
40]. Against this background, we present the LCF clinical framework: a hypothesis-generating, variant-aware tool that links imaging features to decision-relevant thresholds and procedure-specific precautions. It is not a de novo anatomical taxonomy or standard. Point-of-use thresholds and confirmation criteria are summarized in
Box 1; procedure-specific adaptations are summarized in
Table 5. These thresholds anchor the decision-changing triggers summarized in
Section 7.2. Representative variant patterns are illustrated in
Figure 7.
7.2. Clinical Justification
LCF prioritizes situations in which imaging changes management. Based on the operational thresholds in
Section 7.1, imaging is escalated only when the following decision triggers are present:
PR conflicting/equivocal findings.
Planned implant in variant-rich zones (premolars 3–5; retromolar/ramus).
Uncertain MeF location on PR.
AL ≥1–3 mm on CBCT (confirmed in ≥2 planes).
Planned ramus/sagittal split or complex M3 procedures.
MC–crest clearance ≤4–5 mm at the planned site (CBCT, ≥2 planes).
Repeated IANB failure.
(Selective MRI is reserved for soft-tissue tract visualization when the result would change management.)
In practice, this means (1) PR for screening; (2) CBCT selectively when predefined, decision-changing criteria are met; and (3) selective MRI where soft-tissue tract visualization could alter the plan. LCF maps common variants to concrete adjustments—e.g., adapt osteotomy corridors/drill trajectories for bifid/trifid canals [
12,
16]; individualize margins in the premolar (3–5) region where AL length (commonly ≥1–3 mm) affects safety [
18,
24]; reconsider implant length/angulation or use guidance when the MC–crest distance ≤4–5 mm at the target site; and exercise caution around RMC/AMF because of bleeding/neurosensory risk [
15,
19,
29]. See
Figure 4 (workflow) and
Table 2.
7.3. Variant-Aware Clinical Workflow
Step 1—PR (screening). Look for overt anomalies (e.g., radiolucent duplication/retromolar tract suggestion) [
4].
Step 2—CBCT (selective). Obtain only when decision-changing criteria are present: suspected BMC/TMC/RMC/AMF on PR or clinical grounds; short MC–crest distance at the planned site; AL influencing premolar planning; repeated IANB failure; complex M3—consistent with ALARA/ALADA principles [
25,
39].
Step 3—MRI (selective). Selective MRI (3T): soft-tissue tract visualization of neurovascular bundles; consider it only when equivocal CBCT or a soft-tissue question would change the plan [
7,
11,
20].
Step 4—LCF notation in the record. Use concise patient-level labels (e.g., “LCF-BMC buccolingual; LCF-RMC unilateral; LCF-HMC at 36”).
Step 5—Procedure tailoring (examples).
LCF-BMC/LC-TMC: adjust osteotomy corridors and drilling trajectories; consider multi-site anesthesia [
3,
12].
LCF-RMC: modify flap design; anticipate/control retromolar vessels; consider piezosurgery [
12,
16,
23].
LCF-AL (≥1–3 mm): individualize margins in premolars (3–5) from CBCT; avoid fixed distances a priori [
18,
24].
LCF-AMF: avoid osteotomies across corticated accessory foramina; consider guidance [
19,
29].
LCF-HMC (MC–crest ≤4–5 mm at target site): reconsider implant length/angulation or use guided surgery; avoid vertical crest-adjacent cuts [
2,
26].
Step 6—Post-event documentation. If unexpected neurosensory change occurs, document LCF category, preoperative imaging and intraoperative modifications; consider targeted postoperative imaging when clinically justified.
7.4. Clinical Vignettes (LCF in Practice)
Vignette 1—Implant in the 3–5 region (suspected AL). PR is equivocal for an AL. CBCT confirms AL = 2.1 mm, measured and confirmed in ≥2 orthogonal planes (within the ≥1–3 mm operational threshold). Before LCF: a 4.3 × 10 implant with a standard safety margin was planned. LCF-guided change: switch to 3.8 × 8, adjust trajectory to preserve a patient-specific offset beyond the AL, and use guided surgery; MRI is not indicated (no soft-tissue question).
Vignette 2—Third molar with suspected RMC. PR is unclear for accessory branching. CBCT maps an RMC coursing toward the retromolar fossa. Before LCF: routine triangular flap and rotary osteotomy were planned. LCF-guided change: modify the flap and elevation, apply pre-emptive hemostasis, use a conservative osteotomy corridor (consider piezosurgery), and avoid suction trauma; MRI is not indicated (no soft-tissue question).
Vignette 3—Recurrent IAN block failure (suspected BMC). PR is non-diagnostic for variant branching. Selective CBCT (isotropic voxel ≈ 0.20–0.25 mm) demonstrates a BMC with a buccal accessory limb toward the molar apex, confirmed in ≥2 orthogonal planes. Before LCF: repeat conventional IAN block and routine flap/osteotomy were planned. LCF-guided change: switch to Gow–Gates/Vazirani–Akinosi plus targeted buccal/lingual infiltrations along the accessory tract; modify flap/osteotomy corridor to avoid the canal and prepare hemostasis; MRI not indicated (no soft-tissue question).
