Overcoming Oral Cavity Barriers for Peptide Delivery Using Advanced Pharmaceutical Techniques and Nano-Formulation Platforms
Abstract
1. Introduction
Importance of Peptide-Based Therapeutics
2. Modes of Peptide Administration
2.1. Parental Route
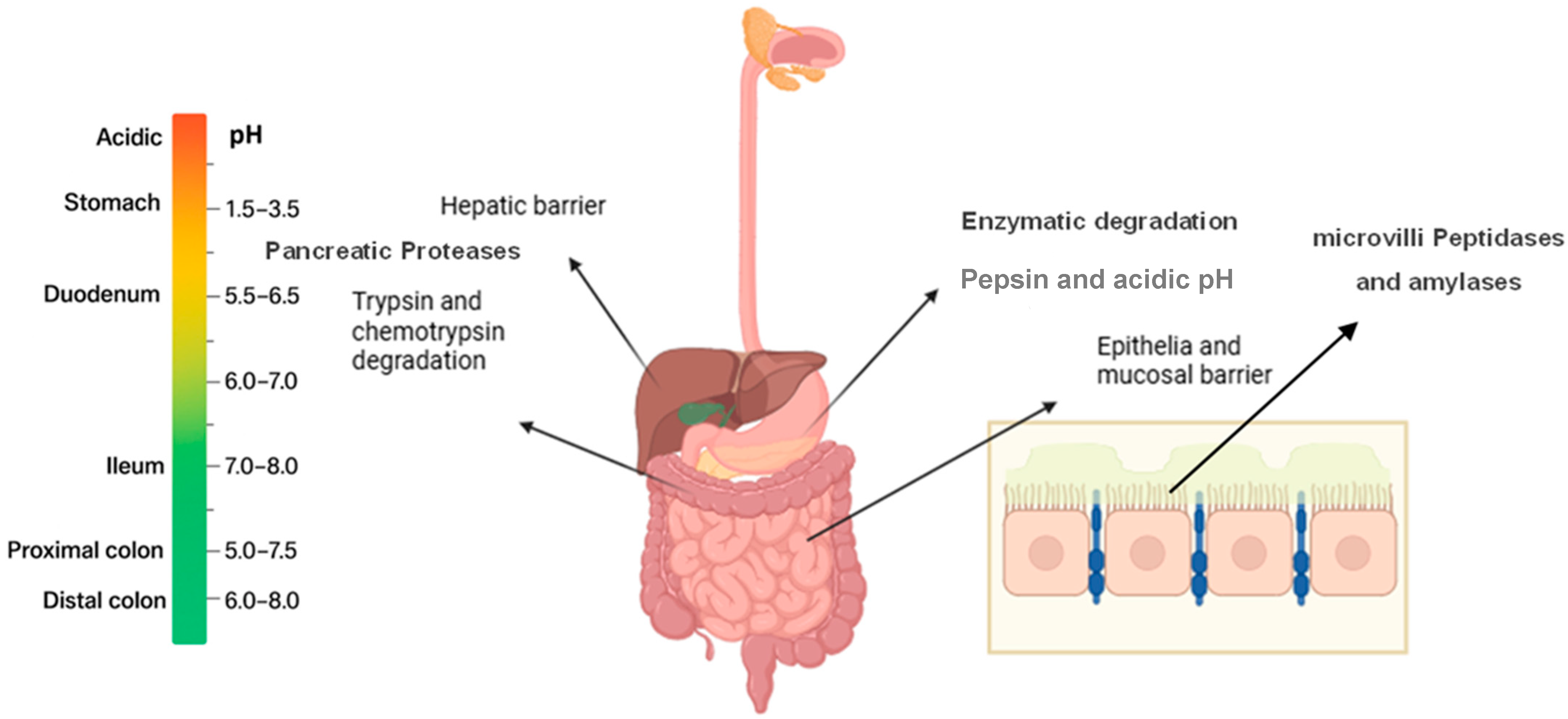
2.2. Oral Peptide Administration Directed to Intestinal Tract
2.3. Peptide Administration via Oral Cavity
3. Mechanisms of Peptide Uptake Across Oral Mucosa via Oral Cavity
4. Challenges of Peptide Transport and Absorption via Oral Mucosa
5. Oral Mucosal Permeability of Peptides in the Oral Cavity
5.1. Peptide Molecular Weight and Buccal Mucosal Permeability
5.2. Conformation and Immunogenicity
5.3. Physicochemical Peptide Properties (Solubility, Hydrophilicity, Partition Coefficient, Aggregation and Hydrogen Bonding)
5.4. Electrostatic Charge and Buccal Permeability
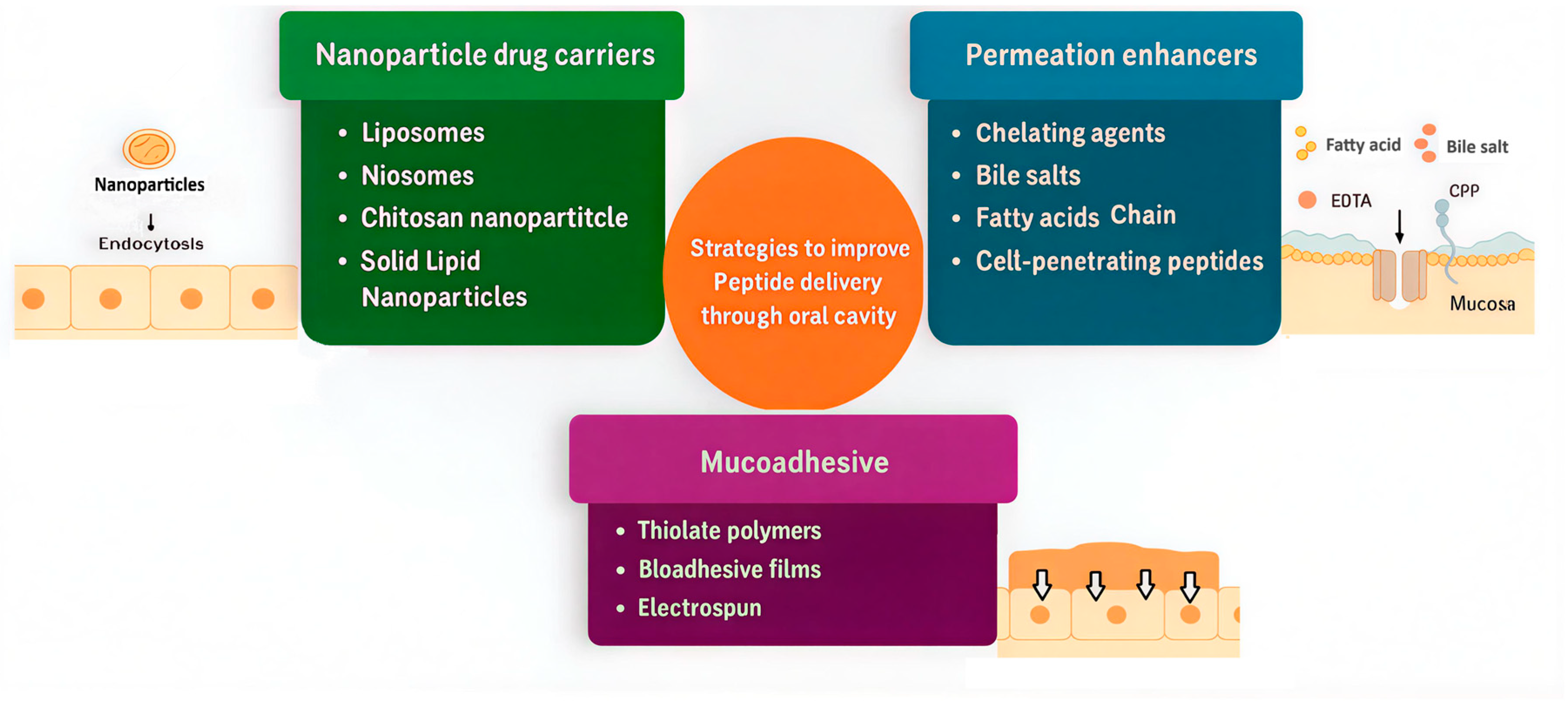
6. Strategies to Enhance Peptide Permeation and Stability via Oral Route
Formulation Strategies to Enhance Peptide Absorption and Retention in the Mouth Cavity
7. Nanoparticulate Strategies for Buccal and Sublingual Peptide Delivery
7.1. Rationale for Nanoparticle Use in Oromucosal Peptide Delivery
7.2. Nanoparticle Classes and Their Comparative Potential
7.2.1. Lipid-Based Nanoparticles
7.2.2. Niosomes
7.2.3. Polymeric Nanoparticles
7.2.4. Hybrid Responsive Nanocarriers
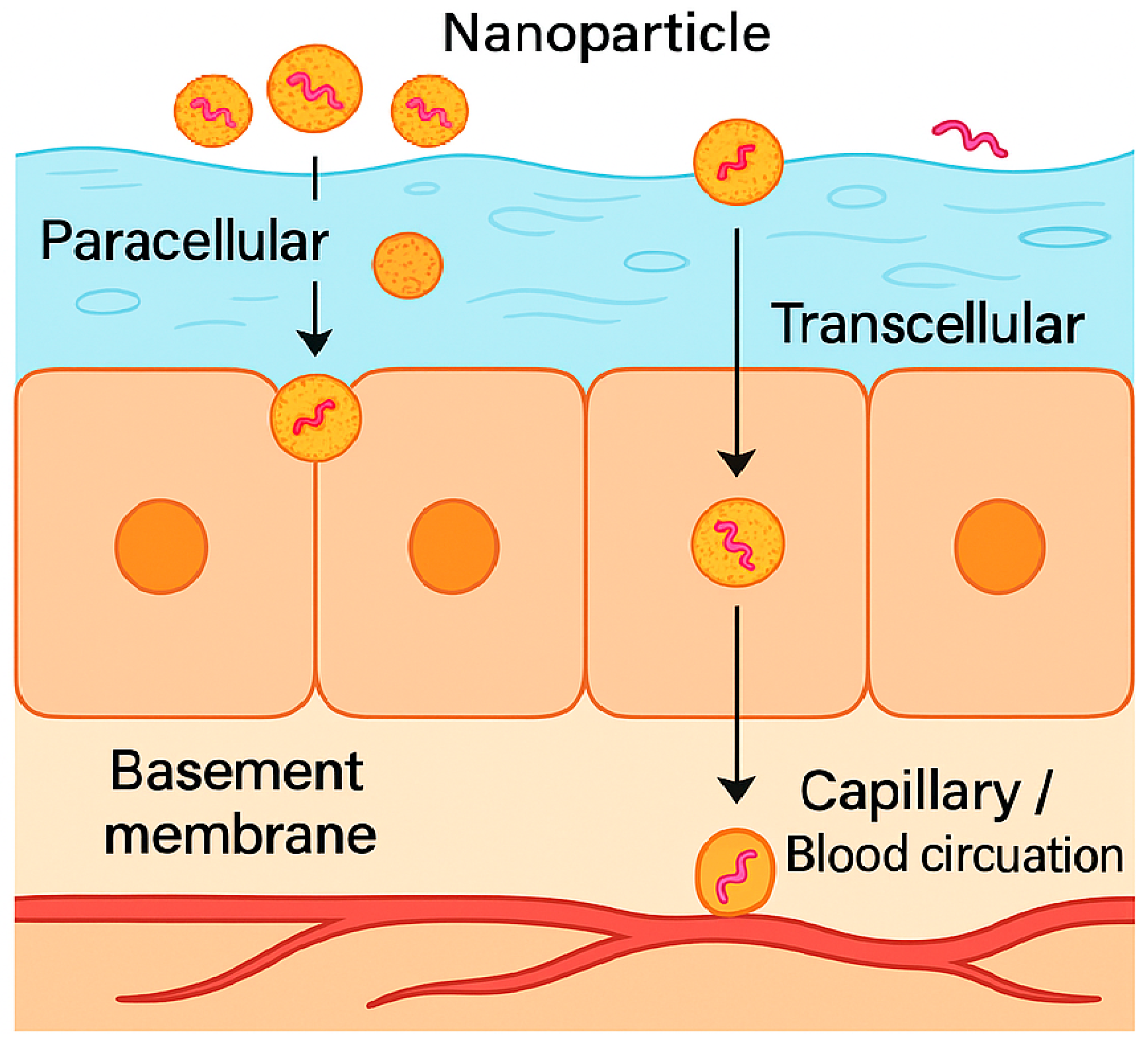
8. Mechanisms and Determinants of Oromucosal Nanoparticle Performance
8.1. Local Microenvironments for Saliva and Mucus
8.2. Nanoparticle Size, Distribution, and Surface Charge
8.3. Nanoparticle Cellular Uptake and Trafficking
8.4. Device Perspective
8.5. Comparative Design and Human Evidence
8.6. Safety and Manufacturability Standards
8.7. Limitations
9. Promising Advances in Nanocarrier-Based Peptide Delivery via the Oral Cavity and Mucosa
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Patel, P. Innovative Strategies In Peptide Therapeutics: Stability Challenges And Advanced Analytical Methods. Int. J. Pharm. Sci. 2024, 2, 97–108. [Google Scholar] [CrossRef]
- Sato, A.K.; Viswanathan, M.; Kent, R.B.; Wood, C.R. Therapeutic peptides: Technological advances driving peptides into development. Curr. Opin. Biotechnol. 2006, 17, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Latham, P.W. Therapeutic peptides revisited. Nat. Biotechnol. 1999, 17, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Pettinato, M. Impact of Intrinsic and Extrinsic Factors on the Pharmacokinetics of Peptides: When Is the Assessment of Certain Factors Warranted? Antibodies 2021, 11, 1. [Google Scholar] [CrossRef]
- Lin, J. Pharmacokinetics of biotech drugs: Peptides, proteins and monoclonal antibodies. Curr. Drug Metab. 2009, 10, 661–691. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef]