7.5. Clinical Impact
Embedding LCF encourages anticipatory rather than reactive decisions and improves cross-team communication. By stating when and why imaging alters the plan—and by using explicit thresholds (e.g., AL ≥ 1–3 mm, MC–crest ≤ 4–5 mm)—LCF aims to increase predictability and patient safety without promoting indiscriminate imaging (Vranckx, 2022) [
39].
8. Future Directions and Innovations
Critical appraisal: Selective MRI may assist when CBCT findings are equivocal or detailed neurovascular mapping is required, but several constraints limit immediate, broad adoption: (1) cost and access—availability, scheduling and reimbursement vary across settings; (2) validation gaps—there are few prospective, multi-center studies with inter-rater reliability and clinically meaningful endpoints (e.g., neurosensory outcomes); (3) heterogeneity—CBCT parameters (voxel size, FOV, kVp/mA) and MRI protocols (field strength, sequences) are not standardized, which impairs comparability; (4) radiation stewardship—CBCT should follow ALARA/ALADA with decision-changing thresholds. Addressing these points is prerequisite to guideline-level recommendations.
Despite recent progress in understanding MC and IAN variants, several challenges remain in imaging standardization, clinical implementation, and education. Addressing these gaps may enhance procedural safety and long-term outcomes.
8.1. International Variant Registries
There is a critical need for large-scale international registries of CBCT and MRI scans annotated for MC variants. Current studies are often limited by regional sampling. Multi-center repositories would allow for robust mapping of global prevalence and support research into demographics and clinical risk stratification [
8,
22].
8.2. MRI Tractography and Validation
Diffusion tensor imaging tractography enables non-invasive mapping of IAN trajectories, revealing complex branching patterns not visible on CBCT. Cadaveric validation is essential to ensure clinical reliability [
20].
8.3. Augmented Reality and Intraoperative Navigation
Augmented reality (AR) may provide real-time visualization of MC variants during surgery by integrating CBCT data intraoperatively. Preliminary studies suggest improved surgical accuracy, particularly in variant-rich zones. He et al. [
43] demonstrated a 57–62% reduction in osteotomy placement error using AR guidance in mandibular distraction procedures. Similarly, a systematic review by Puleio et al. [
44] concluded that AR consistently enhances precision across dental surgical interventions.
8.4. Educational Integration
Anatomical variability should be reflected in curricula and surgical training. LCF-based frameworks and 3D-printed CBCT-derived models offer enhanced simulation opportunities. A case-based, risk-adapted approach can better prepare trainees [
22].
A structured roadmap for innovation in MC and IAN management is summarized in
Table 6. This table outlines the key research and clinical directions, including AI-based variant detection, MRI validation studies, and surgical navigation tools.
9. Conclusions
This review underscores that anatomical variants of the mandibular canal (MC) and IAN) are far more prevalent than traditionally recognized and carry significant clinical implications. With the advent of advanced imaging modalities, especially CBCT and high-resolution MRI, variants such as bifid and trifid canals, anterior loops, and accessory foramina are now acknowledged as common features, not anomalies.
To translate this anatomical variability into safer clinical practice, we propose the LCF—a system directly linking variant types with procedural risks and recommended imaging protocols. This variant-aware workflow improves preoperative planning, reduces iatrogenic complications, and enhances patient outcomes across surgical, implantological, and anesthetic interventions.
An imaging-led pathway (PR → CBCT → selective MRI) aligned with the LCF translates canal variants into concrete risk–adaptation pairs across anesthesia, implantology, third-molar surgery, and osteotomies. Next steps are prospective, multi-center validation, protocol harmonization, and identifying clear decision-change triggers under ALARA/ALADA.
In sum, integrating LCF and advanced imaging into routine care represents a paradigm shift in oral and maxillofacial surgery—from reactive intervention to proactive, evidence-guided practice.
Author Contributions
I.C.L.—Conceptualization; methodology and methodological corrections; investigation (literature screening and study selection); data curation; formal analysis and synthesis; validation (definitions and clinical thresholds); visualization, figure and schema preparation; writing—original draft preparation. M.Ł.—Investigation (literature screening and study selection); manuscript review. Ł.O.—Writing—review and editing; Clinical validation:critical review; supervision; project administration; final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is a narrative review based solely on previously published data.
Informed Consent Statement
Written informed consent was obtained from the individual(s) whose anonymized CBCT images are reproduced in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank the teams of the VARIANTIS Research Laboratory and the VARIA Student Research Laboratory for their support and constructive input during the preparation of this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AF | Accessory foramen |
| AL | Anterior loop |
| ALADA | As Low As Diagnostically Acceptable |
| ALARA | As Low As Reasonably Achievable |
| AMF | Accessory mental foramen |
| BMC | Bifid mandibular canal |
| CBCT | Cone-beam computed tomography |
| DESS | Double-echo steady-state (MRI sequence) |
| DTI | Diffusion tensor imaging |
| FOV | Field of view |
| HMC | High-positioned mandibular canal |
| IAA | Inferior alveolar artery |
| IAN | Inferior alveolar nerve |
| IANB | Inferior alveolar nerve block |
| ICC | Intraclass correlation coefficient |
| κ | Cohen’s/Fleiss’ kappa |
| kVp/mA | Kilovolt peak/Milliampere (X-ray exposure parameters) |
| LCF | Landfald Clinical Framework |
| LF | Lingual foramen |
| MC | Mandibular canal |
| MeF | Mental foramen |
| MF | Mandibular foramen |
| MRI | Magnetic resonance imaging |
| M3 | Mandibular third molar |
| NV | Neurovascular |
| PR | Panoramic radiography |
| RMC | Retromolar canal |
| TIRM | Turbo inversion recovery magnitude (MRI sequence) |
| TMC | Trifid mandibular canal |
References
- Juodzbalys, G.; Wang, H.L. Identification of the mandibular vital structures: Practical clinical applications of anatomy and radiological examination methods. J. Oral Maxillofac. Res. 2010, 1, e1. [Google Scholar] [CrossRef]
- Yu, S.K.; Lee, M.H.; Jeon, Y.H.; Chung, Y.Y.; Kim, H.J. Anatomical configuration of the inferior alveolar neurovascular bundle: A histomorphometric analysis. Surg. Radiol. Anat. 2016, 38, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Priya, A.; Ravi, K.S.; Iwanaga, J.; Tubbs, R.S.; Naaz, S.; Panchal, P. An evaluation of mandibular canal variations: A systematic review and meta-analysis. Anat. Sci. Int. 2023, 98, 176–184. [Google Scholar] [CrossRef]
- Langlais, R.P.; Broadus, R.; Glass, B.J. Bifid mandibular canals in panoramic radiographs. J. Am. Dent. Assoc. 1985, 110, 923–926. [Google Scholar] [CrossRef]
- Sanchis, J.M.; Peñarrocha, M.; Soler, F. Bifid mandibular canal. J. Oral Maxillofac. Surg. 2003, 61, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Elnadoury, E.A.; Gaweesh, Y.S.E.; Abu El Sadat, S.M.; Anwar, S.K. Prevalence of bifid and trifid mandibular canals with unusual patterns of nerve branching using cone beam computed tomography. Odontology 2022, 110, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Öçbe, M.; Borahan, M.O. Identifying the Anatomical Variations of the Inferior Alveolar Nerve with Magnetic Resonance Imaging. Imaging Niger. J. Clin. Pract. 2024, 27, 136–142. [Google Scholar] [CrossRef]
- Dos Santos Oliveira, R.; Maria Gomes Oliveira, A.; Cintra Junqueira, J.L.; Kühl Panzarella, F. Association between the Anatomy of the Mandibular Canal and Facial Types: A Cone-Beam Computed Tomography Analysis. Int. J. Dent. 2018, 2018, 5481383. [Google Scholar] [CrossRef]
- Altındağ, A.; Yalın, H.; Yüksel, İ.B. Pattern diversity and prevalence of bifid mandibular canal: A CBCT-based cross-sectional study: Evaluation of Bifid Mandibular Canal via CBCT. Oral Maxillofac. Surg. 2025, 29, 110. [Google Scholar] [CrossRef]
- Wadhwani, P.; Mathur, R.M.; Kohli, M.; Sahu, R. Mandibular canal variant: A case report. J. Oral Pathol. Med. 2008, 37, 122–124. [Google Scholar] [CrossRef]
- Krasny, A.; Krasny, N.; Prescher, A. Anatomic variations of neural canal structures of the mandible observed by 3-tesla magnetic resonance imaging. J. Comput. Assist. Tomogr. 2012, 36, 150–153. [Google Scholar] [CrossRef][Green Version]
- von Arx, T.; Hänni, A.; Sendi, P.; Buser, D.; Bornstein, M.M. Radiographic study of the mandibular retromolar canal: An anatomic structure with clinical importance. J. Endod. 2011, 37, 1630–1635. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kuribayashi, A.; Watanabe, H.; Imaizumi, A.; Tantanapornkul, W.; Katakami, K.; Kurabayashi, T. Bifid mandibular canals: Cone beam computed tomography evaluation. Dentomaxillofac. Radiol. 2010, 39, 235–239. [Google Scholar] [CrossRef]
- Sisman, Y.; Ercan-Sekerci, A.; Payveren-Arikan, M.; Sahman, H. Diagnostic accuracy of cone-beam CT compared with panoramic images in predicting retromolar canal during extraction of impacted mandibular third molars. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e74–e81. [Google Scholar] [CrossRef]