- Mehrotra, S.; Bg, P.K.; Nayak, P.G.; Joseph, A.; Manikkath, J. Recent Progress in the Oral Delivery of Therapeutic Peptides and Proteins: Overview of Pharmaceutical Strategies to Overcome Absorption Hurdles. Adv. Pharm. Bull. 2024, 14, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Brinck, J.E.; Sinha, A.K.; Laursen, M.F.; Dragsted, L.O.; Raes, J.; Uribe, R.V.; Walter, J.; Roager, H.M.; Licht, T.R. Intestinal pH: A major driver of human gut microbiota composition and metabolism. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 639–656. [Google Scholar] [CrossRef]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Toniolo, C. The Chemistry of Tryptophan in Peptides and Proteins. In Fortschritte der Chemie Organischer Naturstoffe; Springer: Vienna, Austria, 1976; Volume 33, pp. 309–449. [Google Scholar] [CrossRef]
- Badelin, V.G.; Kulikov, O.; Vatagin, V.; Udzig, E.; Zielenkiewicz, A.; Zielenkiewicz, W.; Krestov, G. Physico-chemical properties of peptides and their solutions. Thermochim. Acta 1990, 169, 81–93. [Google Scholar] [CrossRef]
- Jacob, D.; Taylor, M.J.; Tomlins, P.; Sahota, T. Insulin Solution Stability and Biocompatibility with Materials Used for an Implantable Insulin Delivery Device Using Reverse Phase HPLC Methods. Appl. Sci. 2019, 9, 4794. [Google Scholar] [CrossRef]
- Granero, L.; Polache, A. Absorption of Drugs after Oral Administration. In Pharmaceutical Sciences Encyclopedia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 1, pp. 1–42. [Google Scholar] [CrossRef]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef]
- Campos, L.A.; Sancho, J. The active site of pepsin is formed in the intermediate conformation dominant at mildly acidic pH. FEBS Lett. 2003, 538, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.S. Modeling of intestinal drug absorption: Roles of transporters and metabolic enzymes (for the Gillette Review Series). Drug Metab. Dispos. 2003, 31, 1507–1519. [Google Scholar] [CrossRef]
- Langguth, P.; Bohner, V.; Heizmann, J.; Merkle, H.; Wolffram, S.; Amidon, G.; Yamashita, S. The challenge of proteolytic enzymes in intestinal peptide delivery. J. Control. Release 1997, 46, 39–57. [Google Scholar] [CrossRef]
- Mahato, R.I.; Narang, A.S.; Thoma, L.; Miller, D.D. Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 153–214. [Google Scholar] [CrossRef]
- Mödl, B.; Schmidt, K.; Moser, D.; Eferl, R. The intermicrovillar adhesion complex in gut barrier function and inflammation. Explor. Dig. Dis. 2022, 1, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef]
- Ferraris, R.P. Dietary and developmental regulation of intestinal sugar transport. Biochem. J. 2001, 360, 265–276. [Google Scholar] [CrossRef]