- Shen, Y.W.; Chang, W.C.; Huang, H.L.; Tsai, M.T.; Fuh, L.J.; Hsu, J.T. Assessment of the Retromolar Canal in Taiwan Subpopulation: A Cross-Sectional Cone-Beam Computed Tomography Study in a Medical Center. Tomography 2021, 7, 219–227. [Google Scholar] [CrossRef]
- Nascimento, E.H.L.D.; Pontual, M.L.d.A.; Pontual, A.d.A.; Perez, D.E.d.C.; Figueiroa, J.N.; Frazão, M.A.G.; Ramos-Perez, F.M.d.M. Assessment of the anterior loop of the mandibular canal: A study using cone-beam computed tomography. Imaging Sci. Dent. 2016, 46, 69–75. [Google Scholar] [CrossRef]
- Uchida, Y.; Yamashita, Y.; Goto, M.; Hanihara, T. Measurement of anterior loop length for the mandibular canal and diameter of the mandibular incisive canal to avoid nerve damage when installing endosseous implants in the interforaminal region. J. Oral Maxillofac. Surg. 2007, 65, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, M.; Zarch, S.H.H.; Eshghpour, M.; Khodadadzadeh, P. Prevalence of accessory mental foramen and lateral lingual foramen using cone beam computed tomography: A single-center cross-sectional study. Oral Maxillofac. Surg. 2024, 28, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, S.; Sakamoto, J.; Kretapirom, K.; Supak, N.; Sumi, Y.; Kurabayashi, T. Diffusion tensor imaging of the inferior alveolar nerve using 3T MRI: A study for quantitative evaluation and fibre tracking. Dentomaxillofac. Radiol. 2016, 45, 20160200. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, M.; Hiraiwa, Y.; Aimiya, H.; Ariji, E. Observation of bifid mandibular canal using cone-beam computerized tomography. Int. J. Oral Maxillofac. Implants 2009, 24, 155–159. [Google Scholar] [PubMed]
- Göller Bulut, D.; Kartal Yalçın, G.; Tanrıseven, Z.; Taşkın, B.; Aydın, B. Prevalence and topography of bifid and trifid mandibular canal in Turkish Western Anatolia Population: Evaluation of the inferior alveolar canal with CBCT. Surg. Radiol. Anat. 2024, 46, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Park, C.S. Cone beam CT findings of retromolar canals: Report of cases and literature review. Imaging Sci. Dent. 2013, 43, 309–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosa, M.B.; Sotto-Maior, B.S.; Machado Vde, C.; Francischone, C.E. Retrospective study of the anterior loop of the inferior alveolar nerve and the incisive canal using cone beam computed tomography. Int. J. Oral Maxillofac. Implants 2013, 28, 388–392. [Google Scholar] [CrossRef]
- Khorshidi, H.; Raoofi, S.; Ghapanchi, J.; Shahidi, S.; Paknahad, M. Cone Beam Computed Tomographic Analysis of the Course and Position of Mandibular Canal. J. Maxillofac. Oral Surg. 2017, 16, 306–311. [Google Scholar] [CrossRef]
- Heasman, P.A. Variation in the position of the inferior dental canal and its significance to restorative dentistry. J. Dent. 1988, 16, 36–39. [Google Scholar] [CrossRef]
- Polland, K.E.; Munro, S.; Reford, G.; Lockhart, A.; Logan, G.; Brocklebank, L.; McDonald, S.W. The mandibular canal of the edentulous jaw. Clin. Anat. 2001, 14, 445–452. [Google Scholar] [CrossRef]
- Kim, S.T.; Hu, K.S.; Song, W.C.; Kang, M.K.; Park, H.D.; Kim, H.J. Location of the mandibular canal and the topography of its neurovascular structures. J. Craniofac. Surg. 2009, 20, 936–939. [Google Scholar] [CrossRef]
- Varvara, G.; Feragalli, B.; Turkyilmaz, I.; D’Alonzo, A.; Rinaldi, F.; Bianchi, S.; Piattelli, M.; Macchiarelli, G.; Bernardi, S. Prevalence and Characteristics of Accessory Mandibular Canals: A Cone-Beam Computed Tomography Study in a European Adult Population. Diagnostics 2022, 12, 1885. [Google Scholar] [CrossRef]
- Landfald, I.C.; Adamek, J.; Kumar, Y.A.S.; Olewnik, Ł.; Coleman, J.; Labetowicz, P. Anatomy Reimagined: The Landfald Classification as a Transformative Surgical and Radiological Guide to Facial Artery Variants. Ann. Anat.-Anat. Anz. 2025, 263, 152712. [Google Scholar] [CrossRef]
- Landfald, I.C.; Vazquez, T.; Okoń, A.; Olewnik, Ł. Temporalis muscle flap in craniofacial reconstruction: Anatomy, techniques, outcomes, and innovations. Front. Surg. 2025, 12, 1678935. [Google Scholar] [CrossRef] [PubMed]
- Okoń, A.; Landfald, I.C.; Olewnik, Ł. The Deep Head of the Masseter Muscle: A Classification-Based Anatomical and Surgical Framework. Biomedicines 2025, 13, 2201. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, J.F.; Mérida-Velasco, J.R.; Mérida-Velasco, J.A.; Sánchez-Montesinos, I.; Espín-Ferra, J.; Jiménez-Collado, J. Development of Meckel’s cartilage in the symphyseal region in man. Anat. Rec. 1997, 249, 249–254. [Google Scholar] [CrossRef]
- Wyganowska-Świątkowska, M.; Przystańska, A. The Meckel’s cartilage in human embryonic and early fetal periods. Anat. Sci. Int. 2011, 86, 98–107. [Google Scholar] [CrossRef]
- Chávez-Lomeli, M.E.; Mansilla Lory, J.; Pompa, J.A.; Kjaer, I. The human mandibular canal arises from three separate canals innervating different tooth groups. J. Dent. Res. 1996, 75, 1540–1544. [Google Scholar] [CrossRef]
- Iwanaga, J.; Takeshita, Y.; Matsushita, Y.; Hur, M.S.; Ibaragi, S.; Tubbs, R.S. What are the retromolar and bifid/trifid mandibular canals as seen on cone-beam computed tomography? Revisiting classic gross anatomy of the inferior alveolar nerve and correcting terminology. Surg. Radiol. Anat. 2022, 44, 147–156. [Google Scholar] [CrossRef]
- Lipski, M.; Tomaszewska, I.M.; Lipska, W.; Lis, G.J.; Tomaszewski, K.A. The mandible and its foramen: Anatomy, anthropology, embryology and resulting clinical implications. Folia Morphol. 2013, 72, 285–292. [Google Scholar] [CrossRef]
- Murlimanju, B.V.; Latha, V.; Prameela, M.D.; Ashraf, C.M. Accessory mandibular foramina: Prevalence, embryological basis and surgical implications. J. Clin. Diagn. Res. 2011, 5, 1137–1139. [Google Scholar]
- Vranckx, M.; Geerinckx, H.; Gaêta-Araujo, H.; Leite, A.F.; Politis, C.; Jacobs, R. Do anatomical variations of the mandibular canal pose an increased risk of inferior alveolar nerve injury after third molar removal? Clin. Oral Investig. 2022, 26, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Muinelo-Lorenzo, J.; Fernández-Alonso, A.; Smyth-Chamosa, E.; Suárez-Quintanilla, J.A.; Varela-Mallou, J.; Suárez-Cunqueiro, M.M. Predictive factors of the dimensions and location of mental foramen using cone beam computed tomography. PLoS ONE 2017, 12, e0179704. [Google Scholar] [CrossRef]
- Claeys, V.; Wackens, G. Bifid mandibular canal: Literature review and case report. Dentomaxillofac. Radiol. 2005, 34, 55–58. [Google Scholar] [CrossRef]
- Rouas, P.; Nancy, J.; Bar, D. Identification of double mandibular canals: Literature review and three case reports with CTscans and cone beam, C.T. Dentomaxillofac. Radiol. 2007, 36, 34–38. [Google Scholar] [CrossRef] [PubMed]
- He, S.-X.; Ma, C.; Yuan, Z.-Y.; Xu, T.-F.; Xie, Q.-T.; Wang, Y.-X.; Huang, X.-P. Feasibility of augmented reality using dental arch-based registration applied to navigation in mandibular distraction osteogenesis: A phantom experiment. BMC Oral Health 2024, 24, 1321. [Google Scholar] [CrossRef] [PubMed]
- Puleio, F.; Tosco, V.; Pirri, R.; Simeone, M.; Monterubbianesi, R.; Giudice, G.L.; Giudice, R.L. Augmented Reality in Dentistry: Enhancing Precision in Clinical Procedures–A Systematic Review. Clin. Pract. 2024, 14, 2267–2283. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
PRISMA 2020 flow diagram for study selection (PubMed/MEDLINE and Scopus; last search: 6 October 2025).
Figure 1.
PRISMA 2020 flow diagram for study selection (PubMed/MEDLINE and Scopus; last search: 6 October 2025).
Figure 2.
CBCT multiplanar visualization of the mandibular canal (MC). (A) Axial view showing the corticated cross-section of the mandibular canal (yellow dotted circle). (B) Sagittal reconstruction demonstrating two parallel corticated tracts suggestive of a bifid configuration of the mandibular canal (yellow lines). (C) Coronal reconstruction outlining the course of the main mandibular canal within the mandibular body (yellow dotted outline).
Figure 2.
CBCT multiplanar visualization of the mandibular canal (MC). (A) Axial view showing the corticated cross-section of the mandibular canal (yellow dotted circle). (B) Sagittal reconstruction demonstrating two parallel corticated tracts suggestive of a bifid configuration of the mandibular canal (yellow lines). (C) Coronal reconstruction outlining the course of the main mandibular canal within the mandibular body (yellow dotted outline).
Figure 3.
Axial CBCT demonstrating the mandibular canal (MC) bilaterally. Corticated cross-sections of the mandibular canals (yellow arrows) are visible within the mandibular bodies on both sides, allowing direct assessment of canal morphology and symmetry.
Figure 3.
Axial CBCT demonstrating the mandibular canal (MC) bilaterally. Corticated cross-sections of the mandibular canals (yellow arrows) are visible within the mandibular bodies on both sides, allowing direct assessment of canal morphology and symmetry.
Figure 4.
Variant-aware imaging workflow (PR → CBCT → selective MRI). Sequential decision pathway illustrating when to proceed from panoramic radiography to CBCT or MRI based on red flags and clinical relevance, following ALARA/ALADA principles.