- Mustata, G.; Dinh, S.M. Approaches to oral drug delivery for challenging molecules. Crit. Rev. Ther. Drug Carr. Syst. 2006, 23, 111–135. [Google Scholar] [CrossRef]
- Goldberg, M.; Gomez-Orellana, I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2003, 2, 289–295. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Mishra, R.; Rana, D.; Kundu, P.P. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Prog. Polym. Sci. 2012, 37, 1457–1475. [Google Scholar] [CrossRef]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef] [PubMed]
- Kremsmayr, T.; Aljnabi, A.; Blanco-Canosa, J.B.; Tran, H.N.T.; Emidio, N.B.; Muttenthaler, M. On the Utility of Chemical Strategies to Improve Peptide Gut Stability. J. Med. Chem. 2022, 65, 6191–6206. [Google Scholar] [CrossRef]
- Nicze, M.; Borówka, M.; Dec, A.; Niemiec, A.; Bułdak, Ł.; Okopień, B. The Current and Promising Oral Delivery Methods for Protein- and Peptide-Based Drugs. Int. J. Mol. Sci. 2024, 25, 815. [Google Scholar] [CrossRef] [PubMed]
- Asano, D.; Takakusa, H.; Nakai, D. Oral Absorption of Middle-to-Large Molecules and Its Improvement, with a Focus on New Modality Drugs. Pharmaceutics 2024, 16, 47. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. JNCI Monogr. 2001, 2001, 7–15. [Google Scholar] [CrossRef]
- Brizuela, M.; Winters, R. Histology, Oral Mucosa; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gavriiloglou, M.; Hammad, M.; Iliopoulos, J.M.; Layrolle, P.; Apatzidou, D.A. Bioengineering the Junctional Epithelium in 3D Oral Mucosa Models. J. Funct. Biomater. 2024, 15, 330. [Google Scholar] [CrossRef]
- Wanasathop, A.; Patel, P.B.; Choi, H.A.; Li, S.K. Permeability of Buccal Mucosa. Pharmaceutics 2021, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A. Buccal bioadhesive drug delivery—A promising option for orally less efficient drugs. J. Control. Release 2006, 114, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-G.; Singh, A.P. Emerging strategies for enhancing buccal and sublingual administration of nutraceuticals and pharamaceuticals. J. Drug Deliv. Sci. Technol. 2019, 52, 440–451. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Coderch, L.; Alonso, C.; Calpena, A.C.; Pérez-García, M.L.; Clares-Naveros, B.; Ramos, A.; Martí, M. Permeation Protection by Waterproofing Mucosal Membranes. Pharmaceutics 2023, 15, 2698. [Google Scholar] [CrossRef]
- Tharini, L. A review: Treatment of gingivitis by buccal patches. Int. J. Pharm. Pharm. Sci. 2023, 5, 7–14. [Google Scholar] [CrossRef]
- Marc, V.F.P.; Brown, B. Buccal and Sublingual Drug Delivery|Basicmedical Key. Available online: https://basicmedicalkey.com/buccal-and-sublingual-drug-delivery (accessed on 28 June 2025).