Figure 4.
Variant-aware imaging workflow (PR → CBCT → selective MRI). Sequential decision pathway illustrating when to proceed from panoramic radiography to CBCT or MRI based on red flags and clinical relevance, following ALARA/ALADA principles.
Figure 5.
Panoramic radiograph (PR) showing a suspected bifid mandibular canal (BMC; blue arrow). The blue arrow indicates the cortical outline suggestive of canal duplication. PR alone cannot confirm this finding; CBCT is recommended when confirmation would change management (CBCT not shown).
Figure 5.
Panoramic radiograph (PR) showing a suspected bifid mandibular canal (BMC; blue arrow). The blue arrow indicates the cortical outline suggestive of canal duplication. PR alone cannot confirm this finding; CBCT is recommended when confirmation would change management (CBCT not shown).
Figure 6.
Oblique-sagittal CBCT reconstruction demonstrating the course of the mandibular canal (MC). The yellow arrows indicate the canal trajectory within the mandibular body and its proximity to the molar roots, serving as key landmarks for preoperative assessment and surgical planning.
Figure 6.
Oblique-sagittal CBCT reconstruction demonstrating the course of the mandibular canal (MC). The yellow arrows indicate the canal trajectory within the mandibular body and its proximity to the molar roots, serving as key landmarks for preoperative assessment and surgical planning.
Figure 7.
LCF-guided imaging variants. Panels (I–VI) illustrate representative mandibular canal configurations used within the Landfald Clinical Framework to guide imaging and planning (e.g., single course; bifid/trifid or retromolar branching; anterior loop–dominant course; high-positioned canal; accessory exits). The yellow line marks the inferred inferior alveolar neurovascular pathway/mandibular canal; AF denotes an accessory foramen.
Figure 7.
LCF-guided imaging variants. Panels (I–VI) illustrate representative mandibular canal configurations used within the Landfald Clinical Framework to guide imaging and planning (e.g., single course; bifid/trifid or retromolar branching; anterior loop–dominant course; high-positioned canal; accessory exits). The yellow line marks the inferred inferior alveolar neurovascular pathway/mandibular canal; AF denotes an accessory foramen.
Table 1.
Per-study characteristics and outcomes (complete rows).
Table 1.
Per-study characteristics and outcomes (complete rows).
| Study (First Author, Year) | Modality | Voxel/Sequence | Operational Definition/Threshold | Population | N (Unit) | Variant | Prevalence (%) | Morphometrics (Units) | Reader Method |
|---|
| Langlais [4] | PR | — | Duplicate/parallel corticated tract visible on panoramic radiograph | Adults; routine dental radiography | 6000 PRs | BMC | 1.0 (57/6000) | — | NR |
| Kuribayashi [14] | CBCT | 0.125 mm (3DX micro CT); 1 mm slice | BMC present; Nortjé-type classification; ≥2-plane confirmation | Adults (mean age 33; 18–74); impacted third molars | 252 pts/301 sides | BMC | 15.6 (47/301) | Mean canal diameter 1.68 mm (0.88–3.40); main canal 3.28 mm (2.02–4.63) | Two oral radiologists; independent readings; consensus after discussion |
| Sisman [15] | PR vs. CBCT | PR: —; CBCT: 0.15 mm voxel (NewTom 5G typical) | RMC present (comparison PR vs. CBCT) | Adults (632 individuals; 947 hemimandibles) with impacted M3 (mean age 27.47 ± 8.74 years) | 632 pts/947 hemimandibles | RMC | CBCT: 26.7; PR: 3.06 | Canal height 11.4 ± 2.61 mm; horizontal distance to second molar 15.45 ± 3.12 mm; origin width 2.24 ± 0.94 mm; exit width 1.64 ± 0.64 mm | Two dentomaxillofacial radiologists; independent evaluation; consensus when disagreement; measurements repeated after 2 weeks |
| Shen [16] | CBCT | 0.155 mm (AZ3000; 85 kVp, 12.5 mA; FOV 70 mm) | RMF present (distinct foramen/tract in ≥2 planes) | Adults; Taiwan, medical center | 68 hemimandibles | RMC/RMF | 10.3 (7/68) | RMF Ø 1.41 ± 0.30 mm; MeF–RMF 11.57 ± 2.70 mm; RMF–crest 13.62 ± 1.34 mm | NR |
| von Arx [12] | CBCT | NR | RMF present on CBCT (corticated tract/foramen) | Adults; imaging study (CH) | 100 pts/121 sides | RMC/RMF | 25.6 (sides) | Distance to second molar = 15.16 ± 2.39 mm; canal height = 11.34 ± 2.36 mm; canal width = 0.99 ± 0.31 mm | NR |
| do Nascimento [17] | CBCT | 0.25 mm (i-CAT) | AL present; length measured in orthogonal planes | Adults; Brazil (radiology clinic) | 250 pts/500 hemimandibles | AL | 41.6 | AL length mean 1.1 ± 0.8 mm (0.25–4.00) | Two trained and calibrated observers; consensus after discussion |
| Uchida [18] | Anatomy vs. CBCT (subset) | — | AL length measured directly (cadaveric) | 38 cadavers/75 hemimandibles | 75 hemimandibles | AL | — | AL mean 1.5 ± 1.4 mm (0–6) | Direct measurements |
| Mostafavi [19] | CBCT | NR | AMF per CBCT criteria; foramen-level reporting | Adults; single center | 2082 CBCTs | AMF | 11.8 | NR (size not reported) | NR |
| Krasny [11] | MRI (3T) | 3T; T1/T2/TIRM (conventional) | Visualization of neurovascular tract (soft-tissue)—no bony canal prevalence | Adults; dentate | 64 patients | Soft-tissue tract | — | NR (soft-tissue only) | NR |
| Kotaki [20] | MRI-DTI (3T) | 3T MRI; single-shot EPI DTI with STIR fat suppression; 3D T1 MP-RAGE; 16-channel head/neck coil | Neurovascular bundle course mapped by fiber tracking | Healthy volunteers | 46 volunteers (92 IANs) | Soft-tissue tract | — | NR (soft-tissue only) | NR |
Table 2.
Quick Clinical Keys for variant-aware decision-making.
Table 2.
Quick Clinical Keys for variant-aware decision-making.
| Variant | Key Clinical Risk | Mechanism | Imaging Trigger | Planning/Actions | Key Sources |
|---|
| Bifid/Trifid mandibular canal (BMC/TMC) | Failed anesthesia; neurosensory injury; intraoperative bleeding | Duplicated neurovascular pathway with accessory limbs/exits | PR equivocal or repeated anesthesia failure → fine-voxel CBCT (≥2 planes); MRI only for specific soft-tissue questions | Use Gow-Gates or Vazirani–Akinosi plus supplementary infiltrations; avoid instrumentation between limbs; plan shorter implants/altered trajectory; plan conservative osteotomy; hemostasis readiness | Langlais [4]; Naitoh [21]; Göller Bulut [22]; Wadhwani et al. [10]; Asghar et al. [3]; von Arx [12] |
| Retromolar canal/foramen (RMC) | Hemorrhage; neurosensory disturbance | Accessory neurovascular bundle in the retromolar triangle connecting to MC or crest | PR low sensitivity; PR suspicion or surgery near retromolar area → CBCT to map canal/foramen; MRI rarely needed | Supplemental infiltration in retromolar region; modify flap to avoid foramen; avoid crossing RMC with implants/osteotomy; pre-emptive hemostasis; use piezosurgery if needed | von Arx [12]; Sisman [15]; Shen [16]; Han and Park [23] |
| Anterior loop (AL) | Mental nerve injury in premolar implant placement | Anterior extension of the IAN beyond the mental foramen | PR unreliable; CBCT to measure loop length and orientation (≥1–3 mm) in ≥2 planes; MRI only for soft-tissue pathway | Individualize safety margin based on CBCT (≥1–3 mm beyond loop); adjust implant length and angulation; plan incision posterior to mental foramen; consider guided surgery; caution with infiltration near MeF | Uchida [18]; Rosa [24]; Khorshidi et al. [25] |
| High-positioned mandibular canal (HMC) | IAN injury; limited bone height | Short distance between MC and alveolar crest | If PR suggests reduced height or planned split/implant surgery → CBCT to measure MC–crest clearance; treat ≤4–5 mm as high risk | Choose shorter implants or alternative site; modify osteotomy trajectory; consider guided surgery; avoid vertical crest cuts; monitor sensation | Heasman [26]; Polland [27]; Kim [28]; Yu [2] |
| Accessory mental foramen/accessory exits (AMF/LF) | Accessory nerve injury; bleeding; anesthesia failure | Presence of accessory mental or lateral foramen with additional neurovascular bundle | Plan lateral cortical drilling in premolar region or suspicious PR → CBCT to locate AF; MRI rarely needed | Localize AF on CBCT; reposition incision or fixation to avoid channel; targeted anesthesia away from accessory exits; consider guided surgery; counsel patient on paresthesia risk | Mostafavi [19]; Varvara [29]; Krasny [11]; Öçbe & Borahan [7] |
Table 3.
Embryological Origins of MC and IAN Variants.
Table 3.
Embryological Origins of MC and IAN Variants.
| Variant | Origin | Clinical Implication | References |
|---|
| BMC, TMC | Incomplete fusion of IAN nerve bundles | Anesthesia failure, nerve injury | Rodríguez-Vázquez et al. [33]; Chávez-Lomeli et al. [35] |
| RMC | Persistent secondary nerve branches | Bleeding, neuropathy | Lipski et al. [38]; Rodríguez-Vázquez et al. [33] |
| AF (AMF, LF) | Persistent embryonic vascular channels | Unexpected bleeding, incomplete anesthesia | Murlimanju et al. [38]; Lipski et al. [37] |
| High MC | Differential mandibular growth | Surgical complications, implant risk | Wyganowska-Świątkowska and Przystańska [34] |
Table 4.
Imaging modalities for MC variants—roles, performance, and limitations (framework summary).