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]
- He, Y.; Vasilev, K.; Zilm, P. pH-Responsive Biomaterials for the Treatment of Dental Caries—A Focussed and Critical Review. Pharmaceutics 2023, 15, 1837. [Google Scholar] [CrossRef]
- Hua, S. Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Authimoolam, S.P.; Dziubla, T.D. Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications. Polymers 2016, 8, 71. [Google Scholar] [CrossRef]
- Walia, C.; Arya, A. Review on Buccal Drug Delivery System. Int. J. Inf. Syst. Manag. Sci. 2023, 8, 2456–4184. [Google Scholar]
- Chen, C.; Patel, A.; Demirkhanyan, L.; Gondi, C.S. The Role of Mucins in Cancer and Cancer Progression: A Comprehensive Review. Curr. Issues Mol. Biol. 2025, 47, 406. [Google Scholar] [CrossRef]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery—Understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chavan, G.A.; Tawani, A.S.; Gavhane, Y.N. Buccal Patches: A Promising Buccal Bioadhesive Drug Delivery System—A Review. Int. J. Pharm. Pharm. Res. 2020, 19, 533–572. [Google Scholar]
- Akbar, S.; Hohman, M.H. Anatomy, Head and Neck, Styloglossus; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ismail, A.S.; Hooper, L.V. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G779–G784. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Passel, M.W.J.; van de Bovenkamp, J.H.B.; Schipper, R.G.; de Vos, W.M.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Rathbone, M.J. Oral Mucosal Drug Delivery; Marcel Dekker: New York, NY, USA, 1996; Volume 74, p. 440. Available online: https://catalog.nlm.nih.gov/discovery/fulldisplay/alma998072333406676/01NLM_INST:01NLM_INST (accessed on 27 October 2025).
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for Enhancing Oral Bioavailability of Peptides and Proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef]
- Malhotra, S.; Lijnse, T.; Cearbhaill, E.O.; Brayden, D.J. Devices to overcome the buccal mucosal barrier to administer therapeutic peptides. Adv. Drug Deliv. Rev. 2025, 220, 115572. [Google Scholar] [CrossRef] [PubMed]
- Rehmani, S.; Dixon, J.E. Oral delivery of anti-diabetes therapeutics using cell penetrating and transcytosing peptide strategies. Peptides 2018, 100, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.C.; Tevini, J.; Mair, L.; Friedl, H.-P.; Fuchs, D.; Felder, T.; Gostner, J.M.; Neuhaus, W. Investigations Towards Tryptophan Uptake and Transport Across an In Vitro Model of the Oral Mucosa Epithelium. Int. J. Tryptophan Res. 2024, 17, 1–10. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, I.J.; Kim, H.; Kim, H.-J.; Jeong, M.J.; Ahn, S.G.; A Kim, S.; Lee, C.H.; Choi, B.K.; Kim, J.-K.; et al. Amino acid transport system L is differently expressed in human normal oral keratinocytes and human oral cancer cells. Cancer Lett. 2005, 222, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Oyama, Y.; Yamano, H.; Ohkuma, A.; Ogawara, K.I.; Higaki, K.; Kimura, T. Carrier-mediated transport systems for glucose in mucosal cells of the human oral cavity. J. Pharm. Sci. 1999, 88, 830–834. [Google Scholar] [CrossRef]
- Walker, G.F.; Langoth, N.; Bernkop-Schnürch, A. Peptidase activity on the surface of the porcine buccal mucosa. Int. J. Pharm. 2002, 233, 141–147. [Google Scholar] [CrossRef]
- Gao, X.; Bhattacharya, S.; Chan, W.K.; Jasti, B.R.; Upadrashta, B.; Li, X. Expression of P-glycoprotein and CYP3A4 along the porcine oral-gastrointestinal tract: Implications on oral mucosal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 599–603. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Petrova, V.A.; Skorik, Y.A. Biopolymer Drug Delivery Systems for Oromucosal Application: Recent Trends in Pharmaceutical R&D. Int. J. Mol. Sci. 2024, 25, 5359. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Valarmathi, S.; Siddique, M.A.M.; Kumar, T.A.; Chiranjeevi, K. Mucoadhesive buccal drug delivery system—An overview. Int. J. Pharm. Technol. 2012, 4, 3951–3969. [Google Scholar]
- Patel, D.A.; Patel, M.R.; Patel, K.R.; Patel, N.M. Buccal mucosa as a route for systemic drug delivery: A review. Int. J. Drug Dev. Res. 2012, 4, 99–116. [Google Scholar]
- Hoogstraate, A.J.; Cullander, C.; Nagelkerke, J.F.; Senel, S.; Verhoef, J.C.; Junginger, H.E.; Boddé, H.E. Diffusion Rates and Transport Pathways of Fluorescein Isothiocyanate (FITC)-Labeled Model Compounds Through Buccal Epithelium. Pharm. Res. 1994, 11, 83–89. [Google Scholar] [CrossRef]
- Fantini, A.; Giulio, L.; Delledonne, A.; Pescina, S.; Sissa, C.; Nicoli, S.; Santi, P.; Padula, C. Buccal Permeation of Polysaccharide High Molecular Weight Compounds: Effect of Chemical Permeation Enhancers. Pharmaceutics 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Keum, T.; Noh, G.; Seo, J.-E.; Bashyal, S.; Lee, S. In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues. Pharmaceuticals 2020, 13, 408. [Google Scholar] [CrossRef]
- Berka, P.; Stránská, D.; Semecký, V.; Berka, K.; Doležal, P. In vitro testing of flash-frozen sublingual membranes for storage and reproducible permeability studies of macromolecular drugs from solution or nanofiber mats. Int. J. Pharm. 2019, 572, 118711. [Google Scholar] [CrossRef] [PubMed]
- Veuillez, F.; Kalia, Y.N.; Jacques, Y.; Deshusses, J.; Buri, P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 2001, 51, 93–109. [Google Scholar] [CrossRef]