Table 4.
Imaging modalities for MC variants—roles, performance, and limitations (framework summary).
Imaging
Modality | Detection
Sensitivity | Soft-Tissue
Detail | Radiation
Exposure | Clinical
Applications | Limitations | References |
|---|
| PR | Low for bony variants; susceptible to superimposition and projection geometry | None | Minimal | Initial screening; baseline orientation of dentition and MeF region | Poor spatial resolution; superimposition; cannot confirm small/accessory canals; 2D only | Langlais [4]; Sanchis [5] |
| CBCT | High for bony canal variants when ≥2-plane confirmation and appropriate voxel size are used; definition- and voxel-dependent | Limited (osseous detail only) | Moderate (apply ALADA principles) | Implant planning; third-molar/ramus surgery; variant mapping (BMC/RMC/AMF); MC–crest clearance; AL assessment | Does not visualize nerve tissue directly; artifacts at very low voxels/metal; targeted cohorts can bias apparent frequencies | Naitoh [21]; Göller Bulut [22]; Asghar [3] |
| MRI (3T) | Soft-tissue tract visualization (not directly comparable to CBCT “prevalence” of bony canals) | Excellent for neurovascular bundles and perineural pathology | None | Selective use for soft-tissue questions when results would change management (complex branching, perineural/inflammatory pathology) | Cost/availability; motion and metal susceptibility; limited standardization across centers | Krasny [11]; Öçbe [7] |
| MRI-DTI | Experimental; tractography feasibility in selected cohorts | Excellent (directional information) | None | Advanced nerve pathway mapping in research or highly selected clinical scenarios | Requires further validation; longer acquisition; artifacts and expertise requirements | Kotaki [20] |
Table 5.
Domain-specific adaptations by variant (anesthesia, implantology, third-molar, osteotomy).
Table 5.
Domain-specific adaptations by variant (anesthesia, implantology, third-molar, osteotomy).
| Variant | Anesthesia Adaptation | Implantology/Third-Molar/Osteotomy Adaptation | Imaging & Trigger/Key References |
|---|
| Bifid/Trifid mandibular canal (BMC/TMC) | Prefer Gow-Gates or Vazirani–Akinosi; add buccal/lingual infiltrations; target accessory foramina | Use shorter implant or altered trajectory/site; avoid instrumentation between canal limbs; conservative ostectomy/flap; hemostasis readiness | PR often equivocal → CBCT to map both limbs; MRI only for specific neurovascular questions; revise plan if canal within planned corridor
von Arx et al. [12]; Asghar et al. [3]; Wadhwani et al. [10] |
| Retromolar canal/foramen (RMC) | Supplemental infiltration in retromolar area or alternative block | Avoid crossing RMC with implants/osteotomy/screws; modify flap to spare foramen; vessel control; consider piezosurgery | PR low sensitivity → CBCT to confirm canal/foramen; revise plan if canal intersects surgical path
von Arx et al. [12]; Shen et al. [16]; Han & Park [23] |
| Anterior loop (AL) | Adjust infiltration/incisions near mental foramen; avoid injuring loop | Keep entry/exit corridor posterior to MeF by loop length + buffer; use shorter fixture or adjust angulation; consider guided surgery | PR unreliable → CBCT to estimate loop length/orientation; posteriorize plan or choose shorter implant if uncertain
Uchida et al. [18]; Rosa et al. [24]; Khorshidi et al. [25] |
| High-positioned mandibular canal (HMC) | Consider supplemental infiltrations if block efficacy uncertain | Shorter implant; alter trajectory/site; consider coronectomy for M3 when close; guided surgery; avoid vertical crest cuts | CBCT to measure crest/apex–MC distances; if clearance ≤4–5 mm, shorten implant or change site
Heasman [26]; Kim et al. [28]; Yu et al. [2] |
| Accessory mental foramen/accessory exits (AMF/LF) | Targeted infiltration away from accessory exits | Re-route incisions; avoid screws/fixtures crossing accessory channels; consider guided approach | PR often misses → CBCT mapping; revise plan if accessory exit lies in surgical corridor; MRI rarely required
Varvara et al. [29]; Mostafavi et al. [19]; Krasny et al. [11] |
Table 6.
Summary of Future Directions for MC and IAN Management.
Table 6.
Summary of Future Directions for MC and IAN Management.
| Future Direction | Objectives | Clinical Impact | Implementation Strategies |
|---|
| Variant Registries & Big Data | Global MC variant prevalence mapping; outcome correlation | Improved risk stratification, surgical planning | International standardized registry protocols |
| MRI Tractography Validation | Anatomical validation of MRI nerve tractography with dissections | Precise preoperative nerve mapping | Cadaveric MRI–anatomy correlation studies |
| AR & Surgical Navigation | Real-time MC visualization during mandibular surgery | Improved intraoperative precision, reduced injuries | Development and validation of AR navigation systems |
| Educational Reform | Curriculum integration of anatomical variant clinical significance | Enhanced trainee preparedness, proactive mindset | Textbook updates, case-based learning, 3D models |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).