- Linker, S.M.; Schellhaas, C.; Kamenik, A.S.; Veldhuizen, M.M.; Waibl, F.; Roth, H.-J.; Fouché, M.; Rodde, S.; Riniker, S. Lessons for Oral Bioavailability: How Conformationally Flexible Cyclic Peptides Enter and Cross Lipid Membranes. J. Med. Chem. 2023, 66, 2773–2788. [Google Scholar] [CrossRef]
- Sivasankaran, R.P.; Snell, K.; Kunkel, G.; Georgiou, P.G.; Puente, E.G.; Maynard, H.D. Polymer-mediated protein/peptide therapeutic stabilization: Current progress and future directions. Prog. Polym. Sci. 2024, 156, 101867. [Google Scholar] [CrossRef]
- Dan, N.; Samanta, K.; Almoazen, H. An Update on Pharmaceutical Strategies for Oral Delivery of Therapeutic Peptides and Proteins in Adults and Pediatrics. Children 2020, 7, 307. [Google Scholar] [CrossRef]
- Schumacher-Klinger, A.; Fanous, J.; Merzbach, S.; Weinmueller, M.; Reichart, F.; Räder, A.F.B.; Gitlin-Domagalska, A.; Gilon, C.; Kessler, H.; Hoffman, A. Enhancing Oral Bioavailability of Cyclic RGD Hexa-peptides by the Lipophilic Prodrug Charge Masking Approach: Redirection of Peptide Intestinal Permeability from a Paracellular to Transcellular Pathway. Mol. Pharm. 2018, 15, 3468–3477. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. The Effect of Various In Vitro Conditions on the Permeability Characteristics of the Buccal Mucosa. J. Pharm. Sci. 2003, 92, 2399–2410. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Kurotani, A.; Sato, K.-I. Protein pI and Intracellular Localization. Front. Mol. Biosci. 2021, 8, 775736. [Google Scholar] [CrossRef]
- Zhang, L.; Bulaj, G. Converting Peptides into Drug Leads by Lipidation. Curr. Med. Chem. 2012, 19, 1602–1618. [Google Scholar] [CrossRef]
- Augustijns, P.F.; Brown, S.C.; Willard, D.H.; Consler, T.G.; Annaert, P.P.; Hendren, R.W.; Bradshaw, T.P. Hydration changes implicated in the remarkable temperature-dependent membrane permeation of cyclosporin A. Biochemistry 2000, 39, 7621–7630. [Google Scholar] [CrossRef] [PubMed]
- Liras, S.; Mcclure, K.F. Permeability of Cyclic Peptide Macrocycles and Cyclotides and Their Potential as Therapeutics. ACS Med. Chem. Lett. 2019, 10, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Raj, N.; Verma, H.; Patel, M.; Chakraborti, S.; Khatri, B.; Doreswamy, C.M.; Anandakumar, S.R.; Seekallu, S.; Dinesh, M.B.; et al. An amide to thioamide substitution improves the permeability and bioavailability of macrocyclic peptides. Nat. Commun. 2023, 14, 6050. [Google Scholar] [CrossRef]
- Li, J.; Yanagisawa, K.; Akiyama, Y. CycPeptMP: Enhancing membrane permeability prediction of cyclic peptides with multi-level molecular features and data augmentation. Brief. Bioinform. 2024, 25, bbae417. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.L.; Habeshian, S.; Li, B.; David, J.-A.G.L.; Nielsen, A.L.; Ji, X.; Khwildy, K.I.; Benitez, M.M.D.; Phothirath, P.; Heinis, C. De novo development of small cyclic peptides that are orally bioavailable. Nat. Chem. Biol. 2023, 20, 624–633. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Guo, Y.; Baldelli, A.; Singh, A. Concept for a Unidirectional Release Mucoadhesive Buccal Tablet for Oral Delivery of Antidiabetic Peptide Drugs Such as Insulin, Glucagon-like Peptide 1 (GLP-1), and their Analogs. Pharmaceutics 2023, 15, 2265. [Google Scholar] [CrossRef]
- Garcia, A.; Eljack, N.D.; Sani, M.-A.; Separovic, F.; Rasmussen, H.H.; Kopec, W.; Khandelia, H.; Cornelius, F.; Clarke, R.J. Membrane accessibility of glutathione. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2430–2436. [Google Scholar] [CrossRef]
- Möbitz, H.; Dittrich, B.; Rodde, S.; Strang, R. Nonclassical Zwitterions as a Design Principle to Reduce Lipophilicity without Impacting Permeability. J. Med. Chem. 2024, 67, 9485–9494. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhu, X.; Tao, W.; Cui, Y.; Liu, M.; Wu, L.; Li, L.; Zheng, Y.; Huang, Y. Enhanced Oral Delivery of Protein Drugs Using Zwitterion-Functionalized Nanoparticles to Overcome both the Diffusion and Absorption Barriers. ACS Appl. Mater. Interfaces 2016, 8, 25444–25453. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.; Witten, J.; Grodzinsky, A.J.; Ribbeck, K. Spatial configuration of charge and hydrophobicity tune particle transport through mucus. Biophys. J. 2022, 121, 277–287. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Lamichhane, S.; Kim, J.H.; Kim, C.H.; Choi, Y.W.; Lee, S. Facilitated buccal insulin delivery via hydrophobic ion-pairing approach: In vitro and ex vivo evaluation. Int. J. Nanomed. 2021, 16, 4677–4691. [Google Scholar] [CrossRef] [PubMed]
- Dowty, M.E.; Knuth, K.E.; Irons, B.K.; Robinson, J.R. Transport of Thyrotropin Releasing Hormone in Rabbit Buccal Mucosa In Vitro. Pharm. Res. 1992, 9, 1113–1122. [Google Scholar] [CrossRef]
- Baral, K.C.; Choi, K.Y. Barriers and Strategies for Oral Peptide and Protein Therapeutics Delivery: Update on Clinical Advances. Pharmaceutics 2025, 17, 397. [Google Scholar] [CrossRef]
- Couto, R.O.D.; Thomaz, D.V.; Duarte, M.P.F.; Lopez, R.F.V.; Pedrazzi, V.; de Freitas, O.; Tartaglia, G.M. Assessing α-Bisabolol as a Transmucosal Permeation Enhancer of Buccal Local Anesthetics. Pharmaceutics 2024, 16, 1198. [Google Scholar] [CrossRef]
- Panou, D.A.; Pedersen, S.F.; Kristensen, M.; Nielsen, H.M. Epithelium dynamics differ in time and space when exposed to the permeation enhancers penetramax and EGTA. A head-to-head mechanistic comparison. Front. Drug Deliv. 2023, 3, 1221628. [Google Scholar] [CrossRef]
- Del Vecchio, G.; Tscheik, C.; Tenz, K.; Helms, H.C.; Winkler, L.; Blasig, R.; Blasig, I.E. Sodium caprate transiently opens claudin-5-containing barriers at tight junctions of epithelial and endothelial cells. Mol. Pharm. 2012, 9, 2523–2533. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Kane, M.P.; Triplitt, C. Current Understanding of Sodium N-(8-[2-Hydroxylbenzoyl] Amino) Caprylate (SNAC) as an Absorption Enhancer: The Oral Semaglutide Experience. Clin. Diabetes 2023, 42, 74–86. [Google Scholar] [CrossRef]
- Wu, J.; Roesger, S.; Jones, N.; Hu, C.-M.J.; Li, S.-D. Cell-penetrating peptides for transmucosal delivery of proteins. J. Control. Release 2024, 366, 864–878. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Wang, N.; Pei, X.; Guo, Y.; Wang, J.; Barth, S.; Yu, F.; Lee, S.J.; He, H.; et al. Cell-penetrating peptide enhanced insulin buccal absorption. Int. J. Pharm. 2020, 584, 119469. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Mitchell, J.; Pawar, H.; Ayensu, I. Functional Characterisation and Permeation Studies of Lyophilised Thiolated Chitosan Xerogels for Buccal Delivery of Insulin. Protein Pept. Lett. 2014, 21, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Mfoafo, K.; Mittal, R.; Eshraghi, A.; Omidi, Y.; Omidian, H. Thiolated polymers: An overview of mucoadhesive properties and their potential in drug delivery via mucosal tissues. J. Drug Deliv. Sci. Technol. 2023, 85, 104596. [Google Scholar] [CrossRef]
- Kali, G.; Taha, A.M.M.M.; Campanella, E.; Truszkowska, M.; Haddadzadegan, S.; Denora, N.; Bernkop-Schnürch, A. Enhanced Mucoadhesion of Thiolated β-Cyclodextrin by S-Protection with 2-Mercaptoethanesulfonic Acid. ACS Omega 2024, 9, 5819–5828. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef]
- Hu, S.; Pei, X.; Duan, L.; Zhu, Z.; Liu, Y.; Chen, J.; Chen, T.; Ji, P.; Wan, Q.; Wang, J. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 2021, 12, 1689, Erratum in Nat. Commun. 2024, 15, 3713. [Google Scholar] [CrossRef]
- Edmans, J.G.; Murdoch, C.; Hatton, P.V.; Madsen, L.S.; Santocildes-Romero, M.E.; Spain, S.G.; Colley, H.E. Bioactive Protein and Peptide Release from a Mucoadhesive Electrospun Membrane. Biomed. Mater. Devices 2024, 2, 444–453. [Google Scholar] [CrossRef]
- Amer, A.A.; Bingle, L.; Chaw, C.S.; Elkordy, A.A. Development of Vancomycin, a Glycopeptide Antibiotic, in a Suitable Nanoform for Oral Delivery. Molecules 2025, 30, 1624. [Google Scholar] [CrossRef]
- Vaidya, A.; Mitragotri, S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J. Control. Release 2020, 327, 26–34. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Santos, H.A. Improving Oral Absorption Via Drug-Loaded Nanocarriers: Absorption Mechanisms, Intestinal Models and Rational Fabrication. Curr. Drug Metab. 2012, 14, 28–56. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-loaded nanocarriers: Passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Damgé, C.; Reis, C.P.; Maincent, P. Nanoparticle strategies for the oral delivery of insulin. Expert Opin. Drug Deliv. 2008, 5, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J.; Chen, Y. Nanoparticles–A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Teubl, B.J.; Meindl, C.; Eitzlmayr, A.; Zimmer, A.; Fröhlich, E.; Roblegg, E. In-vitro permeability of neutral polystyrene particles via buccal mucosa. Small 2013, 9, 457–466. [Google Scholar] [CrossRef]
- Amer, A.A.; Karkar, Y.; Bingle, L.; Elkordy, A.A.; Chaw, C.S. Fast-Disintegrating Oral Films Containing Nisin-Loaded Niosomes. Molecules 2025, 30, 3715. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Svirskis, D.; Proft, T.; Loh, J.; Huang, Y.; Wen, J. Cellular Uptake and Transport Mechanism Investigations of PEGylated Niosomes for Improving the Oral Delivery of Thymopentin. Pharmaceutics 2024, 16, 397. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Lin, G.C.; Leitgeb, T.; Vladetic, A.; Friedl, H.-P.; Rhodes, N.; Rossi, A.; Roblegg, E.; Neuhaus, W. Optimization of an oral mucosa in vitro model based on cell line TR146. Tissue Barriers 2020, 8, 1748459, Erratum in Tissue Barriers 2020, 8, 1784644. [Google Scholar] [CrossRef]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef]
- Lancina, M.G.; Shankar, R.K.; Yang, H. Chitosan Nanofibers for Transbuccal Insulin Delivery. J. Biomed. Mater. Res. Part A 2017, 105, 1252–1259, Erratum in J. Biomed. Mater. Res. Part A 2017, 105, 3520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Deng, H.; Zhang, Y.; Wu, P.; He, B.; Dai, W.; Zhang, H.; Zhang, Q.; Zhao, R.; Wang, X. Thiolated Nanoparticles Overcome the Mucus Barrier and Epithelial Barrier for Oral Delivery of Insulin. Mol. Pharm. 2020, 17, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Kaur, P.; Kaur, V.; Mishra, N.; Vaidya, B. Lipid polymer hybrid as emerging tool in nanocarriers for oral drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 334–349. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Xu, Y.; Meng, Y.; Zhang, X.; Xia, X.; Liu, Y. Factors affecting the buccal delivery of deformable nanovesicles based on insulin–phospholipid complex: An in vivo investigation. Drug Deliv. 2020, 27, 900–908. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Wojnarowicz, J.; Zareba, T.; Koltsov, I.; Lojkowski, W.; Tyski, S.; Mielczarek, A.; Zawadzki, P. Nanoparticles And Human Saliva: A Step Towards Drug Delivery Systems For Dental And Craniofacial Biomaterials. Int. J. Nanomed. 2019, 14, 9235–9257. [Google Scholar] [CrossRef]
- Koller, G.; Schürholz, E.; Ziebart, T.; Neff, A.; Frankenberger, R.; Bartsch, J.W. Clinical Evaluation of Pathognomonic Salivary Protease Fingerprinting for Oral Disease Diagnosis. J. Pers. Med. 2021, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, T.; Ahmed, N.; Hassan, N.U.; Badshah, M.; Khan, S.; Rehman, A.U. Voriconazole nanoparticles-based film forming spray: An efficient approach for potential treatment of topical fungal infections. J. Drug Deliv. Sci. Technol. 2022, 70, 102973. [Google Scholar] [CrossRef]
- Jeitler, R.; Glader, C.; Tetyczka, C.; Zeiringer, S.; Absenger-Novak, M.; Selmani, A.; Fröhlich, E.; Roblegg, E. Investigation of Cellular Interactions of Lipid-Structured Nanoparticles With Oral Mucosal Epithelial Cells. Front. Mol. Biosci. 2022, 9, 917921. [Google Scholar] [CrossRef]
- Mahor, A.K.; Singh, P.P.; Gupta, R.; Bhardwaj, P.; Rathore, P.; Kishore, A.; Goyal, R.; Sharma, N.; Verma, J.; Rosenholm, J.M.; et al. Nanostructured Lipid Carriers for Improved Delivery of Therapeutics via the Oral Route. J. Nanotechnol. 2023, 2023, 4687959. [Google Scholar] [CrossRef]
- Twarog, C.; Fattah, S.; Heade, J.; Maher, S.; Fattal, E.; Brayden, D.J. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10). Pharmaceutics 2019, 11, 78. [Google Scholar] [CrossRef]
- Keum, T.; Noh, G.; Seo, J.-E.; Bashyal, S.; Sohn, D.H.; Lee, S. Examination of Effective Buccal Absorption of Salmon Calcitonin Using Cell-Penetrating Peptide-Conjugated Liposomal Drug Delivery System. Int. J. Nanomed. 2022, 17, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Xu, Y.; Meng, Y.; Ye, J.; Xia, X.; Liu, Y. Deformable Nanovesicle-Loaded Gel for Buccal Insulin Delivery. Pharmaceutics 2022, 14, 2262. [Google Scholar] [CrossRef]
- Sangnim, T.; Dheer, D.; Jangra, N.; Huanbutta, K.; Puri, V.; Sharma, A. Chitosan in Oral Drug Delivery Formulations: A Review. Pharmaceutics 2023, 15, 2361. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Brayden, D.J.; Casettari, L.; Illum, L. Application of Permeation Enhancers in Oral Delivery of Macromolecules: An Update. Pharmaceutics 2019, 11, 41. [Google Scholar] [CrossRef]
- Midaform® Insulin PharmFilm. Phase 2a Trial for Oral Insulin Film|Aquestive. Available online: https://aquestive.com/monosol-rx-announces-initiation-of-phase-2a-trial-for-oral-insulin-film (accessed on 10 October 2025).
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems? Expert Opin. Drug Metab. Toxicol. 2011, 8, 47–69. [Google Scholar] [CrossRef]
- Bierbaumer, L.; Schwarze, U.Y.; Gruber, R.; Neuhaus, W. Cell culture models of oral mucosal barriers: A review with a focus on applications, culture conditions and barrier properties. Tissue Barriers 2018, 6, 1479568. [Google Scholar] [CrossRef]
- McCartney, F.; Gleeson, J.P.; Brayden, D.J. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers 2016, 4, e1176822. [Google Scholar] [CrossRef] [PubMed]
- Shipp, L.; Liu, F.; Kerai-Varsani, L.; Okwuosa, T.C. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. J. Control. Release 2022, 352, 1071–1092. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Jacques, Y. Oral Insulin and Buccal Insulin: A Critical Reappraisal. J. Diabetes Sci. Technol. 2009, 3, 568–584. [Google Scholar] [CrossRef]
- Feitosa, R.C.; Geraldes, D.C.; Beraldo-De-Araújo, V.L.; Costa, J.S.R.; Oliveira-Nascimento, L. Pharmacokinetic aspects of nanoparticle-in-matrix drug delivery systems for oral/buccal delivery. Front. Pharmacol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]

| Peptide | MW (Da) | Therapeutic Uses | Dosage Form |
|---|---|---|---|
| Insulin | ~5800 | Diabetes mellitus | Injection (subcutaneous) |
| GLP-1 analogs (Liraglutide, Semaglutide) | ~3751 (Liraglutide) | Type 2 diabetes, obesity | Injection (subcutaneous and oral) |
| Oxytocin | 1007 | Labor induction, postpartum bleeding | Injection, IV infusion |
| Vasopressin | 1084 | Diabetes insipidus, bleeding control | Injection, nasal spray |
| Calcitonin | 3432 | Osteoporosis, hypercalcemia | Injection, nasal spray |
| Enfuvirtide | 4492 | HIV treatment (fusion inhibitor) | Injection (subcutaneous) |
| Leuprolide | 1209 | Prostate/breast cancer, endometriosis | Injection (IM, SC), implant |
| Desmopressin | 1069 | Diabetes insipidus, bleeding disorders | Nasal spray, oral |
| Somatostatin/Octreotide | 1637 (Somatostatin)/1019 (Octreotide) | Acromegaly, neuroendocrine tumors | Injection (IM, SC), depot injection |
| Exenatide | 4186 | Type 2 diabetes | Injection (SC), extended-release injection |
| Bremelanotide | 1024 | Hypoactive sexual desire disorder | Injection (SC) |
| Thymosin Alpha-1 | 3108 | Immunomodulation (Hepatitis B, C) | Injection (SC, IM) |
| BPC-157 | 1419 | Experimental: wound healing, inflammation | Experimental; injection (SC or IM) |
| Glucagon | 3485 | Severe hypoglycemia emergency treatment | Injection (IM, SC, IV) |
| Melanotan II | 1025 | Investigation: skin tanning, sexual dysfunction | Injection (SC) |
| Thyrotropin-releasing hormone (TRH) | 362 | Diagnostic for pituitary disorders; neuroregulation | Injection (IV) |
| Bivalirudin | 2180 | Anticoagulant | Injection (IV) |
| Goserelin | 1282 | Prostate cancer, breast cancer, endometriosis | Implant, injection (SC) |
| Follitropin alfa | ~30,000 | Fertility treatment (FSH analog) | Injection (SC, IM) |
| Vasopressin analog (Terlipressin) | 1056 | Acute variceal bleeding, septic shock | Injection (IV) |
| Daptomycin (cyclic lipopeptide) | 1620 | Gram-positive bacterial infections (MRSA, VRE) | Injection (IV) |
| Colistin (Polymyxin E) | ~1155 | Multidrug-resistant Gram-negative infections | Injection (IV, IM) |
| Teicoplanin | ~1883 | Gram-positive infections, including MRSA | Injection (IV, IM) |
| Gramicidin | ~1900 | Topical infections | Topical (ointment) |
| Vancomycin (glycopeptide) | 1449 | Serious Gram-positive infections | Injection (IV), Oral (for C. diff) |
| Pexiganan (synthetic magainin analog) | 2249 | Topical diabetic foot infections (under evaluation) | Topical cream |
| Bacitracin | ~1422 | Topical antibacterial for minor skin infections | Topical ointment |
| Nisin | ~3350 | Food preservation, investigational clinical use | Topical, experimental |
| Features | Intestinal Tract | Oral Cavity |
|---|---|---|
| Epithelium cell type | Single-layer columnar epithelium with mucus layer (non-keratinized) | Stratified squamous epithelium (keratinized or non-keratinized) |
| Epithelial Thickness | Thin (20–30 µm) [55] | Thicker (100–200 μm) for non-keratinized epithelium |
| Surface Area | Very large (due to villi and microvilli) | Limited surface area |
| Mucus Layer | Variable thickness and the thinnest in small intestine | Thinner mucus layer [56] |
| Enzymatic Barriers | High activity of digestive enzymes (proteases, peptidases) in lumen and brush border | Proteolytic enzymes are present but less extensive than GIT lumen |
| pH Environment | Variable, acidic (stomach pH 1–3), neutral to basic in intestines | Neutral pH (6.5–7) |
| First-Pass Metabolism | Significant hepatic first-pass metabolism | Bypasses hepatic first-pass metabolism |
| Transit Time | Rapid and variable; exposure limited by gastric emptying and intestinal transit | Short residence time and increase duration of stay with special aids |
| Absorption Route | Transcellular, paracellular and endocytic | Transcellular, paracellular and some endocytic [57] |
| Salivary Washout | Not applicable | Present, reduces drug residence time |
| Bioavailability | Low | Low |
| Accessibility and Control | Anatomically deep within the body, limiting ease of access and direct control over administration conditions | Easily accessible and visible, allowing for precise administration and better control of local environment |
| Advantages | Large surface area and non- invasive | Potential for rapid onset of drug action and may be removed, less metabolism |
| Disadvantages | Harsh enzymatic and acidic environment, first-pass metabolism | Small surface area and presence of salivary clearance |
| Route/Model | Compound and MW | Permeability Enhancer | Permeability/Outcome | Reference |
|---|---|---|---|---|
| Buccal (in vivo, pig) | FD-4 (4 kDa) | without enhancement | Bioavailability 1.8% | Hoogstraate et al., 1994 |
| Buccal (in vivo, pig) | FD-4 (4 kDa) | 10 mM sodium glycodeoxycholate (GDC) | Bioavailability increased to 12.7% | [69] |
| Sublingual (porcine) | FD70 dextran (~70 kDa) | without enhancement | Papp 2 × 10−10 cm/s extremely low permeability | Berka et al., 2019 |
| Sublingual (porcine) | FITC-BSA (66 kDa) As nanofiber mats | without enhancement | Papp1 × 10−7 cm/s 1000× faster than FD70 dextran (~70 kDa) | [72] |
| Esophageal porcine mucosa (a buccal model) | FITC-dextrans (4–70 kDa) | without enhancement | P_app ≤ 10−7 cm/s for >40 kDa FD-70 and FD-150 did not cross the membrane in detectable amounts | Fantini et al., 2022 [70] |
| Esophageal porcine mucosa (a buccal model) | FITC-dextrans + enhancer | +caprylic acid/taurocholate | P_app significantly increased, enabling >10 kDa transport | |
| Buccal (TR146 cells) | Salmon calcitonin (3.4 kDa) | +12.2 µM penetratin (CPP) | 5.5× increase in permeability over passive | Keum et al., 2020 |
| Buccal (porcine tissue) | Salmon calcitonin (3.4 kDa) | +12.2 µM penetratin (CPP) | 93.7× increase in permeability over passive | [71] |
| Nanosystem | Peptide | Key Benefits | References |
|---|---|---|---|
| Liposomes | Salmon calcitonin (sCT) | Cell-penetrating peptide liposomes enhance epithelial uptake and mucoadhesion | [131] |
| Niosomes | Thymopentin (TP5), Vancomycin, Nisin | Pegylated niosomes improve uptake via endocytosis; high encapsulation | [107,117] |
| Chitosan-based PEG-b-PLA nanoparticles | Insulin | Strong mucoadhesion, protection from enzymatic degradation | [120] |
| Thiolated chitosan NPs | Insulin, GLP-1 | Open tight junctions, enhanced paracellular transport | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, A.A.; Bingle, L.; Elkordy, A.A.; Chaw, C.S. Overcoming Oral Cavity Barriers for Peptide Delivery Using Advanced Pharmaceutical Techniques and Nano-Formulation Platforms. Biomedicines 2025, 13, 2735. https://doi.org/10.3390/biomedicines13112735
Amer AA, Bingle L, Elkordy AA, Chaw CS. Overcoming Oral Cavity Barriers for Peptide Delivery Using Advanced Pharmaceutical Techniques and Nano-Formulation Platforms. Biomedicines. 2025; 13(11):2735. https://doi.org/10.3390/biomedicines13112735
Chicago/Turabian StyleAmer, Ali A., Lewis Bingle, Amal Ali Elkordy, and Cheng Shu Chaw. 2025. "Overcoming Oral Cavity Barriers for Peptide Delivery Using Advanced Pharmaceutical Techniques and Nano-Formulation Platforms" Biomedicines 13, no. 11: 2735. https://doi.org/10.3390/biomedicines13112735
APA StyleAmer, A. A., Bingle, L., Elkordy, A. A., & Chaw, C. S. (2025). Overcoming Oral Cavity Barriers for Peptide Delivery Using Advanced Pharmaceutical Techniques and Nano-Formulation Platforms. Biomedicines, 13(11), 2735. https://doi.org/10.3390/biomedicines13112735







